Abstract
Background
Multiple myeloma is a common disease, accounting for about 10% of hematologic malignancies in the United States. For eligible patients, the treatment of choice includes induction therapy (usually involving newer biologic agents) followed by autologous stem cell transplant; however, this treatment is generally not considered curative, and relapses usually occur. However, extramedullary relapse is an uncommon presentation, and relapses that involve the lungs have only rarely been described.
Case Report
We report the case of a patient who underwent an autologous stem cell transplant for multiple myeloma and subsequently relapsed with diffuse pulmonary nodules. She then had a rapid clinical and serologic response following initiation of salvage therapy.
Conclusion
This case is remarkable for both the radiographic appearance of the pulmonary involvement, as well as the rapid resolution of these findings after 2 cycles of treatment with bortezomib, dexamethasone, and lenalidomide.
Keywords: Multiple myeloma, multiple pulmonary nodules, plasmacytoma, recurrence, stem cell transplantation
INTRODUCTION
Accounting for approximately 10% of hematologic malignancies, multiple myeloma is a common hematologic malignancy with an annual incidence of 20,000 people in the United States.1,2 It is characterized by an abnormal clonal proliferation of plasma cells and classically presents with renal failure, anemia, hypercalcemia, bone pain, or some combination of these symptoms.3 In young (<65 years), healthy patients, autologous stem cell transplant after initial treatment with traditional chemotherapeutic agents has been shown to increase response rates and median survival compared to conventional chemotherapy. Newer biologic agents are frequently used initially as these have been shown to increase the complete response rate. Unfortunately, even with such measures, most patients with multiple myeloma relapse.
Relapsed or progressive multiple myeloma is frequently revealed by an increase in a serum and/or urine monoclonal protein or by an increase in bone marrow plasma cell percentage in patients with no identifiable monoclonal protein. Extramedullary plasmacytomas are less frequent than bone marrow involvement at relapse but occur in about 10% of patients.4,5 Isolated pulmonary relapse is rare.
We report the case of a patient with multiple myeloma who achieved a very good partial response after an autologous stem cell transplant preceded by treatment with lenalidomide and dexamethasone. She relapsed 8 months later with the unusual presentation of respiratory symptoms and diffuse pulmonary nodules. Also of interest is the rapid resolution of her symptoms and radiographic findings after treatment with 2 cycles of bortezomib, lenalidomide, and dexamethasone.
CASE REPORT
A 62-year-old African-American female initially presented with left hip pain. Imaging studies revealed a large area of lytic bone destruction in the L proximal femur. She underwent an open biopsy and prophylactic intramedullary nailing for impending fracture. The biopsy revealed sheets of plasma cells. Additional studies demonstrated normal calcium, hemoglobin, and creatinine. The skeletal survey was notable for faint lucencies throughout the calvarium and degenerative changes throughout the spine. Serum protein electrophoresis (SPEP) demonstrated a paraprotein measuring 0.52 g/dL in the second beta band. Immunofixation identified this paraprotein as IgA kappa. The bone marrow contained about 15% plasma cells at that time. Cytogenetics revealed a normal karyotype.
The patient was treated with thalidomide and dexamethasone for 8 months until discontinuation secondary to peripheral neuropathy. At that time, she had achieved at least a very good partial response by all peripheral measures. A repeat bone marrow aspirate performed at another facility was reported to contain 5% plasma cells, but no mention was made regarding the kappa/lambda ratio by immunohistochemistry or immunofluorescence. A discussion about autologous stem cell transplant was held; however, the patient elected observation at that time.
Two years after discontinuation of her myeloma therapy, she relapsed with an IgA kappa paraprotein measuring 1.93 g/dL in the beta-1 band on SPEP and an elevated serum kappa light chain level of 5.88 mg/dL. Calcium and creatinine remained normal. Normocytic anemia was present but was not significantly changed since its onset shortly after her initial diagnosis.
The patient then took 4 cycles of lenalidomide and dexamethasone and achieved a partial response. She maintained a 0.62 g/dL monoclonal protein on SPEP, and bone marrow biopsy demonstrated 15% plasma cells with in situ hybridization showing polytypic plasma cells. This treatment was followed by an autologous stem cell transplant after melphalan. Her initial posttransplant studies completed 10 weeks after her transplant revealed a 0.38 g/dL paraprotein and undetectable serum free kappa light chains. Follow-up studies performed 5 months posttransplant revealed a persistent 0.36 g/dL paraprotein band in the beta-1 region (which was also the nadir), normal urine protein electrophoresis, normal serum free kappa light chains, normal quantitative IgA, and a bone marrow aspirate that revealed only occasional plasma cells with no kappa or lambda light chain restriction.
