Abstract
Polyunsaturated fatty acids (PUFA) play important roles in the normal physiology and in pathological states including inflammation and cancer. While much is known about the biosynthesis and biological activities of eicosanoids derived from ω6 PUFA, our understanding of the corresponding ω3 series lipid mediators is still rudimentary. The purpose of this review is not to offer a comprehensive summary of the literature on fatty acids in prostate cancer but rather to highlight some of the areas where key questions remain to be addressed. These include substrate preference and polymorphic variants of enzymes involved in the metabolism of PUFA, the relationship between de novo lipid synthesis and dietary lipid metabolism pathways, the contribution of cyclooxygenases and lipoxygenases as well as terminal synthases and prostanoid receptors in prostate cancer, and the potential role of PUFA in angiogenesis and cell surface receptor signaling.
Keywords: Prostate cancer, Polyunsaturated fatty acids, Metabolism, Cyclooxygenase, Eicosanoids
1 Introduction
Consumption of high levels of dietary lipids is usually associated with increased risk of cancer, with the notable exception of ω3 polyunsaturated fatty acids (PUFA), which show protective effects against colon, breast, and prostate cancer in a number of experimental systems [1–5]. Since ω3 and ω6 PUFA cannot be synthesized de novo, they are essential fatty acids and must be taken in from the diet. However, PUFA elongation to longer chain species shares malonyl-CoA as a common substrate with de novo fatty acid synthesis. Hence, levels of specific PUFA in an organism depend on dietary intake and on metabolism of these fatty acids by desaturases, elongases, cyclooxygenases (COXs), lipoxygenases (LOXs), and other enzymes [5]. In turn, metabolism shows interindividual differences due to polymorphism in the genes encoding the various metabolic enzymes [6]. Bioactive metabolites of ω6 PUFA generated by COXs and LOXs (eicosanoids) have been extensively investigated and play various roles in inflammation, cancer cell proliferation, and metastasis. Overall, metabolites of ω3 PUFA oppose these actions, but their generation is still poorly understood. In this review, we highlight the interplay between dietary PUFA intake, elongation, β-oxidation, storage, and eicosanoid synthesis and de novo fatty acid synthesis. We further discuss the potential roles of PUFA and their metabolites in prostate cancer, with an emphasis on angiogenesis and cell surface receptors, and contrast the wealth of information available on ω6 PUFA metabolism to the relative scarcity of knowledge on ω3 PUFA metabolism.
2 Source of ω6 and ω3 PUFA and conversion within each series
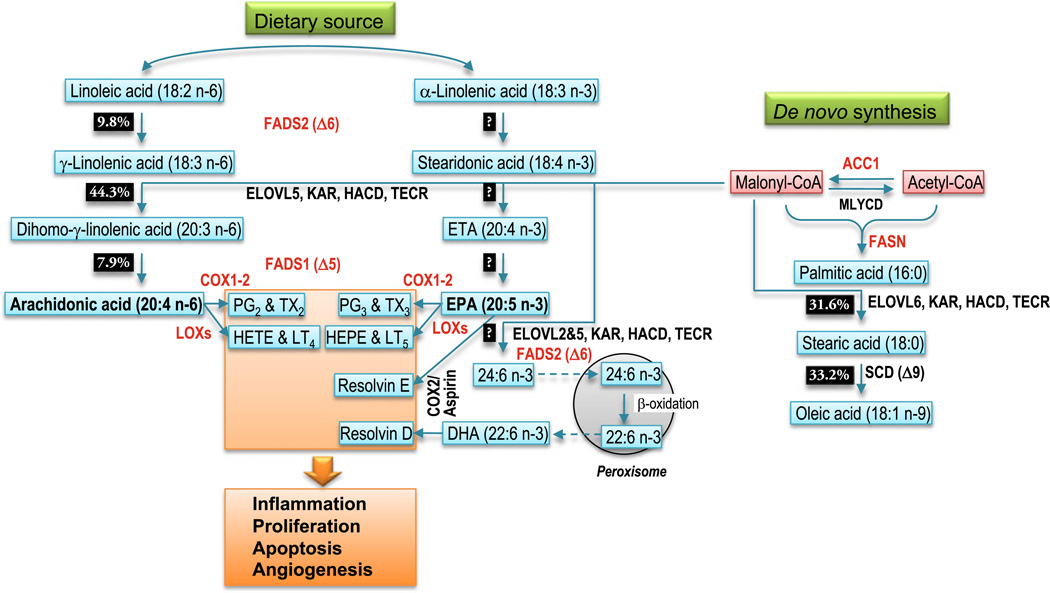
ω6 and ω3 PUFA cannot be interconverted in mammals, but within each series, metabolism can produce various lipids that differ in chain lengths and number of unsaturated bonds. Linoleic acid (LA, 18:2n-6) is an ω6 PUFA found in abundant supply in vegetable oils; it is metabolized primarily to arachidonic acid (AA, 20:4n-6) through a series of alternating oxidative desaturation and elongation steps. In the ω3 series, alpha linolenic acid (α-LNA, 18:3n-3), found at moderate levels in most terrestrial plants, is not converted efficiently to long-chain ω3 PUFA such as eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3; see below). Therefore, in humans the main source for these long-chain ω3 PUFA is through dietary intake of fish or supplementation with fish oil.
During ω6 PUFA conversion, fatty acid desaturase 2 (FADS2) or delta-6 desaturase converts LA to gamma linolenic acid (γ-LNA, 18:3 n-6). This enzyme represents a rate-limiting step in the synthesis of AA from dietary LA [7] (Fig. 1). γ-LNA is elongated to dihomo-gammalinolenic acid (DGLA, 20:3 n-6) through a process of four enzymatic reactions. The first of these is condensation of the fatty acyl chain with malonyl-CoA, catalyzed by an enzyme encoded by the ELOVL5 gene (elongation of very long-chain fatty acids, family member 5). This is followed by a reduction reaction mediated by 3-ketoacyl-CoA reductase (KAR, also known as HSD17B12), a dehydration reaction catalyzed by 3-hydroxyacyl-CoA dehydratase (HACD), and finally a second reduction reaction catalyzed by trans-2,3-enoyl-CoA reductase (TECR). After chain elongation, fatty acid desaturase 1 (FADS1) or delta-5 desaturase converts DGLA to AA. Again, this step is relatively inefficient [7] (Fig. 1).
Fig. 1.
