Abstract
Background
Robotic-assisted surgery has evolved over the past 2 decades with constantly improving technology that assists surgeons in multiple subspecialty disciplines. The surgical requirements of lithotomy and steep Trendelenburg positions, along with the creation of a pneumoperitoneum and lack of direct access to the patient all present management challenges in gynecologic surgery. Patient positioning requirements can have significant physiologic effects and can result in many complications.
Methods
This review focuses on the anesthetic and surgical implications of robot-assisted technology in gynecologic surgery.
Conclusion
Good communication among team members and knowledge of the nuances of robotic surgery have the potential to improve patient outcomes, increase efficiency, and reduce complications.
Keywords: Anesthesia, gynecologic surgical procedures, robotics, surgical procedures—minimally invasive
INTRODUCTION
Recent advancements in surgical procedures have led to greater emphasis on minimally invasive techniques with the goal of improving patient outcomes and satisfaction while decreasing surgical morbidity and mortality. Robotic-assisted surgery, the latest innovation in the field of minimally invasive surgeries, first came into medical practice in 1999.1 The basic principle behind this technology is that the robot teleports the surgeon to the operating site and enables operation on the patient from an ergonomic console using 3-dimensional vision and autonomous control of wristed laparoscopic surgical instruments.2,3 Advantages of robotic-assisted surgery include improved precision and enhanced accuracy of movement, both of which translate into potential benefits for patients.4,5
Laparoscopic surgery, introduced in the late 1980s, had certain limitations, such as loss of typical 3-dimensional vision, reduced surgeon coordination, and greatly limited touch.1 Robotic technology overcame many of these obstacles as the technology improved over the years.6 The da Vinci Surgical System mimics a human wrist and includes 3 distinct pieces: a console, a surgical cart with 4 arms (2 representing a surgeon's left and right arms, 1 arm to hold and position the endoscope, and an optional fourth arm to perform other tasks), and an optical 3-dimensional tower that provides stereoscopic vision and runs the software.1
The first robot-assisted surgical procedure was a laparoscopic cholecystectomy in 1997; one of the first gynecologic surgeries performed with the da Vinci system was a tubal reanastomosis.7 Robotic-assisted techniques are being increasingly used for various gynecologic procedures, including total and supracervical hysterectomy, myomectomy, tubal reanastomosis, ovarian cystectomy, sacrocolpopexy, trachelectomy, lymph node dissection, and surgery for endometriosis and ectopic pregnancy.8-10
In recent years, the number of and indications for robotic-assisted gynecologic surgeries have increased. While a number of excellent surgical reviews are now available on the subject, few focus on anesthetic aspects and specific complications related to gynecologic surgery with robotic technology. The surgical requirements of the steep Trendelenburg position along with creation of a pneumoperitoneum present management challenges in gynecologic surgery. This review focuses, therefore, on anesthetic and surgical implications of robotic-assisted technology in gynecologic surgery.
ROBOTIC-ASSISTED SURGERIES
Robotic-assisted surgeries have gained popularity in gynecologic surgery because the robot can be manipulated through the natural orifices. Wrist-like movements of a robot permit the surgeon to perform with accuracy at the surgical site from a distance. The robotic technique is thus most suitable for operations in a closed and confined space such as the pelvis.10
Compared to conventional laparoscopy, robotic-assisted gynecologic surgeries help the surgeon perform complicated procedures with ease, such as securing uterine vessels and cardinal ligaments, performing an accurate colpotomy, and oversewing the vaginal cuff.11 Several study groups have reported on the feasibility of complex endometriosis surgery with the da Vinci Surgical System.10,12,13 Areas in which robotic surgeries seem to be making a mark are complex cases with severe adhesions, scarring, and difficult anatomical conditions where fertility preservation would be extremely difficult with conventional laparoscopy or laparotomy. In addition, recent reports by Tan et al,6 Liu et al,11 Weinberg et al,14 Swan and Advincula,15 Kalmar et al,16 and Lowery et al17 have shown that robotic surgeries are feasible and safe for patients with complicated gynecologic diseases compared to the conventional laparoscopic or open surgeries.
CENTRAL ISSUES FOR THE ANESTHESIOLOGIST
Critical issues for the anesthesiologist during robotic procedures include steep Trendelenburg position, the physiologic consequences of pneumoperitoneum and patient positioning, hypothermia, restricted access to the patient, venous gas embolism, and subcutaneous emphysema. Some of the physiologic changes and complications associated with robotic surgery are outlined in Tables 1 and 2.
Table 1.