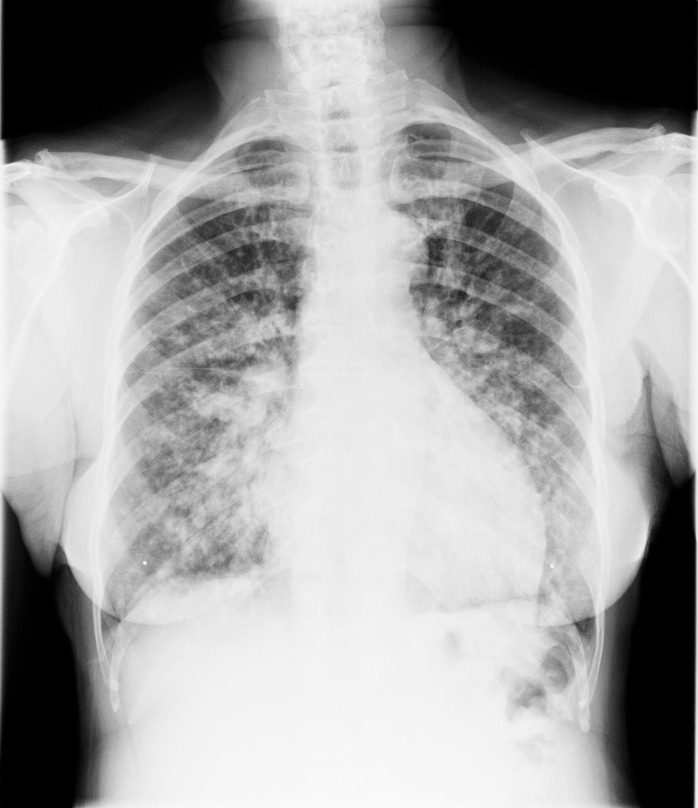
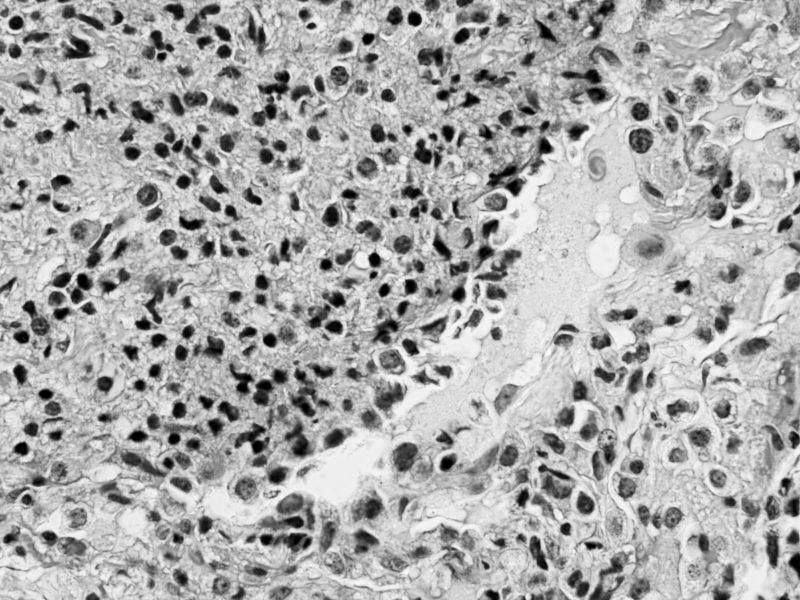
Eight months after transplant, the patient returned with cough, shortness of breath, night sweats, and fatigue. She was hypoxemic on room air with an oxygen saturation of 87%. Chest x-rays demonstrated multiple pulmonary nodules in a perihilar distribution (Figure 1). A noncontrast chest computed tomography scan revealed innumerable noncalcified bilateral pulmonary nodules clustered about the airways (Figures 2 and 3). Most of these nodules were 3-5 mm but some exceeded 10 mm in maximal diameter. Material obtained from a bronchoscopy with transbronchial brushing and transbronchial biopsies revealed neoplastic plasma cells positive for CD138 and CD56 and negative for CD20 and cyclin D1 (Figure 4). Immunostaining showed a kappa light chain restricted plasma cell population consistent with plasma cell neoplasm. Skeletal survey disclosed multiple small lucencies in the calvarium that were unchanged. There were no new lytic lesions. SPEP revealed a 1.55 g/dL paraprotein band (IgA kappa by immunofixation) in the beta-1 region. Serum free kappa light chains measured 5.43 mg/dL with a kappa to lambda free light chain ratio of 5.62. Quantitative IgA was 1,650 mg/dL. A bone marrow biopsy contained 2%-3% plasma cells, with in situ hybridization for kappa and lambda showing a polytypic plasma cell population.