Conversion of ω6 and ω3 series PUFA by metabolic enzymes and interaction with de novo fatty acid synthesis. The main dietary ω6 and ω3 PUFA (LA, 18:2 n-6 and αLNA, 18:3 n-3) undergo a series of desaturation (FADS2, FADS1) and elongation (ELOVL5, KAR, HACD, TECR) steps converting them to AA and EPA, respectively. The in vitro conversion rates of these enzymatic steps are indicated as percent of product formed from 150 nmol substrate incubated with 5 mg of rat liver microsomal protein for 3 min [7]. Long-chain PUFA are converted to prostaglandins (PG) and thromboxanes (TX) by cyclooxygenases (COX1–2) or to leukotrienes (LT) and hydroxyeicosatetraenoic acids (HETE) by lipoxygenases (LOX). EPA can be further elongated and desaturated to DHA in a pathway involving β-oxidation in the peroxisome. In the presence of aspirin, COX2 metabolizes EPA and DHA to resolvins. The various products play important roles in inflammation, cell proliferation and apoptosis, and angiogenesis. In the de novo lipid synthesis pathway, acetyl-CoA and malonyl-CoA can be interconverted by acetyl-CoA carboxylase (ACC1) and malonyl-CoA decarboxylase (MLYCD). Acetyl-CoA and malonyl-CoA are used as substrates by fatty acid synthase (FASN) to generate long-chain saturated fatty acids, which can be elongated further (ELOVL6) or desaturated (SCD) to form monounsaturated fatty acids. Malonyl-CoA is also required for elongation of ω6 and ω3 PUFA
The same enzymes are involved in ω3 PUFA conversion. However, conversion of α-LNA to DHA in humans appears to be very inefficient. Both α-LNA-feeding studies and stable isotope studies have consistently demonstrated that increased consumption of α-LNA does not result in increased DHA in plasma or cell lipids (reviewed in [8, 9]). Instead of being further anabolized, most of the ingested α-LNA is subject to β-oxidation to provide energy and only a small fraction is converted to EPA. From kinetic analyses of fatty acid conversion, it was estimated that conversion of α-LNA to EPA might be as low as 0.2%, conversion of EPA to DPA was estimated at 64%, and conversion of DPA to DHA at 37% [10]. Therefore, the overall amount of DPA and DHA made from α-LNA would only represent 0.13% and 0.05% of the starting α-LNA amount, respectively. These data suggest that FADS2, the first enzyme in the conversion sequence, is rate-limiting. Recently, it was proposed that the limited conversion of α-LNA to DHA in HepG2 cells was in part due to a competition of α-LNA and tetracosapentaenoic acid (24:5n-3) for FADS2 [11]. There is some evidence of gender differences in efficiency of the elongation–desaturation pathway suggesting that sex hormones may play a regulatory role [9, 12, 13].
3 Polymorphism of the FADS gene cluster and potential impact on PUFA conversion
The human FADS gene cluster (FADS1, FADS2, and FADS3) is located on chromosome 11q12–13.1. Approximately 207 single-nucleotide polymorphisms (SNPs) were identified on FADS1, 610 SNPs on FADS2, and 246 SNPs on FADS3 (http://www.ncbi.nlm.nih.gov/snp/). Interestingly, microRNA hsa-mir-1908, which is expressed in embryonic stem cells, cancer, and the female reproductive tract [14–16], is located in the first intron of the FADS1. One SNP (rs174561) is present within the miRNA, and two additional SNPs (rs73487465 and rs75810419) flank it. Whether these SNPs have an effect on hsa-mir-1908 expression is currently unclear.
Schaeffer et al. [17] first reported an association between genetic variants of the FADS gene cluster and PUFA composition in phospholipids. Several SNP studies [18–28] and a genome-wide association study [29] have replicated the observation. FADS polymorphisms may affect both ω3 and ω6 PUFA desaturation–elongation. The topic has been the subject of multiple reviews [30–34]. Recent evidence suggests that polymorphisms in the FADS gene cluster which alters desaturase activity might differ between Caucasians and Asians [35]. An intriguing question is whether this polymorphic difference, if confirmed, has an impact on the effect of ω3 and ω6 PUFA on cancer risk among different populations.
4 Dependence of tumor cells on de novo fatty acid synthesis
Although fatty acids are consumed at high levels in a typical western diet, tumor cells display an obligate requirement to synthesize fatty acid de novo [36, 37]. This is evidenced by high expression levels of enzymes in the pathway in multiple types of cancer, including prostate cancer. For example, fatty acid synthase (FASN), the enzyme that catalyzes the synthesis of fatty acid, is expressed at high levels in prostate cancer and its expression is correlated with disease progression and outcome [36, 38–43]. The fatty acid synthesis pathway is turned on early in prostate cancer development and is regulated by androgen [44–46]. In addition, the pathway is driven by the same processes that drive prostate cancer. As one example, the PI3-kinase pathway is the primary driver of FASN expression in prostate cancer [47]. Pharmacological blockade of the PI3-kinase pathway reduces FASN expression, as does reexpression of PTEN in PTEN-null cells [48]. Moreover, patients with tumors that are PTEN negative and express high levels of FASN have decreased disease-free survival [38]. Conversely, the prostate-specific expression of FASN is sufficient to induce prostatic intraepithelial neoplasia (PIN) lesions in mice [49]. Collectively, these findings strongly suggest that that the de novo pathway of fatty acid synthesis is not only required for prostate cancer but may have a role in promoting disease progression.
There are other enzymes upstream of FASN that are involved in fatty acid synthesis. Fatty acid synthesis primarily uses glucose as the primary carbon source, although recent evidence demonstrates that glutamine also serves as a carbon source in tumor cells [50–52]. To generate the substrates for fatty acid synthesis, several enzymatic steps are required. First, citrate is shunted out of the TCA cycle into the cytoplasm and converted to acetyl-CoA by ATP-citrate lyase (ACLY) [50–52]. The resulting acetyl-CoA is converted to malonyl-CoA by the cytosolic acetyl-CoA carboxylase 1 (ACC1) through an ATP-dependent carboxylation reaction [53, 54]. Generation of malonyl-CoA is the rate-limiting step of fatty acid synthesis. The ultimate steps of fatty acid synthesis are performed by FASN, which also resides in the cytosol, by combining 1 acetyl-CoA with 7 malonyl-CoA to synthesize the 16-carbon fatty acid palmitate [55–58]. FASN also synthesizes the 14-carbon fatty acid myristate and the 18-carbon fatty acid stearate, albeit to lesser degrees. Palmitate can undergo a series of modifications before it is utilized in the cells. It can be elongated by two carbons to stearate using malonyl-CoA as the elongation substrate, just as it is for PUFA. Palmitate and stearate can also be desaturated by stearoyl-CoA desaturase-1 to form the monounsaturated palmitoleate and oleate, respectively. Thus, the fatty acid synthesis pathway generates the saturated fatty acid component of the cell but also provides the precursor for the monounsaturated component of the cell.