Issues for the Anesthesiologist in Robotic-Assisted Gynecologic Surgery
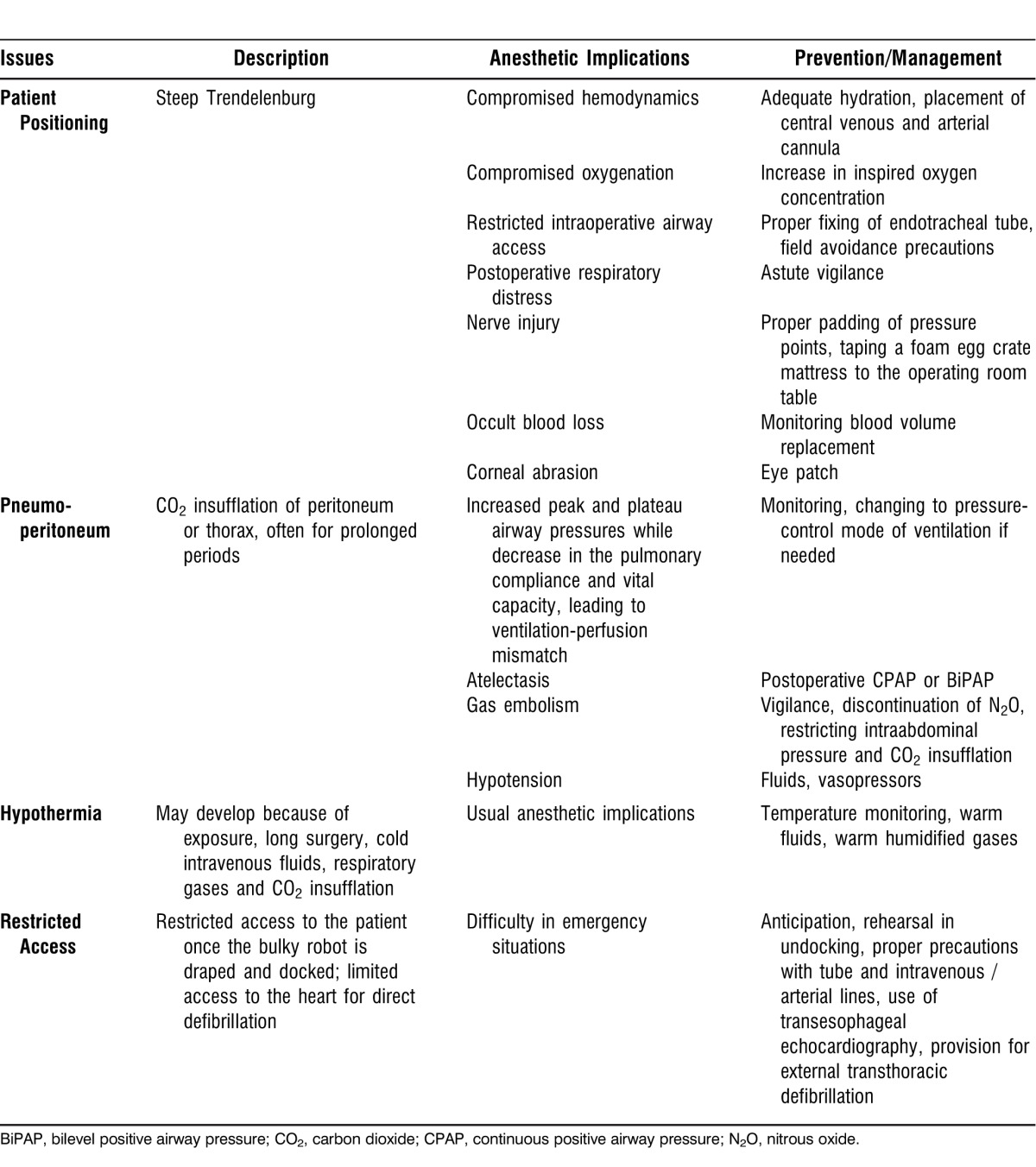
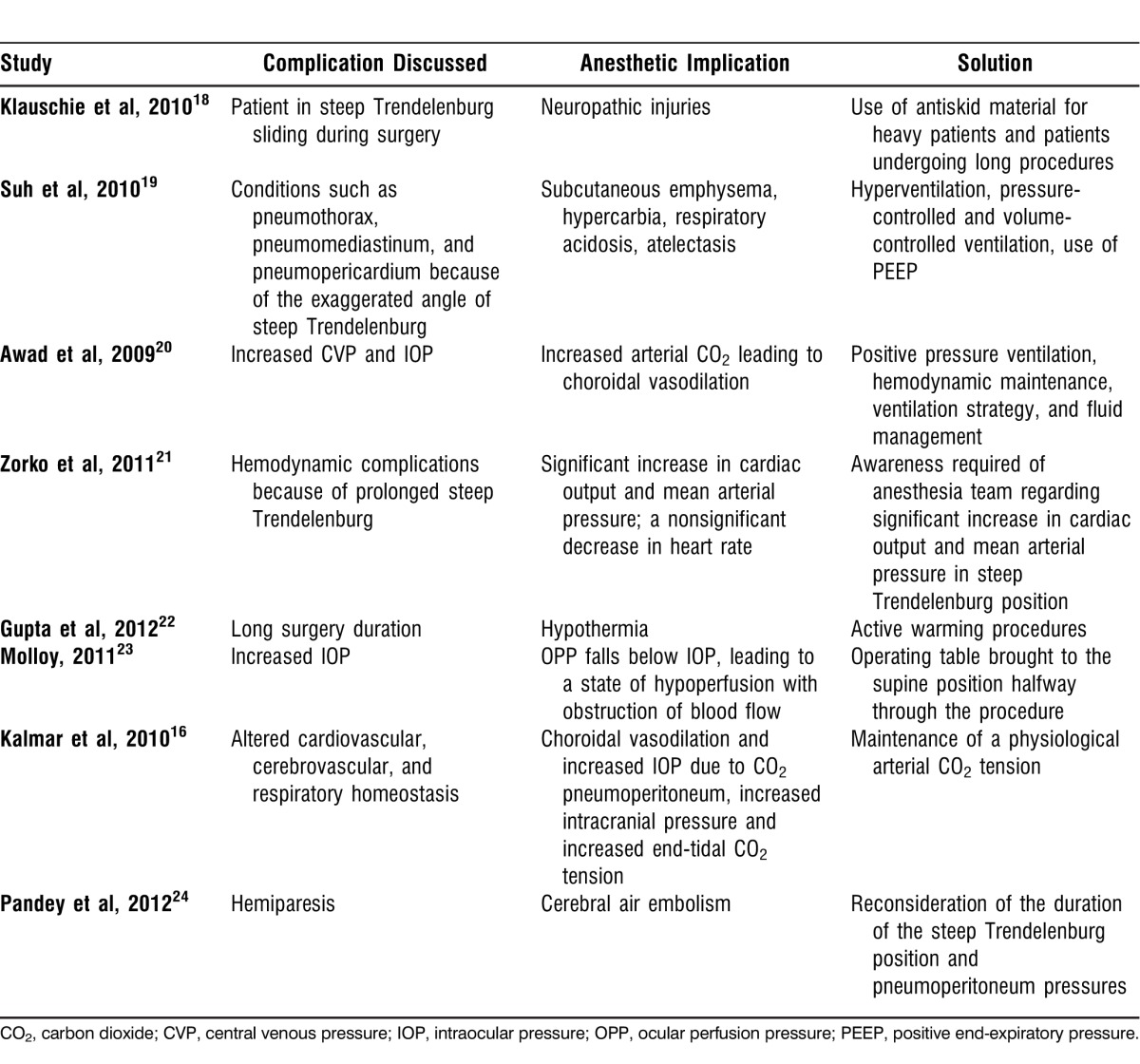
Table 2.
Complications and Their Anesthetic Implications in Robotic-Assisted Gynecologic Surgeries
Patient Positioning
Patient positioning is the most critical part of any robotic-assisted surgery. Without proper patient positioning and port placement, robotic-assisted procedures are tedious to perform and patient outcomes are compromised.25 Once the surgery begins, the patient cannot be moved to any other position during the entire robotic part of the procedure, making the positioning of the patient even more challenging.
The steep Trendelenburg position provides the optimal exposure of the pelvis and the lower abdomen.18 Placing the patients in this position for extended periods can lead to significant physiologic consequences. For example, the downward movement of the diaphragm by abdominal contents and pneumoperitoneum can decrease pulmonary compliance and functional residual capacity, cause pulmonary edema, and exacerbate ventilation/perfusion mismatch.16,26,27 Additional effects on the cardiopulmonary system are discussed in the next section. These effects may further complicate clinical management of patients with underlying chronic lung disease or the morbidly obese. By pushing the trachea cephalad, Trendelenburg position can lead to displacement of the endotracheal tube by pushing it further in, resulting in mainstem intubation.28
Steep inclination of 25-45 degrees for a prolonged period can lead to upper airway and brain edema1 and an increase in intracranial pressure and cerebral blood flow. To preserve cerebrovascular homeostasis, normocarbia should be maintained. Previous studies have also shown that the steep Trendelenburg position for long hours during gynecologic procedures has led to postoperative vision loss.20 Also, facial engorgement and edema are quite substantial. These physiological changes led Molloy23 to hypothesize that under anesthesia in steep Trendelenburg position, cerebrovascular and ophthalmic