Figure 1.
Posteroanterior chest radiograph reveals innumerable nodules in perihilar distribution with basilar predominance. Perihilar stripe and hilar contours appear normal, arguing against lymph node enlargement. No pleural fluid is evident.
Figure 2.
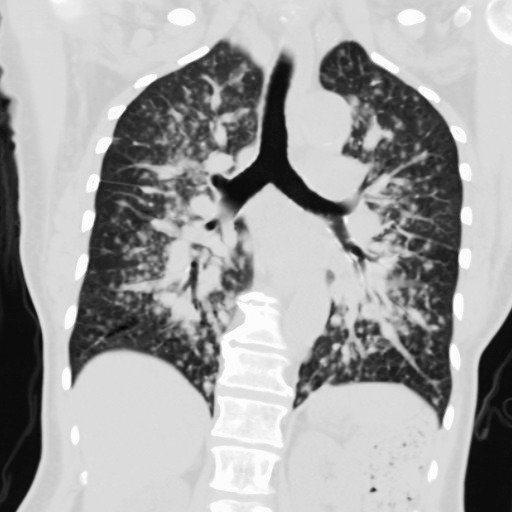
Computed tomographic multiplanar image reconstructed in the coronal plane at the level of the carina reveals multiple pulmonary nodules in perihilar distribution, corresponding to the abnormality seen on frontal chest radiograph.
Figure 3.
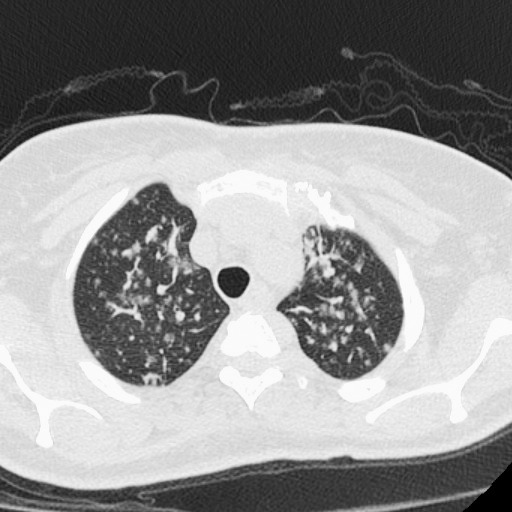
High-resolution computed tomography image with 0.625 mm craniocaudal thickness and high spatial frequency reconstruction algorithm reveals subpleural location of some nodules. Pulmonary vascular margins are smooth, excluding perivascular nodules. The presence of subpleural nodules and the absence of beaded vascular margins argue for random nodular distribution due to hematogenous dissemination.
Figure 4.
Right middle lobe transbronchial biopsy demonstrating plasma cells (hematoxylin and eosin stain magnified 400×). The neoplastic cells were positive for CD138 and CD56.
After her pulmonary relapse, the patient restarted treatment and received bortezomib, dexamethasone, and lenalidomide. After 2 cycles, her pulmonary symptoms and hypoxemia resolved. Chest x-rays at that time demonstrated total resolution of her pulmonary nodules within 6 weeks after her initial presentation. Serum protein electrophoresis and serum immunofixation detected no monoclonal proteins. The quantitative IgA and free light chain ratio returned to normal. She attained at least a very good partial response with no detectable paraprotein markers. A bone marrow biopsy has not been repeated. She was treated with 2 additional cycles of bortezomib, dexamethasone, and lenalidomide and has subsequently remained on maintenance lenalidomide with no recurrence of her paraprotein.