The ubiquitous expression of FASN in tumor cells is paralleled by an absolute requirement for de novo fatty acid synthesis to proliferate and survive [36, 43]. Because of the cellular dependence on fatty acid synthesis to facilitate membrane biogenesis and other aspects of cell biology, fatty acid synthesis impacts on virtually every aspect of cellular activity. For example, it is required for cell cycle progression, protein synthesis, mitochondrial function, and protein targeting [59–63]. Accordingly, blocking the activity of ACLY, ACC1 and FASN reduces proliferation or induces cell death in vitro and inhibits tumor growth in vivo [51, 59, 64–69].
5 Relationship between de novo lipid synthesis and dietary lipid metabolism pathways
The dependence of tumor cells on de novo fatty acid synthesis suggests that these cells have different requirements for fatty acid compared to normal cells. Some portion of the increased demand can be attributed to increased proliferation of tumor cells compared to normal cells. It may also be possible that dietary and de novo fatty acids are subject to different fates in a cell. In such a case, it may be that dietary fatty acid is unable to meet to demands of the tumor cell. The fate of newly synthesized fatty acid in a tumor cells has been well defined. Most of it is used to support membrane biogenesis in the form of phospholipids, although some portion is used for protein palmitoylation and protein targeting [63, 70]. Interestingly, recent evidence demonstrates that de novo synthesized fatty acid is primarily used to support phosphatidylcholine (PC) synthesis [71]. Blockade of the pathway has little effect on phosphatidylserine, phosphatidylethanolamine, and phosphatidylinositol. It is tempting to speculate that dietary fat is required to support the non-PC portion of tumor cell membranes. The pool of newly synthesized fatty acid used for membrane biogenesis is preferentially enriched into detergent-insoluble microdomains known as lipid rafts [70]. This is consistent with the finding that lipid rafts are rich in lipids containing saturated fatty acid. It also suggests that fatty acid synthesis supports signaling functions that are associated with lipid rafts.
Although the expression and activity of enzymes in the fatty acid synthesis pathway is highly correlated with cancer, there are several examples of normal biology in which the pathway is active. Embryonic development is an example of when the pathway appears to be active and required as Fasn- and Acc1-deficient mice display an embryonic mutant phenotype [53, 55]. Fasn-deficient embryos display lethality prior to implantation and heterozygotes die at multiple stages during development [55]. Similarly, Acc1-deficient embryos display lethality at around day 8.5 [53]. It is interesting to note that placing pregnant mothers on a diet enriched in saturated fatty acid is not sufficient to protect embryos from the effects of Fasn deletion. Whether this is because of differential utilization of dietary fat compared to synthesized fat or from having to cross the placenta is unknown.
The liver and adipose tissue are two other examples in which the fatty acid synthesis pathway is active. In these tissues, fatty acid is generated and, following excessive caloric consumption, stored as triglyceride for future energy. Mice with liver-specific deletion of Acc1 and Fasn have also been generated [54, 72]. Mice engineered to have targeted Fasn deletion in the liver display a phenotype that can be reversed with a peroxisome proliferator-activated receptor (PPAR) α agonist, but not with a high-fat diet [72, 73]. It was subsequently demonstrated that Fasn-dependent fatty acid synthesis was responsible for the formation of the PPARα ligand 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (16:0/18:1-GPC) [73]. Of note, this lipid is among the most abundant of all lipid species. That a high-fat diet could not recapitulate the wild-type liver phenotype of the wild-type mice suggests that dietary fat may be utilized differently than de novo synthesized fat in cells.
Unlike what has been a demonstrated in vivo using total or tissue-specific knockout of Fasn, exogenous fatty acid is able to protect cells from the effects of inhibiting FASN and ACC1 in tumor cell lines in vitro. As discussed earlier, when tumor cell lines are treated with FASN and ACC1 inhibitors or transfected with siRNA against either of the two enzymes, cell cycle blockade and cell death ensue. Many of the cellular effects can be delayed or ameliorated by supplementing cells with exogenous fatty acid in their culture medium. Palmitate supplementation can restore cell cycle progression and survival following siRNA knockdown of FASN and ACC1 [59, 65, 74]. Recent studies also suggest that tumor cells may obtain fatty acid from the circulation through a lipoprotein lipase and CD36-dependent mechanism [75]. Moreover, it appears that this mechanism has the potential to protect tumor cells from the effects of inhibitors of de novo fatty acid synthesis. Although in vitro experimentation does not always adequately reflect in vivo biology, it is interesting to consider that dietary fat may have a different influence on tumor cells than on normal cells. This could be related to the fact that tumor cells have an absolute requirement for the synthesis of saturated fatty acid.
Development of FASN, ACC1, and ACLY inhibitors is the subject of intense investigation. Because PUFA elongation requires malonyl-CoA, one interesting possibility is that inhibition of ACC1 or ACLY could reduce cellular malonyl-CoA level and consequently diminish the LA to AA conversion. Since elongation of dietary ω6 PUFA appears to be more efficient than that of ω3 PUFA, it is tempting to speculate that ACC1 and ACLY inhibitors may preferentially reduce the formation of AA and consequently that of the ω6-series eicosanoids.
6 PUFA oxidation and cycling
Human beings first evolved while consuming a diet containing roughly equivalent amounts of ω6 and ω3 PUFA [76]. During the last two centuries, however, the consumption of ω6 PUFA has increased dramatically due to the increased intake of vegetable oils. Today, the ratio of ω6 and ω3 PUFA in western diets is approximately 30:1. The low intake of α-LNA from the diet is consistent with the observation that α-LNA is underrepresented relative to LA in various tissues and organs in the body [77–79]. Even with an equal amount of ω6 and ω3 PUFA intake, some evidence suggests that this phenomenon may also be the result of rapid β-oxidation of α-LNA relative to other fatty acids [80, 81]. Indeed, carnitine palmitoyltransferase (CPT1), a key enzyme in fatty acid β-oxidation, exhibits the fastest maximum turnover capacity with α-LNA as substrate relative to other fatty acids, whether the substrates are presented as nonesterified fatty acids or as acyl-CoA esters [82].
Although the rate of esterification may be comparable between ω6 and ω3 PUFA [83], data indicate that the release of fatty acids from phospholipids could be quite different. Three major groups of phospholipase A2 (PLA2) enzymes have been identified: secreted PLA2 or sPLA2, cytosolic PLA2 or cPLA2, and calcium-independent PLA2 or iPLA2. sPLA2 and cPLA2 can selectively release AA from the sn-2 position of phospholipids and the released AA is then the substrate of oxygenases. Interestingly, phospholipids with ω3 PUFA (EPA or DHA) in the sn-2 position are poor substrates for cPLA2 [84, 85]. In contrast, iPLA2 may selectively release DHA [86]. Therefore, the extent to which ω3 and ω6 PUFA are released from phospholipids would be expected to depend on the relative expression of the various phospholipases.