circulatory autoregulation do not prevent increases in intraocular pressure (IOP) and decreases in ocular perfusion pressure (OPP), which is mean arterial pressure (MAP) minus IOP. The Malloy study showed that even under anesthesia, cerebrovascular and ophthalmic circulatory autoregulation do not prevent complications such as increased IOP.23
Pneumoperitoneum
Pneumoperitoneum refers to the presence of air within the peritoneal cavity. Despite other options such as oxygen, helium, argon, and nitrous oxide, carbon dioxide (CO2) remains the agent most commonly used for creating the pneumoperitoneum because of the problems associated with other gases, such as their combustible nature and the possibility of intravascular embolism on insufflation. Intraperitoneal insufflation with CO2 is performed in Trendelenburg position when the patient is positioned at an angle of 15-20 degrees. There is also a significant effect on respiratory mechanics. Lung compliance can decrease by more than 50%, and mean pulmonary arterial pressure and pulmonary capillary wedge pressure also decrease.29 In addition, there is an increase in peak inspiratory pressure, plateau pressure, and end-tidal CO2 tension.19 The CO2 insufflation can result in increased postoperative complications in patients with underlying lung disease. For example, patients with conditions such as chronic obstructive pulmonary disease are less efficient in eliminating excessive CO2 even with increased minute volume of ventilation. This deficiency can lead to postoperative respiratory hypercarbia and acidosis, requiring prolonged mechanical ventilation.30,31
The combination of the steep Trendelenburg position with pneumoperitoneum influences cardiopulmonary physiology in many ways.32 Pneumoperitoneum and a 45-degree Trendelenburg position have been shown to cause 2- to 3-fold increases in left ventricular filling pressures,33 and cardiac output may decrease.34 Systemic vascular resistance and MAP also increase, whereas renal, splanchnic, and portal flows decrease. Activation of the renin-angiotensin system increases the levels of vasopressin.