DISCUSSION
Plasmacytomas are tumors composed of clonal plasma cells. They can occur both within the bones (plasmacytomas of the bone) or outside the bones (extramedullary plasmacytomas). At diagnosis of multiple myeloma, the incidence of extramedullary disease is reported to be from 7% to 19%.4,5 Another 6% to 20% of patients subsequently develop plasmacytomas.4-6 Rates of extramedullary disease at relapse ranging from 20% to 37% have been described in patients following allogeneic transplantation with dose-reduced intensity conditioning.7,8 The frequency of extramedullary involvement in patients who relapsed after myeloablative allogeneic transplantation was similar at 32%.9 Reports of extramedullary relapse rates after autologous stem cell transplantation are lower at 9% to 14%.9-11 Although the reason for the lower incidence following autologous stem cell transplant compared to allogeneic stem cell transplant is unknown, possible explanations include differences in patient and disease characteristics or because the graft versus myeloma effect is more effective on bone marrow than at extramedullary sites.12
Extramedullary plasmacytomas may occur as a consequence of several factors, including local growth, hematogenous spread, or invasive or surgical procedures.12 Sites of hematogenous spread include the subcutaneous tissues, skin, liver,13 breast,14 kidney,15 lymph nodes,16 and central nervous system.17 Pulmonary plasmacytomas are rare. One review of 958 patients with multiple myeloma found that 46% of these patients had thoracic skeletal or pleuropulmonary abnormalities or both.18 In this series, 11 patients developed extramedullary plasmacytoma in the thorax and 4 had diffuse infiltrates thought to be caused by plasma cell infiltrates (biopsy proven in 1). Patients with pulmonary plasmacytomas are infrequently described, but cases involving a solitary nodule, interstitial infiltrates,19 bilateral pulmonary nodules,20 and diffuse alveolar infiltrates 21 have been reported, often associated with bone marrow involvement. Some reports suggest pulmonary involvement is frequently associated with rapidly progressive disease.
Our patient with multiple myeloma after autologous stem cell transplantation had a recurrence with innumerable bilateral pulmonary nodules without evidence of disease progression elsewhere. The random distribution of nodules, including the subpleural location as well as the intrapulmonary nodules that do not conform to the vascular margins, is a pattern typically seen in hematogenous dissemination of disease.
CONCLUSION
This case is remarkable for both the radiographic appearance of the pulmonary involvement, as well as the rapid resolution of these findings after 2 cycles of treatment that included newer biologic agents. Also of interest is the deep response obtained and maintained utilizing bortezomib, dexamethasone, and lenalidomide with maintenance lenalidomide.
Footnotes
Dr. Sumrall now practices at Central Georgia Cancer Care in Macon, GA.
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008 Mar 15;111(6):2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations), (eds). National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER website, April 2012. Accessed August 28, 2013.
- 3.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003 Jan;78(1):21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 4.Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol. 2010 Feb;21(2):325–330. doi: 10.1093/annonc/mdp329. Epub 2009 Jul 24. [DOI] [PubMed] [Google Scholar]
- 5.Bladé J, Lust JA, Kyle RA. Immunoglobulin D multiple myeloma: presenting features, response to therapy, and survival in a series of 53 cases. J Clin Oncol. 1994 Nov;12(11):2398–2404. doi: 10.1200/JCO.1994.12.11.2398. [DOI] [PubMed] [Google Scholar]
- 6.Bladé J, Kyle RA, Greipp PR. Presenting features and prognosis in 72 patients with multiple myeloma who were younger than 40 years. Br J Haematol. 1996 May;93(2):345–351. doi: 10.1046/j.1365-2141.1996.5191061.x. [DOI] [PubMed] [Google Scholar]