To date, it is unclear whether ω6 and ω3 PUFA are differently β-oxidized and how ω6 and ω3 PUFA are cycled in tumor cells. The role of cPLA2 and sPLA2 in cancer has been discussed previously [87–89]. A study found that sPLA2 was upregulated and that endogenous inhibitors of cPLA2 were downregulated in prostate cancer, with resulting effects on cancer cell growth [90]. In another study, the mRNA and protein levels of sPLA2 were significantly higher in Gleason pattern grade 2–4 carcinomas than benign prostate samples and correlated with proliferation, but the expression was lower in metastases [91]. Compared to cytosolic and secreted phospholipases, little is known about the expression and function of iPLA2 enzymes in prostate cancer.
7 Relative contribution of oxygenases in prostate cancer
AA is metabolized by a number of enzymes belonging to the COX and LOX families as well as cytochrome P450 epoxygenases. COXs catalyze the first reaction in the conversion of AA to prostaglandins (PG) G and H, which are further metabolized into other prostaglandins (PGE, PGF, PGJ), prostacyclins (PGI), and thromboxanes (TXA, TXB), whereas LOXs mediate the first step in the conversion of AA to leukotrienes and hydroxyeicosatetraenoic acids (HETEs). Cytochrome P450 oxygenases convert AA to HETEs by the action of omega-hydroxylase activity and to epoxyeicosatrienoic acids (EETs) by epoxygenase activity. The resulting lipid metabolites have multiple biological activities and have been implicated in various pathological processes, including inflammation, autoimmunity, and cancer [92, 93]. Long-chain ω3 fatty acids are also believed to be substrates of COX, LOX, and P450 enzymes, and the resulting products tend to have opposing effects to their ω6 counterpart [94]. However, in contrast to ω6 PUFA, the metabolism of ω3 PUFA is not well understood.
COXs (also known as prostaglandin G/H synthases, PTGS) have two well-characterized isoforms, namely COX1 or PTGS1 and COX2 or PTGS2. COX1 is a constitutively expressed gene in most tissues whereas COX2 is an immediate-early response gene, highly induced during tumor progression [95]. A third isoform, COX3, appears to be a splice variant of the COX1 gene. Due to its induction in inflammation and cancer, COX2 has been the object of intense study and proposed as a target for cancer therapy. Numerous review articles have been written on this topic [96–99]. There are several types of LOXs in mammals, the most widely studies of which are LOX5 (ALOX5), LOX12 (ALOX12) and LOX15 (ALOX15), where the names reflects the substrate specificity in terms of the carbon number [100]. LOXs have been suggested to play roles in cancer as well [101].
Due to the existence of multiple oxygenases, the role of specific enzymes in the development of prostate cancer has not been studied systematically in a single system or animal model. In addition, studies performed in animals rarely take diet into account for the design and interpretation of the experiments. For this reason, it is still unclear whether all oxygenases play an important role or some oxygenases are more critical in the development of prostate cancer in animals consuming different diets. To systematically assess the interaction between oxygenases and dietary PUFA in a single in vivo model of prostate cancer, we knocked out Cox1, Cox2, Lox5, Lox12, or Lox15 in prostate-specific Pten-null mice. We have previously demonstrate that ω3 and ω6 PUFA differentially modulate the growth and progression of prostate tumors in this model [3]. Preliminary results indicate that loss of Cox1 had significant effects on prostate tumor growth in a PUFA-dependent manner; namely, tumor growth was significantly increased in Cox1 knockout mice on ω3 diet compared to Pten-null, Cox1-positive mice on the same diet, essentially negating the protective effects of ω3 PUFA. In other words, Cox1 appears to be required for the protective effects of ω3 PUFA, suggesting that ω3 metabolites of Cox1 reduce cancer formation. On the other hand, tumor growth was decreased in mice on ω6 diet compared to Pten-null, Cox1-positive mice on the same diet, suggesting that ω6 metabolites of Cox1 (e.g., PGE2) play a promoting role on tumor formation. Loss of Cox2 reduced prostate tumor growth regardless of diet, suggesting that metabolites of Cox2 promote tumor growth and that suppressive effects of ω3 PUFA do not depend upon Cox2. Loss of Lox5 reduced prostate tumor growth on ω6 diet but had no effect on ω3 diet, suggesting that ω6 metabolites of Lox5 promote tumor growth, and protective effects of ω3 PUFA are independent of Lox5. Loss of Lox12 or Lox15 did not affect prostate tumor growth on either diet, suggesting that either PUFA metabolites are not generated from these two enzymes, or metabolites generated are not critical for prostate tumor in this animal model (Chen et al., unpublished). It is clear from these studies that the interaction between diet and metabolic genes can play an important role in determining cancer risk and response of cancer to dietary PUFA.
Substantial evidence from human studies supports the important role of COXs in PUFA metabolism and cancer [92]. LOX5 has also been implicated in the progression of several types of human cancers [102–106] including that of the prostate [107–110]. Interestingly, interactions between dietary intake of PUFA and polymorphisms in COX2 and LOX5 genes may determine cancer risk [111–114]. Such reports are consistent with our animal studies, but the role of COX1 as well as specific polymorphisms of eicosanoid metabolic enzymes in human prostate cancer warrants further investigations.