PERIOPERATIVE COMPLICATIONS
The mere availability of robotic surgical capability cannot by itself guarantee a successful surgical program. Teamwork is essential for successful patient outcomes. The anesthesiologist must be ready to deal with the consequences of the steep learning curve, stressed surgeons, and the long duration of most procedures. Also, the anesthesiologist must be prepared to handle new challenges associated with proper patient selection and screening, as well as intraoperative care challenges.22,35 Invasion of the anesthetic workspace with the robotic system is almost unavoidable, and anesthesiologists must be aware that the size of the robot might interfere with their ability to quickly access the patient.36 Proper positioning of the patient is a necessary first step for robotic-assisted laparoscopic procedures. Without proper patient positioning and port placement, robotic-assisted procedures are tedious to perform and patient outcomes are compromised.25 The most common complications and their anesthetic implications are summarized in Table 2.
Patient Positioning
Obtaining the proper patient position is a dynamic process that requires the supervision of the surgeon. Not only should the patient be protected from injuries, but the optimal position must also allow for safe docking of the robot as well as for access of the bedside surgeon to the surgical assistant ports.23 Once the procedure begins, the anesthesiologist and the surgeon are limited in making any changes to or improving the positioning of the patient. Consequently, the anesthesiologist must carefully arrange intravenous access and arterial lines (if required) prior to positioning because access will be limited once the robotic portion of the procedure starts. Bilateral peripheral intravenous access is generally advised.
During the steep Trendelenburg position in gynecologic surgeries, shifting the patient's trunk often leads to suboptimal positioning of the extremities, increasing the risk of nerve injury from stretch and compression. Lower extremity acute compartment syndrome requiring fasciotomy and rhabdomyolysis resulting in renal failure as a result of prolonged intraoperative lithotomy position have been reported.37,38
Groups around the world have suggested methods to prevent patient shifting during the steep Trendelenburg position, including braces, leg suspension, and iliac supports. However, all of these methods can potentially result in nerve injury,39-42 and the shoulder braces and straps used to prevent the patient from shifting can cause neuropathic injury.32 During robotic-assisted gynecologic surgeries, the trocars and instruments are fixed, so the prevention of patient sliding becomes all the more important. The risk of stretching and tearing of the incisions, which may increase the risk of an incisional hernia, is a concern. Klauschie et al18 demonstrated for the first time the use of an antiskid foam material for patient positioning. Although they observed small shifts in patient positioning, no clinical neurologic injuries were noted.
Most vulnerable to the head-down extreme position are the cardiac, respiratory, and central nervous systems.10 Because any intraoperative movement can be catastrophic, muscle relaxation is critical for success. Other complications include unrecognized surgical injury, occult blood loss, and risk of hypothermia.1,43
Cardiopulmonary Complications
As discussed previously, the combination of pneumoperitoneum and steep Trendelenburg causes pulmonary problems such as atelectasis and ventilation-perfusion mismatch.1 A decrease in the pulmonary compliance and functional residual capacity is observed, but the peak airway pressures increase. White and Freire44 demonstrated how subcutaneous emphysema occurs frequently with the steep Trendelenburg position and may contribute significantly to the total amount of CO2 absorbed in addition to the absorption of peritoneal CO2 insufflation. Ideally, hyperventilation is the solution to the hypercarbia and respiratory acidosis, but in the steep Trendelenburg position, hyperventilation is limited during robotic surgery by a higher ventilator-inspired pressure. Plus, the abdominal CO2 insufflation also limits diaphragmatic excursion.27 In this setting, Oğurlu et al45 observed lower peak airway pressure and plateau pressure with higher lung compliance with the use of pressure-controlled ventilation. This use of pressure-controlled ventilation—allowing a larger tidal volume for the same inspired pressure—might be particularly useful for patients for whom it is difficult to achieve adequate oxygenation.46
Positive end-expiratory pressure (PEEP) can help decrease atelectasis. PEEP improves intraoperative oxygenation and lung mechanics, impedes the venous blood return from the lower extremities, and decreases cardiac output, but these effects are likely to be negated by the steep Trendelenburg position. Limiting the amount of CO2 insufflation causing increased venous congestion in the upper extremity can help prevent facial and airway edema.47
Many patients with endometrial cancer are obese and have less efficient ventilation during pneumoperitoneum.48 These patients present with further challenges in airway management, and they may be at higher risk of coronary artery disease, pulmonary dysfunction, and diabetes.17 In general, the hindrance to normal diaphragmatic excursion is substantial when these patients are placed in the steep Trendelenburg position.49
With the creation of pneumoperitoneum, immediate gas embolism may occur, and in very rare cases it can cause severe cardiovascular failure, reduction of pulmonary blood flow, and death. The clinical manifestations generally include a sudden increase followed by a rapid drop in end-tidal CO2, tachycardia, hypotension, diminished breath sounds in a specific lung field on auscultation, cyanosis, and a classic cardiac murmur (mill-wheel murmur) associated with gas embolization. The mechanism is perceived to be infiltration of insufflated CO2 into venous/lymphatic channels with pulmonary migration, presumed to occur from rapid insufflation of gas directly into the bloodstream.36 Certain measures to avoid and to treat this complication include rapid removal of pneumoperitoneum, hyperventilation with oxygen, placing the patient in the left lateral decubitus and Trendelenburg positions, cardiopulmonary resuscitation, and potentially aspirating the embolus via a central venous catheter or needle insertion directly into the right ventricle via a substernal approach aimed toward the left shoulder with subsequent therapeutic aspiration of gas.36,48 During the procedure, CO2 should be used for insufflation because of its high diffusion coefficient to minimize the risk of gas emboli.21 The anesthesiologist needs to use extreme caution and measure CO2 levels at the end of exhalation so he/she can adjust the ventilator to remove excess CO2 and help prevent hypercarbia and acidosis.