- 7.Pérez-Simón JA, Sureda A, Fernández-Aviles F, et al. Grupo Español de Mieloma. Reduced-intensity conditioning allogeneic transplantation is associated with a high incidence of extramedullary relapses in multiple myeloma patients. Leukemia. 2006 Mar;20(3):542–545. doi: 10.1038/sj.leu.2404085. [DOI] [PubMed] [Google Scholar]
- 8.Minnema MC, van de Donk NW, Zweegman S, et al. Extramedullary relapses after allogeneic non-myeloablative stem cell transplantation in multiple myeloma patients do not negatively affect treatment outcome. Bone Marrow Transplant. 2008 May;41(9):779–784. doi: 10.1038/sj.bmt.1705982. Epub 2008 Jan 14. [DOI] [PubMed] [Google Scholar]
- 9.Zeiser R, Deschler B, Bertz H, Finke J, Engelhardt M. Extramedullary vs medullary relapse after autologous or allogeneic hematopoietic stem cell transplantation (HSCT) in multiple myeloma (MM) and its correlation to clinical outcome. Bone Marrow Transplant. 2004 Dec;34(12):1057–1065. doi: 10.1038/sj.bmt.1704713. [DOI] [PubMed] [Google Scholar]
- 10.Alegre A, Granda A, Martínez-Chamorro C, et al. Spanish Registry of Transplants in Multiple Myelomas; Spanish Group of Hemopoietic Transplant (GETH); PETHEMA. Different patterns of relapse after autologous peripheral blood stem cell transplantation in multiple myeloma: clinical results of 280 cases from the Spanish Registry. Haematologica. 2002 Jun;87(6):609–614. [PubMed] [Google Scholar]
- 11.Lenhoff S, Hjorth M, Turesson I, et al. Nordic Myeloma Study Group. Intensive therapy for multiple myeloma in patients younger than 60 years. Long-term results focusing on the effect of the degree of response on survival and relapse pattern after transplantation. Haematologica. 2006 Sep;91(9):1228–1233. [PubMed] [Google Scholar]
- 12.Bladé J, Fernández de Larrea C, Rosiñol L, Cibeira MT, Jiménez R, Powles R. Soft-tissue plasmacytomas in multiple myeloma: incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol. 2011 Oct 1;29(28):3805–3812. doi: 10.1200/JCO.2011.34.9290. Epub 2011 Sep 6. [DOI] [PubMed] [Google Scholar]
- 13.Mayr NA, Wen BC, Hussey DH, et al. The role of radiation therapy in the treatment of solitary plasmacytomas. Radiother Oncol. 1990 Apr;17(4):293–303. doi: 10.1016/0167-8140(90)90003-f. [DOI] [PubMed] [Google Scholar]
- 14.Lamy O, von Bremen K, Burckhardt P. Breast plasmacytoma. Leuk Lymphoma. 2000 May;37((5-6)):611–615. doi: 10.3109/10428190009058514. [DOI] [PubMed] [Google Scholar]
- 15.Kanoh T, Yago K, Iwata H, Tei K, Higashino T. IgM-producing renal plasmacytoma. Urology. 1992 Nov;40(5):484–488. doi: 10.1016/0090-4295(92)90471-8. [DOI] [PubMed] [Google Scholar]
- 16.Holland J, Trenkner DA, Wasserman TH, Fineberg B. Plasmacytoma. Treatment results and conversion to myeloma. Cancer. 1992 Mar 15;69(6):1513–1517. doi: 10.1002/1097-0142(19920315)69:6<1513::aid-cncr2820690633>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 17.Bolek TW, Marcus RB, Jr, Mendenhall NP. Solitary plasmacytoma of bone and soft tissue. Int J Radiat Oncol Biol Phys. 1996 Sep 1;36(2):329–333. doi: 10.1016/s0360-3016(96)00334-3. [DOI] [PubMed] [Google Scholar]
- 18.Kintzer JS, Jr, Rosenow EC, 3rd, Kyle RA. Thoracic and pulmonary abnormalities in multiple myeloma. A review of 958 cases. Arch Intern Med. 1978 May;138(5):727–730. [PubMed] [Google Scholar]
- 19.Oymak FS, Karaman A, Soyuer I, et al. Pulmonary and chest wall involvement in multiple myeloma [in Turkish] Tuberk Toraks. 2003;51(1):27–32. [PubMed] [Google Scholar]
- 20.O'Sullivan P, Müller NL. Pulmonary and nodal multiple myeloma mimicking lymphoma. Br J Radiol. 2006 Jul;79(943):e25–e27. doi: 10.1259/bjr/77207966. [DOI] [PubMed] [Google Scholar]
- 21.Lazarevic V, Cemerikic-Martinovic V, Suvajdzic N, Subotic D, Colovic M. Diffuse primary plasmacytoma of the lung. Haematologia (Budap) 2001;31(2):161–165. doi: 10.1163/15685590152492972. [DOI] [PubMed] [Google Scholar]