8 Role of prostaglandin, prostacyclin and thromboxane synthases, and prostanoid receptors in cancer
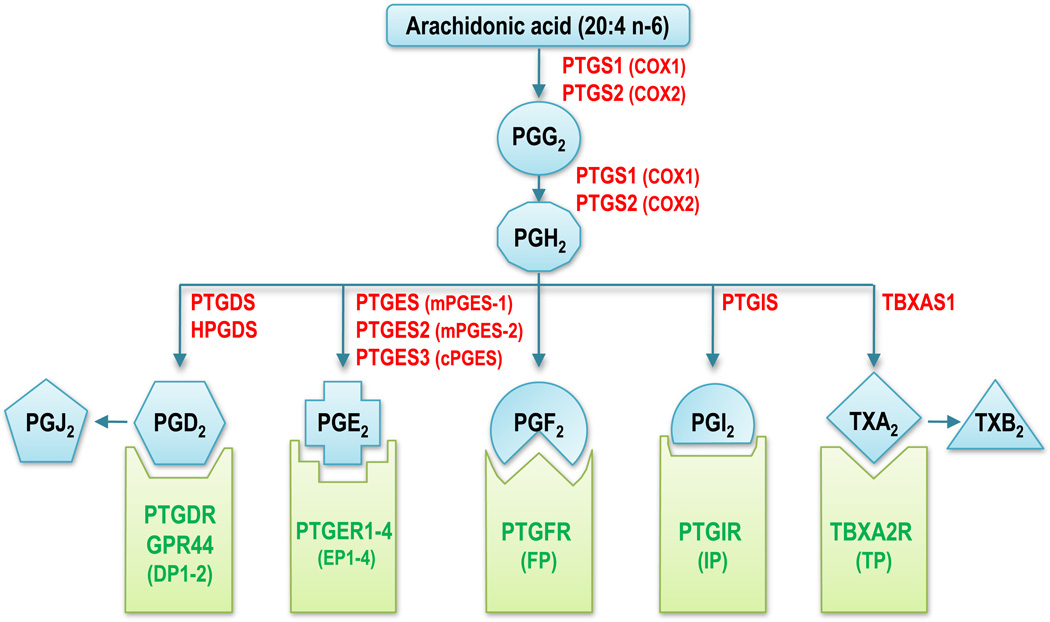
As mentioned above, COX1 and COX2 convert arachidonic acid into prostaglandin G2, which undergoes conversion to PGH2 through the peroxidase activity of COX. In turn, PGH2 is converted to other prostaglandins, prostacyclin, and thromboxanes by the action of several isomerases also called terminal synthases (Fig. 2). Three terminal synthases capable of producing PGE2 from COX-derived PGH2 have been reported: prostaglandin E synthase (PTGES) or microsomal PGES-1 (mPGES-1), PTGES2 or mPGES-2, and PTGES-3 or cPGES. A prostaglandin D2 synthase responsible for converting PGH2 to PGD2 has been described in the brain, whereas in immune cells this conversion is catalyzed by hematopoietic prostaglandin D synthase. Prostaglandin I2 (prostacyclin) synthase (PTGIS) is involved in the conversion of PGH2 to PGI2. In platelets, thromboxane A synthase 1 (TBXAS1) converts PGH2 to TXA2. ω3 PUFA EPA is thought to serve as a substrate of the same set of enzymes to generate 3-series eicosanoids (PGH3, PGE3, etc.) [115]. However, for many of these enzymes, the extent to which ω3 PUFA can serve as substrates is unclear.
Fig. 2.
Main enzymes and G protein-coupled receptors in the cyclooxygenase pathway. Arachidonic acid is converted to PGG2 by cyclooxygenase 1 or 2 and PGG2 then undergoes conversion to PGH2 through the peroxidase activity of COX. Several isomerases convert PGH2 to other 2-series prostaglandins, prostacyclins or thromboxanes, which act in part by binding to specific prostanoid receptors (green). EPA is thought to serve as a substrate for the same set of enzymes to generate three-series eicosanoids (PGH3, PGE3, etc.). Some prostanoids have also been shown to bind to PPARs (not shown). Official protein symbols are shown in red for enzymes and in green for receptors, with common abbreviations in parenthesis. PTGS1–2 prostaglandin-endoperoxide synthase 1–2, PTGDS PGD2 synthase (brain), HPGDS hematopoietic PGD synthase, PTGES1–3 PGE synthase 1–3, PTGIS PGI2 (prostacyclin) synthase, TBXAS1 TXA synthase 1, PTGDR PGD2 receptor, PTGER1–4 PGE receptor 1–4 (subtype EP1–4), PTGFR PG F receptor, PTGIR PGI2 (prostacyclin) receptor, TBXA2R TXA2 receptor
mPGES-1 is a microsomal glutathione-dependent prostaglandin E synthase that can be induced by LPS, the proinflammatory cytokine interleukin 1 beta, by tumor suppressor protein TP53, as well as in pathological conditions such as cancer. Therefore, mPGES-1 has been proposed as a therapeutic target in cancer [116, 117]. mPGES-2 is also a microsomal prostaglandin E synthase, but its expression seems to be independent of inflammation and there is less evidence linking it to cancer. cPGES is a cytosolic protein also known as p23, described as an HSP90 co-chaperone involved in the stabilization of the glucocorticoid receptor complex. A role for cPEGS in PGE2 synthesis has been suggested experimentally [118, 119], but the results of mouse knockout studies failed to support this function [120].
One report showed that mPGES-1 is expressed at high levels in DU145 human prostate cancer cells and in human prostate cancer tissues compared with benign hyperplasia. Short hairpin RNA-mediated mPGES-1 knockdown in DU145 decreased clonogenic survival and increased sensitivity to adriamycin-induced apoptosis, which could be rescued by exogenous PGE2. mPGES-1 knockdown also decreased the tumorigenic potential of xenograft tumors in nude mice [121]. Polymorphisms in the mPGES-2 and leukotriene A4 hydrolase (LTA4H) genes were found to be inversely associated with prostate cancer risk [122].
In contrast with other prostaglandin synthases, PTGIS may reduce cancer risk (reviewed in [123]). The PTGIS gene promoter was identified as being hypermethylated in 43 out of 100 colorectal cancers and in colorectal cancer cell lines [124]. Promoter repeat polymorphism in PTGIS has also been found to be associated with risk of adenomas in a case–control study of individual with colorectal polyps versus polyp-free controls [125]. In human lung cancer, PGI2 and PGE2 appear to play antagonistic roles: PTGIS and PGI2 are downregulated, whereas PGE2 synthase and PGE2 are upregulated [126, 127]. Moreover, transgenic mice with selective pulmonary prostacyclin synthase overexpression are protected from carcinogen-induced lung tumor formation [128]. The role of prostacyclin and PTGIS in prostate cancer is less clear.
Thromboxane A4 synthase (TBXAS1) is overexpressed in a number of cancers and has been proposed to contribute to tumor development and progression by modulating tumor growth, angiogenesis, thrombosis, invasion, and metastasis and by inhibiting apoptosis. The balance in the expression of PTGIS and TBXAS1 has been suggested to be clinically relevant [123]. In prostate cancer, immunohistochemical analysis of tumor samples showed significantly elevated TBXAS1 expression in prostate tumors compared to normal luminal or secretory cells, with a marked increased in tumors presenting perineural invasion [129]. In addition, migration of PC-3 cells, which express high levels of TBXAS1, was reduced by inhibition of this enzyme, whereas motility of TBXAS1-negative DU145 cells was stimulated by its overexpression.