Cardiac arrhythmias and vagal reactions secondary to peritoneal distention during insufflation or viscus manipulation and diminished cardiac preload secondary to caval compression can contribute to a catastrophic outcome and asystolic cardiac arrest. Hypoxia or hypercapnia can result in cardiac arrhythmias. The combination of Trendelenburg positioning and elevated intraabdominal compartment pressures predispose a patient to aspiration, potentially resulting in hypoxia and possibly hypercapnia. Theoretically, hypercapnia can also occur from CO2 absorption during pneumoperitoneum.
Other Complications
Another major anesthetic consideration during robotic-assisted surgery for endometrial cancers is the prolonged anesthesia that accentuates the problems highlighted above by placing a longer challenge on the patient's cardiorespiratory capacity.50 Prolonged anesthesia is a key area of concern with all robotic-assisted gynecologic procedures. Because many patients undergoing gynecologic surgery are discharged home the same day, adequate pain control and postoperative nausea and vomiting (PONV) are significant concerns. Multimodal approaches to pain management and appropriate PONV prophylaxis have been shown to decrease length of stay and improve patient satisfaction.51,52
TEAMWORK AND COMMUNICATION
Given all of the technological aspects of robotic surgery and the potential physiological consequences and risk of morbidity and mortality specific to gynecologic surgeries, the use of robotic surgery simulation programs may afford distinct advantages when preparing personnel for success in the operating room. Simulation has the potential to improve outcomes and reduce complications while enhancing teamwork.53 In addition, good communication among all members of the team, including surgeons, anesthesiologists, and nurses is the key to a safe, effective, and efficient environment. The addition of audio speakers to transmit the surgeon's voice can also improve communication among team members.22
CONCLUSION
In 2 short decades, robotic surgery has grown into its own subspecialty. As with other procedures, gynecologic robotic-assisted procedures are associated with potentially serious complications as a result of steep Trendelenburg positioning, creation of pneumoperitoneum, and difficult access to the patient. Common complications include positioning injuries, upper body edema, cardiopulmonary compromise, subcutaneous emphysema, and hypothermia. In a review of the literature, the American Association of Gynecology reported an incidence of 1 in 2,500 cases of asystole and arrest during laparoscopy, reflecting the potential for catastrophic morbidity and mortality.54,55
Teamwork and communication among surgeons, nurses, and anesthesiologists are essential to minimize complications and improve surgical conditions and patient outcomes.
ACKNOWLEDGMENT
The authors wish to acknowledge Anna Maria Margarella and Rose Julia Margarella for their own clinical experiences involving a catastrophic outcome involving robotic-assisted surgery.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Goswami S, Nishanian E, Mets B. Anesthesia for robotic surgery. In: RD Miller, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL., editors. Miller's Anesthesia. 7th ed. Philadelphia, PA: Elsevier; 2010:1103, 2389-2395. [Google Scholar]
- 2.Mendivil A, Holloway RW, Boggess JF. Emergence of robotic assisted surgery in gynecologic oncology: American perspective. Gynecol Oncol. 2009 Aug;114((2 Suppl)):S24–S31. doi: 10.1016/j.ygyno.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Boggess J. Robotic surgery in gynecologic oncology: evolution of a new surgical paradigm. J Robotic Surg. 2007 Mar;1(1):31–37. doi: 10.1007/s11701-007-0011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mettler L, Schollmeyer T, Boggess J, Magrina JF, Oleszczuk A. Robotic assistance in gynecologic oncology. Curr Opin Oncol. 2008 Sep;20(5):581–589. doi: 10.1097/CCO.0b013e328307c7ec. [DOI] [PubMed] [Google Scholar]
- 5.Kauffman EC, Ng CK, Lee MM, et al. Critical analysis of complications after robotic-assisted radical cystectomy with identification of preoperative and operative risk factors. BJU Int. 2010 Feb;105(4):520–527. doi: 10.1111/j.1464-410X.2009.08843.x. Epub 2009 Sep 4. [DOI] [PubMed] [Google Scholar]
- 6.Tan SJ, Lin CK, Fu PT, et al. Robotic surgery in complicated gynecologic diseases: experience of Tri-Service General Hospital in Taiwan. Taiwan J Obstet Gynecol. 2012 Mar;51(1):18–25. doi: 10.1016/j.tjog.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Degueldre M, Vandromme J, Huong PT, Cadière GB. Robotically assisted laparoscopic microsurgical tubal reanastomosis: a feasibility study. Fertil Steril. 2000 Nov;74(5):1020–1023. doi: 10.1016/s0015-0282(00)01543-0. [DOI] [PubMed] [Google Scholar]
- 8.Advincula AP, Song A. The role of robotic surgery in gynecology. Curr Opin Obstet Gynecol. 2007 Aug;19(4):331–336. doi: 10.1097/GCO.0b013e328216f90b. [DOI] [PubMed] [Google Scholar]
- 9.Swan K, Advincula AP. Role of robotic surgery in urogynecologic surgery and radical hysterectomy: how far can we go? Curr Opin Urol. 2011 Jan;21(1):78–83. doi: 10.1097/MOU.0b013e328340451a. [DOI] [PubMed] [Google Scholar]
- 10.Sener A, Chew BH, Duvdevani M, Brock GB, Vilos GA, Pautler SE. Combined transurethral and laparoscopic partial cystectomy and robot-assisted bladder repair for the treatment of bladder endometrioma. J Minim Invasive Gynecol. 2006 May-Jun;13(3):245–248. doi: 10.1016/j.jmig.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Lu D, Wang L, Shi G, Song H, Clarke J. Robotic surgery for benign gynaecological disease. Cochrane Database Syst Rev. 2012. Feb 15;2:CD008978. [DOI] [PubMed]
- 12.Cadière GB, Himpens J, Germay O, et al. Feasibility of robotic laparoscopic surgery: 146 cases. World J Surg. 2001 Nov;25(11):1467–1477. doi: 10.1007/s00268-001-0132-2. [DOI] [PubMed] [Google Scholar]
- 13.Nezhat C, Saberi NS, Shahmohamady B, Nezhat F. Robotic-assisted laparoscopy in gynecologic surgery. JSLS. 2006 Jul-Sep;10(3):317–320. [PMC free article] [PubMed] [Google Scholar]
- 14.Weinberg L, Rao S, Escobar PF. Robotic surgery in gynecology: an updated systematic review. Obstet Gynecol Int. 2011. 2011:852061. Epub 2011 Nov 28. [DOI] [PMC free article] [PubMed]
- 15.Swan K, Advincula AP. Advances in urogynaecological robotic surgery. BJU Int. 2011 Sep;108((6 Pt 2)):1024–1027. doi: 10.1111/j.1464-410X.2011.10557.x. [DOI] [PubMed] [Google Scholar]
- 16.Kalmar AF, Foubert L, Hendrickx JF, et al. Influence of steep Trendelenburg position and CO(2) pneumoperitoneum on cardiovascular, cerebrovascular, and respiratory homeostasis during robotic prostatectomy. Br J Anaesth. 2010 Apr;104(4):433–439. doi: 10.1093/bja/aeq018. Epub 2010 Feb 18. [DOI] [PubMed] [Google Scholar]
- 17.Lowery WJ, Leath CA, 3rd, Robinson RD. Robotic surgery applications in the management of gynecologic malignancies. J Surg Oncol. 2012 Apr 1;105(5):481–487. doi: 10.1002/jso.22080. [DOI] [PubMed] [Google Scholar]
- 18.Klauschie J, Wechter ME, Jacob K, et al. Use of anti-skid material and patient-positioning to prevent patient shifting during robotic-assisted gynecologic procedures. J Minim Invasive Gynecol. 2010 Jul-Aug;17(4):504–507. doi: 10.1016/j.jmig.2010.03.013. Epub 2010 May 14. [DOI] [PubMed] [Google Scholar]
- 19.Suh MK, Seong KW, Jung SH, Kim SS. The effect of pneumoperitoneum and Trendelenburg position on respiratory mechanics during pelviscopic surgery. Korean J Anesthesiol. 2010 Nov;59(5):329–334. doi: 10.4097/kjae.2010.59.5.329. Epub 2010 Nov 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Awad H, Santilli S, Ohr M, et al. The effects of steep Trendelenburg positioning on intraocular pressure during robotic radical prostatectomy. Anesth Analg. 2009 Aug;109(2):473–478. doi: 10.1213/ane.0b013e3181a9098f. [DOI] [PubMed] [Google Scholar]
- 21.Zorko N, Mekiš D, Kamenik M. The influence of the Trendelenburg position on haemodynamics: comparison of anaesthetized patients with ischaemic heart disease and healthy volunteers. J Int Med Res. 2011;39(3):1084–1089. doi: 10.1177/147323001103900343. [DOI] [PubMed] [Google Scholar]
- 22.Gupta K, Mehta Y. Sarin Jolly A, Khanna S. Anaesthesia for robotic gynaecological surgery. Anaesth Intensive Care. 2012 Jul;40(4):614–621. doi: 10.1177/0310057X1204000406. [DOI] [PubMed] [Google Scholar]