Nine prostanoid receptors have been identified, which belong to the G protein-coupled receptor family and are conserved from mouse to human (Fig. 2). There are two PGD2 receptors, called PTGDR or DP1 and GPR44 or DP2, four subtypes of PGE receptors, named PTGER1–4 or EP1–4, one PGF receptor (PTGFR or FP), one PGI2 receptor (PTGIR or IP), and one TXA2 receptor (TBXA2R or TP) [130]. In mouse prostate cells, expression of Ep1, Ep2, and Ep4 is detectable (Chen et al., unpublished). Only EP2 and EP4 were detected in PC3, DU145, and LNCaP human prostate tumor cells, which is consistent with the literature [131, 132]. Aberrant TXA2 signaling and TP expression is associated with prostate cancer, where a direct correlation was observed with tumor Gleason score and pathological state [133, 134]. However, much remains to be learnt about the expression of these prostanoid receptors and their activation by ω6 and, especially, ω3 prostanoids in cancer cells.
9 Role of PUFA in tumor angiogenesis
Nonsteroidal anti-inflammatory drugs (NSAIDs) exert some of their anti-inflammatory and antitumor effects by reducing prostanoid production through the inhibition of COX enzyme activity. NSAIDs have been reported to have beneficial effects on reducing the risk of developing prostate cancer [135–138]. The role of eicosanoids in tumor inflammation and proliferation has been reviewed extensively [92, 93, 139–143]. Here we will focus on the potential role of PUFA in angiogenesis and cell surface receptor signaling.
Angiogenesis plays a major role in tumor progression by providing a blood supply to the growing tumor and facilitating its dissemination [144, 145]. The significance of angiogenesis in human prostate cancer progression is evidenced by correlations of microvessel density with Gleason score, pathologic stage of disease, and patient survival [146, 147]. The most investigated stimulator of angiogenesis is VEGF (reviewed in [148]). ω3 PUFA were shown to suppress VEGF-stimulated endothelial cell proliferation, migration, and tube formation during sprouting angiogenesis [149, 150], and endothelial cell expression of the VEGF receptor FLK-1 was reduced by EPA [151]. Effects of ω3 PUFA on in vivo models of VEGF-induced angiogenesis are inconsistent. Both EPA and DHA inhibited VEGF expression and reduced microvessels in tumors arising from HT-29 colon cancer cell transplants in nude mice. This involved inhibition of a pathway comprising COX2, PGE2, and HIF-1α [152]. However, in a mouse model of hypoxia-induced retinal angiogenesis, an ω3 PUFA diet or expression of the Caenorhabditis elegans fat-1 gene to endogenously enrich tissues in ω3 PUFA was shown to reduce pathological neovascularization without suppression of VEGF [153–155]. Likewise, an ω3 PUFA diet-induced decrease in tumor microvessel density in a mouse breast cancer xenograft model was not accompanied by a reduction in VEGF mRNA [156]. Using a random crossover design, a Mediterranean diet enriched in ω3 PUFA was compared to an ordinary Swedish diet for the ability to reduce indices of inflammation [157]. A 45% reduction in the ω6 to ω3 PUFA ratio in serum was accompanied by a significant decrease in VEGF following the Mediterranean diet phase.
PDGF is another key regulator of angiogenesis. Studies examining the role of fish oil in reducing risk of cardiovascular disease showed that ω3 PUFA almost completely eliminated endothelial cell production of PDGF in vitro [158]. Further, in human volunteers fed with dietary fish oil, increased monocyte ω3 PUFA levels were associated with a 66% and 70% decrease in mRNA for PDGF-A and PDGF-B, respectively [159]. Similar studies have yet to be conducted with cancer patients.
The FGF family in vertebrates consists of 22 proteins that range in size from 17 to 34 kDa and share 17–31% sequence identity [160, 161]. FGFs display variable affinities for four cognate tyrosine kinase receptors, designated FGFR1–4, and all of which are upregulated in prostate cancer [162]. FGF1, FGF2, FGF6, FGF8, FGF9, and FGF17 show elevated expression in prostate tumors compared to normal human prostate [162–164]. Of these, FGF1 and FGF2 are well-defined promoters of angiogenesis [165]. Over 80% of prostate cancers express FGF1, and expression was shown to positively correlate with Gleason score [166]. The biological significance of FGF2 in prostate cancer was demonstrated in the transgenic adenocarcinoma of the mouse prostate mouse model, in which elimination of one or both Fgf2 alleles reduced metastasis and increased survival [167]. FGF8 has also emerged as an important promoter of angiogenesis. An alternatively spliced isoform, FGF8b is the major isoform of FGF8 present in human prostate cancer and is strongly associated with stage and grade of disease [168]. Xenografts of PC3 cells overexpressing FGF8b in mice demonstrated an extensive capillary network and metastases [169]. Targeted prostate expression of Fgf8b in transgenic mice was shown to induce a hypercellular reactive stroma enriched in vasculature, prior to the development of PIN [170]. Although there is no evidence to date for regulation of any FGF by ω3 PUFA, enrichment of tumor cells with the ω6 PUFA linoleic acid was shown to convert the growth-promoting activity of FGF from a transient to a sustained effect [171].
10 Effects of PUFA on cell surface receptor signaling
Toll-like receptors (TLRs) are a family of transmembrane receptors that mediate an innate immune response through recognition of conserved patterns of microbial and viral components. They play a major role in defense against pathogens, particularly at mucosal surfaces. Although TLRs were originally detected only on immune cells [172], it is now known that tumor cells express functional TLRs that are stimulated by endogenous ligands in addition to those of microbial origin [173–175]. The physiological functions of TLRs in relation to cancer have been reviewed [175]. In prostate cancer, increased risk has been linked to sequence variations in TLR4 [176] and the TLR6–10 cluster [177]. Although the biological consequences of these polymorphisms are not understood, they are consistent with a major role of inflammation in prostate cancer and suppression of TLR activity may be one of the anti-inflammatory roles of ω3 PUFA. Dietary fish oil was shown to inhibit TLR4 activity in peripheral blood monocytes from human volunteers [178], and DHA acted as a pan inhibitor of TLRs in a macrophage cell line [179]. In prostate cancer cells, TLR3 activation was shown to trigger apoptosis and promote angiogenesis through upregulation of HIF-1α [180]. Effects of ω3 PUFA on prostate cancer cell TLRs remain to be determined.