- 23.Molloy BL. Implications for postoperative visual loss: steep Trendelenburg position and effects on intraocular pressure. AANA J. 2011 Apr;79(2):115–121. [PubMed] [Google Scholar]
- 24.Pandey R, Garg R, Darlong V, Punj J. Chandralekha. Hemiparesis after robotic laparoscopic radical cystectomy and ileal conduit formation in steep Trendelenburg position. J Robotic Surg. 2012;6(3):269–271. doi: 10.1007/s11701-011-0302-7. [DOI] [PubMed] [Google Scholar]
- 25.Ramanathan R, Carey RI, Lopez-Pujals A, Leveillee RJ. Patient positioning and trocar placement for robotic urologic procedures. In: Patel VR, editor. Robotic Urologic Surgery. 2nd ed. London: Springer-Verlag;; 2012. pp. 107–120. [Google Scholar]
- 26.Hirvonen EA, Nuutinen LS, Kauko M. Hemodynamic changes due to Trendelenburg positioning and pneumoperitoneum during laparoscopic hysterectomy. Acta Anaesthesiol Scand. 1995 Oct;39(7):949–955. doi: 10.1111/j.1399-6576.1995.tb04203.x. [DOI] [PubMed] [Google Scholar]
- 27.Choi SJ, Gwak MS, Ko JS, et al. The effects of the exaggerated lithotomy position for radical perineal prostatectomy on respiratory mechanics. Anaesthesia. 2006 May;61(5):439–443. doi: 10.1111/j.1365-2044.2006.04614.x. [DOI] [PubMed] [Google Scholar]
- 28.Chang CH, Lee HK, Nam SH. The displacement of the tracheal tube during robot-assisted radical prostatectomy. Eur J Anaesthesiol. 2010 May;27(5):478–480. doi: 10.1097/EJA.0b013e328333d587. [DOI] [PubMed] [Google Scholar]
- 29.Nieminen MS, Böhm M, Cowie MR, et al. ESC Committee for Practice Guideline (CPG). Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005 Feb;26(4):384–416. doi: 10.1093/eurheartj/ehi044. Epub 2005 Jan 28. [DOI] [PubMed] [Google Scholar]
- 30.Carry PY, Gallet D, François Y, et al. Respiratory mechanics during laparoscopic cholecystectomy: the effects of the abdominal wall lift. Anesth Analg. 1998 Dec;87(6):1393–1397. doi: 10.1097/00000539-199812000-00035. [DOI] [PubMed] [Google Scholar]
- 31.Koivusalo AM, Lindgren L. Respiratory mechanics during laparoscopic cholecystectomy. Anesth Analg. 1999 Sep;89(3):800. doi: 10.1097/00000539-199909000-00052. [DOI] [PubMed] [Google Scholar]
- 32.Barnett JC, Hurd WW, Rogers RM, Jr, Williams NL, Shapiro SA. Laparoscopic positioning and nerve injuries. J Minim Invasive Gynecol. 2007 Sep-Oct;14(5):664–672. doi: 10.1016/j.jmig.2007.04.008. ; quiz 673. [DOI] [PubMed] [Google Scholar]
- 33.Lestar M, Gunnarsson L, Lagerstrand L, Wiklund P, Odeberg-Wernerman S. Hemodynamic perturbations during robot-assisted laparoscopic radical prostatectomy in 45° Trendelenburg position. Anesth Analg. 2011 Nov;113(5):1069–1075. doi: 10.1213/ANE.0b013e3182075d1f. Epub 2011 Jan 13. [DOI] [PubMed] [Google Scholar]
- 34.Joris JL, Noirot DP, Legrand MJ, Jacquet NJ, Lamy ML. Hemodynamic changes during laparoscopic cholecystectomy. Anesth Analg. 1993 May;76(5):1067–1071. doi: 10.1213/00000539-199305000-00027. [DOI] [PubMed] [Google Scholar]
- 35.Lanfranco AR, Castellanos AE, Desai JP, Meyers WC. Robotic surgery: a current perspective. Ann Surg. 2004 Jan;239(1):14–21. doi: 10.1097/01.sla.0000103020.19595.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Dawlatly AA, Khairy G, Al-Dohayan A, Alsaigh A, Abdulkarim A, Alotaibi W. Anesthetic considerations with telemanipulative robot-assisted laparoscopic cholecystectomy using the Da Vinci System. Internet J Anesthesiol. 2004;8(2) http://archive.ispub.com/journal/the-internet-journal-of-anesthesiology/volume-8- number-2/anesthetic-considerations-with-telemanipulative- robot-assisted-laparoscopic-cholecystectomy-using-the-da- vinci-system.html#sthash.NGV3eBEV.dpbs. Accessed September 23, 2013. [Google Scholar]
- 37.Beraldo S, Dodds SR. Lower limb acute compartment syndrome after colorectal surgery in prolonged lithotomy position. Dis Colon Rectum. 2006 Nov;49(11):1772–1780. doi: 10.1007/s10350-006-0712-1. [DOI] [PubMed] [Google Scholar]
- 38.Mattei A, Di Pierro GB, Rafeld V, Konrad C, Beutler J, Danuser H. Positioning injury, rhabdomyolysis, and serum creatine kinase-concentration course in patients undergoing robot-assisted radical prostatectomy and extended pelvic lymph node dissection. J Endourol. 2013 Jan;27(1):45–51. doi: 10.1089/end.2012.0169. Epub 2012 Sep 10. [DOI] [PubMed] [Google Scholar]
- 39.Coppieters MW, Van de Velde M, Stappaerts KH. Positioning in anesthesiology: toward a better understanding of stretch-induced perioperative neuropathies. Anesthesiology. 2002 Jul;97(1):75–81. doi: 10.1097/00000542-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Howkins J. The Trendelenburg position. Lancet. 1952 Oct 18;2(6738):759–760. doi: 10.1016/s0140-6736(52)91238-5. [DOI] [PubMed] [Google Scholar]
- 41.Hewer CL. Maintenance of the Trendelenburg position by skin friction. Lancet. 1953 Mar 14;1(6759):522–524. doi: 10.1016/s0140-6736(53)92362-9. [DOI] [PubMed] [Google Scholar]
- 42.Irvin W, Andersen W, Taylor P, Rice L. Minimizing the risk of neurologic injury in gynecologic surgery. Obstet Gynecol. 2004 Feb;103(2):374–382. doi: 10.1097/01.AOG.0000110542.53489.c6. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan MJ, Frost EA, Lew MW. Anesthetic care of the patient for robotic surgery. Middle East J Anesthesiol. 2008 Jun;19(5):967–982. [PubMed] [Google Scholar]
- 44.White PF, Freire AR. Ambulatory (outpatient) anesthesia. In: RD Miller, Fleisher LA, Jones RA, Savarese JJ, Wiener-Kronish JP, Young WL., editors. Miller's Anesthesia. 6th ed. Philadelphia, PA: Churchill Livingstone; 2005. pp. 2589–2623. [Google Scholar]
- 45.Oğurlu M, Küçük M, Bilgin F, et al. Pressure-controlled vs volume-controlled ventilation during laparoscopic gynecologic surgery. J Minim Invasive Gynecol. 2010 May-Jun;17(3):295–300. doi: 10.1016/j.jmig.2009.10.007. Epub 2010 Mar 19. [DOI] [PubMed] [Google Scholar]
- 46.Stolzenburg JU, Aedtner B, Olthoff D, et al. Anaesthetic considerations for endoscopic extraperitoneal and laparoscopic transperitoneal radical prostatectomy. BJU Int. 2006 Sep;98(3):508–513. doi: 10.1111/j.1464-410X.2006.06223.x. [DOI] [PubMed] [Google Scholar]
- 47.Weingarten TN, Whalen FX, Warner DO, et al. Comparison of two ventilatory strategies in elderly patients undergoing major abdominal surgery. Br J Anaesth. 2010 Jan;104(1):16–22. doi: 10.1093/bja/aep319. [DOI] [PubMed] [Google Scholar]
- 48.Sprung J, Whalley DG, Falcone T, Warner DO, Hubmayr RD, Hammel J. The impact of morbid obesity, pneumoperitoneum, and posture on respiratory system mechanics and oxygenation during laparoscopy. Anesth Analg. 2002 May;94(5):1345–1350. doi: 10.1097/00000539-200205000-00056. [DOI] [PubMed] [Google Scholar]
- 49.Danic MJ, Chow M, Alexander G, Bhandari A, Menon M, Brown M. Anesthesia considerations for robotic-assisted laparoscopic prostatectomy: a review of 1,500 cases. J Robotic Surg. 2007 Jul;1(2):119–123. doi: 10.1007/s11701-007-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bird VG, Winfield HN. Laparoscopy in urology: physiologic considerations. In: Fallon B, editor. Hospital Physician Urology Board Review. Wayne, PA: Turner White Communications, Inc.;; 2002. [Google Scholar]
- 51.Trabulsi EJ, Patel J, Viscusi ER, Gomella LG, Lallas CD. Preemptive multimodal pain regimen reduces opioid analgesia for patients undergoing robotic-assisted laparoscopic radical prostatectomy. Urology. 2010 Nov;76(5):1122–1124. doi: 10.1016/j.urology.2010.03.052. Epub 2010 Jun 8. [DOI] [PubMed] [Google Scholar]
- 52.Elvir-Lazo OL, White PF. The role of multimodal analgesia in pain management after ambulatory surgery. Curr Opin Anaesthesiol. 2010 Dec;23(6):697–703. doi: 10.1097/ACO.0b013e32833fad0a. [DOI] [PubMed] [Google Scholar]
- 53.Shamim Khan M, Ahmed K, Gavazzi A. et al. Development and implementation of centralized simulation training: evaluation of feasibility, acceptability and construct validity. BJU Int. 2013 Mar;111(3):518–523. doi: 10.1111/j.1464-410X.2012.11204.x. Epub 2012 Aug 29. [DOI] [PubMed] [Google Scholar]
- 54.Shifren JL, Adlestein L, Finkler NJ. Asystolic cardiac arrest: a rare complication of laparoscopy. Obstet Gynecol. 1992 May;79((5 Pt 2)):840–841. [PubMed] [Google Scholar]
- 55.Loris JL. Anesthesia for laparoscopic surgery. In: RD Miller, Fleisher LA, Jones RA, Savarese JJ, Wiener-Kronish JP, Young WL., editors. Miller's Anesthesia. Philadelphia, PA: Churchill Livingstone; 2005. p. 2289. 6th ed. [Google Scholar]