The syndecan family of cell surface proteoglycans consists of four members with a shared structure of small conserved cytoplasmic and transmembrane domains and larger, distinct ectodomains. The ectodomains are substituted predominantly with heparan sulfate glycosaminoglycan chains, but syndecan-1 can also exist as a hybrid bearing both heparan sulfate and chondroitin sulfate chains [181]. A biologically active ectodomain can be shed by the action of matrix metalloproteinases (reviewed in [182]). Syndecan-1 is the most widely studied in relation to cancer, but its role is complex and far from clear. Its heparan sulfate chains interact with a variety of extracellular matrix components, growth factors, cytokines, and enzymes to facilitate a role in regulation of cell proliferation, apoptosis, adhesion, and migration. Syndecan-1 is expressed primarily by epithelial cells, but with malignant conversion and tumor progression, syndecan-1 expression is reduced and this reduction is associated with poor prognosis in various epithelial-derived tumors [183–186]. However this may be tumor or stage specific, since syndecan-1 increases have been associated with unfavorable prognosis in some cancers [187–189]. Mounting evidence indicates that tumor progression is accompanied by a shift from an epithelial to stromal distribution for syndecan-1 [190–192]. This may result in changes in the fine structure of syndecan-1 that lead to altered function. Syndecan-1 has not been well studied in prostate cancer. An inverse relationship was reported between syndecan-1 and Gleason score [193, 194], but a tissue microarray analysis showed an increase in syndecan-1 with tumor progression [195]. In vitro studies have shown reduced expression of syndecan-1 in prostate cancer cell lines compared to normal prostate epithelial cells and lower expression in androgen-dependent LNCaP cells compared to androgen independent PC3 and DU145 cells [196].
Our studies have shown that in a mouse model of prostate cancer, the reduction in tumor growth as a result of dietaryω3 PUFA [3] is accompanied by an increase in the expression of syndecan-1 [196, 197]. Moreover, in human and mouse prostate cancer cells lines, syndecan-1 was upregulated by DHA but not EPA. This led to increased apoptosis through syndecan-1-dependent suppression of the PDK-1/AKT/BAD signaling pathway [196]. Syndecan-1 upregulation by DHA has also been demonstrated in human breast cancer cells [2, 198, 199] and in ω3 PUFA-enriched mammary glands and liver of Fat-1 mice (Sun et al., in press).
New evidence points to an important role for syndecan-1 as a negative regulator of angiogenesis and suggests that ω3 PUFA modulation of angiogenesis may involve syndecan-1. Shedding of the syndecan-1 ectodomain is triggered by inflammatory conditions [200, 201]. In a mouse model of lung injury, binding of the chemokine CXCL1 to syndecan-1 was shown to induce enzymatic shedding of the ectodomain-CXCL1 complex by MMP-7. This generated a transepithelial chemokine gradient to direct neutrophils to the injury site [201]. In addition, syndecan-1 is a negative regulator of leukocyte-mediated inflammatory responses by binding chemokines CCL7, CCL11, and CCL17 to inhibit CC chemokine-mediated T cell migration [202]. Mice with a deletion of the gene encoding syndecan-1 (Sdc1−/−) demonstrated increased leukocyte adhesion to endothelial cells and increased inflammation-mediated corneal angiogenesis [203, 204]. The inflammatory cytokine, tumor necrosis factor (TNF)α promotes retinal angiogenesis [205]. TNFα was shown to also suppress endothelial cell syndecan-1 expression [206]. Dietary ω3 PUFA reduced retinal TNFα and protected against retinal neovascularization [153]. Further the antiangiogenic effect of ω3 PUFA was shown to be mediated by activation of the nuclear receptor PPARγ [154]. SDC1 is a PPARγ-responsive gene, and we have shown that DHA upregulates syndecan-1 in prostate cancer cells through activation of PPARγ [197]. Critical experiments confirming a role for syndecan-1 in ω3 PUFA suppression of angiogenesis have yet to be conducted.
11 Conclusions
Dietary ω3 and ω6 PUFA are subjected to complex metabolic processes involving genes for lipid release from glycerolipids, elongation, β-oxidation, storage, eicosanoid synthesis and signaling. Much of the knowledge is derived from the study of ω6 PUFA. Relatively little is known about ω3 PUFA metabolism. Moreover, the interplay between dietary fat and de novo synthesis is not fully understood, especially in cancer. We will end this review by posing a series of questions, which we believe are some of the key challenges in the field of PUFA research.
12 Key unanswered questions
Does FADS2 have the same activity towards LA and α-LNA?
Is the low conversion rate of α-LNA to EPA primarily due to low bioavailability of α-LNA or low activity of FADS?
Are there differences in FADS polymorphisms between Caucasians, Asians, and African-American populations, and do these differences have an impact on the effect of PUFA on cancer risk?
Considering the amount of fat in a typical diet, why do tumor cells require de novo fatty acid synthesis?
Are dietary and de novo synthesized fatty acids utilized differently in tumors compared to normal tissues and in vivo compared to cell culture?
Are ACC1 and FASN good cancer therapeutic targets, and can dietary fat compensate for the loss of ACC1 or FASN in tumor tissue?
Can ACC1 inhibition affect arachidonic acid formation and metabolism?
What are the relative contributions of cyclooxygenases and lipoxygenases during human prostate cancer development, and what is the influence of diet?
How do polymorphic variants of lipid metabolizing enzymes interact with dietary fat to modify cancer risk and progression?
Which metabolites of EPA and DHA are found in prostate tissues and play a major role in suppression of prostate cancer?
Contributor Information
Isabelle M. Berquin, Department of Cancer Biology, Wake Forest School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157, USA Comprehensive Cancer Center, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Iris J. Edwards, Department of Pathology, Wake Forest School of Medicine, Winston-Salem, NC, USA Comprehensive Cancer Center, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Steven J. Kridel, Department of Cancer Biology, Wake Forest School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157, USA Comprehensive Cancer Center, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Yong Q. Chen, Email: yqchen@wfubmc.edu, Department of Cancer Biology, Wake Forest School of Medicine, Medical Center Blvd, Winston-Salem, NC 27157, USA; Comprehensive Cancer Center, Wake Forest School of Medicine, Winston-Salem, NC, USA.
References
- 1.Calviello G, Serini S, Piccioni E. n-3 polyunsaturated fatty acids and the prevention of colorectal cancer: molecular mechanisms involved. Current Medicinal Chemistry. 2007;14(29):3059–3069. doi: 10.2174/092986707782793934. [DOI] [PubMed] [Google Scholar]
- 2.Sun H, Berquin IM, Owens RT, O’Flaherty JT, Edwards IJ. Peroxisome proliferator-activated receptor gamma-mediated up-regulation of syndecan-1 by n-3 fatty acids promotes apoptosis of human breast cancer cells. Cancer Research. 2008;68(8):2912–2919. doi: 10.1158/0008-5472.CAN-07-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berquin IM, Min Y, Wu R, et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. The Journal of Clinical Investigation. 2007;117(7):1866–1875. doi: 10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berquin IM, Edwards IJ, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Letters. 2008;269(2):363–377. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen YQ, Edwards IJ, Kridel SJ, Thornburg T, Berquin IM. Dietary fat–gene interactions in cancer. Cancer Metastasis Reviews. 2007;26(3–4):535–551. doi: 10.1007/s10555-007-9075-x. [DOI] [PubMed] [Google Scholar]
- 6.Simopoulos AP. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk. Experimental Biology and Medicine. 2010;235(7):785–795. doi: 10.1258/ebm.2010.009298. [DOI] [PubMed] [Google Scholar]
- 7.Bernert JT, Jr, Sprecher H. Studies to determine the role rates of chain elongation and desaturation play in regulating the unsaturated fatty acid composition of rat liver lipids. Biochimica et Biophysica Acta. 1975;398(3):354–363. doi: 10.1016/0005-2760(75)90186-1. [DOI] [PubMed] [Google Scholar]
- 8.Burdge GC, Calder PC. Conversion of alphalinolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reproduction Nutrition Development. 2005;45(5):581–597. doi: 10.1051/rnd:2005047. [DOI] [PubMed] [Google Scholar]
- 9.Williams CM, Burdge G. Long-chain n-3 PUFA: plant v. marine sources. Proceedings of the Nutrition Society. 2006;65(1):42–50. doi: 10.1079/pns2005473. [DOI] [PubMed] [Google Scholar]
- 10.Pawlosky RJ, Hibbeln JR, Novotny JA, Salem N., Jr Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. Journal of Lipid Research. 2001;42(8):1257–1265. [PubMed] [Google Scholar]
- 11.Portolesi R, Powell BC, Gibson RA. Competition between 24:5n-3 and ALA for Delta 6 desaturase may limit the accumulation of DHA in HepG2 cell membranes. Journal of Lipid Research. 2007;48(7):1592–1598. doi: 10.1194/jlr.M700081-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Childs CE, Romeu-Nadal M, Burdge GC, Calder PC. Gender differences in the n-3 fatty acid content of tissues. Proceedings of the Nutrition Society. 2008;67(1):19–27. doi: 10.1017/S0029665108005983. [DOI] [PubMed] [Google Scholar]
- 13.Kitson AP, Stroud CK, Stark KD. Elevated production of docosahexaenoic acid in females: potential molecular mechanisms. Lipids. 2010;45(3):209–224. doi: 10.1007/s11745-010-3391-6. [DOI] [PubMed] [Google Scholar]
- 14.Bar M, Wyman SK, Fritz BR, et al. MicroRNA discovery and profiling in human embryonic stem cells by deep sequencing of small RNA libraries. Stem Cells. 2008;26(10):2496–2505. doi: 10.1634/stemcells.2008-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nygaard S, Jacobsen A, Lindow M, et al. Identification and analysis of miRNAs in human breast cancer and teratoma samples using deep sequencing. BMC Medical Genomics. 2009;2:35. doi: 10.1186/1755-8794-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creighton CJ, Benham AL, Zhu H, et al. Discovery of novel microRNAs in female reproductive tract using next generation sequencing. PLoS One. 2010;5(3):e9637. doi: 10.1371/journal.pone.0009637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaeffer L, Gohlke H, Muller M, et al. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Human Molecular Genetics. 2006;15(11):1745–1756. doi: 10.1093/hmg/ddl117. [DOI] [PubMed] [Google Scholar]
- 18.Xie L, Innis SM. Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. Journal of Nutrition. 2008;138(11):2222–2228. doi: 10.3945/jn.108.096156. [DOI] [PubMed] [Google Scholar]
- 19.Martinelli N, Girelli D, Malerba G, et al. FADS genotypes and desaturase activity estimated by the ratio of arachidonic acid to linoleic acid are associated with inflammation and coronary artery disease. American Journal of Clinical Nutrition. 2008;88(4):941–949. doi: 10.1093/ajcn/88.4.941. [DOI] [PubMed] [Google Scholar]
- 20.Malerba G, Schaeffer L, Xumerle L, et al. SNPs of the FADS gene cluster are associated with polyunsaturated fatty acids in a cohort of patients with cardiovascular disease. Lipids. 2008;43(4):289–299. doi: 10.1007/s11745-008-3158-5. [DOI] [PubMed] [Google Scholar]
- 21.Rzehak P, Heinrich J, Klopp N, et al. Evidence for an association between genetic variants of the fatty acid desaturase 1 fatty acid desaturase 2 (FADS1 FADS2) gene cluster and the fatty acid composition of erythrocyte membranes. British Journal of Nutrition. 2009;101(1):20–26. doi: 10.1017/S0007114508992564. [DOI] [PubMed] [Google Scholar]
- 22.Bokor S, Dumont J, Spinneker A, et al. Single nucleotide polymorphisms in the FADS gene cluster are associated with delta-5 and delta-6 desaturase activities estimated by serum fatty acid ratios. Journal of Lipid Research. 2010;51(8):2325–2333. doi: 10.1194/jlr.M006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Y, Feskens EJ, Dolle ME, et al. Dietary n-3 and n-6 polyunsaturated fatty acid intake interacts with FADS1 genetic variation to affect total and HDL-cholesterol concentrations in the Doetinchem Cohort Study. American Journal of Clinical Nutrition. 2010;92(1):258–265. doi: 10.3945/ajcn.2009.29130. [DOI] [PubMed] [Google Scholar]
- 24.Mathias RA, Vergara C, Gao L, et al. FADS genetic variants and omega-6 polyunsaturated fatty acid metabolism in a homogeneous island population. Journal of Lipid Research. 2010;51(9):2766–2774. doi: 10.1194/jlr.M008359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molto-Puigmarti C, Plat J, Mensink RP, et al. FADS1 FADS2 gene variants modify the association between fish intake and the docosahexaenoic acid proportions in human milk. American Journal of Clinical Nutrition. 2010;91(5):1368–1376. doi: 10.3945/ajcn.2009.28789. [DOI] [PubMed] [Google Scholar]
- 26.Zietemann V, Kroger J, Enzenbach C, et al. Genetic variation of the FADS1 FADS2 gene cluster and n-6 PUFA composition in erythrocyte membranes in the European Prospective Investigation into Cancer and Nutrition-Potsdam study. British Journal of Nutrition. 2010;104(12):1748–1759. doi: 10.1017/S0007114510002916. [DOI] [PubMed] [Google Scholar]
- 27.Koletzko B, Lattka E, Zeilinger S, Illig T, Steer C. Genetic variants of the fatty acid desaturase gene cluster predict amounts of red blood cell docosahexaenoic and other polyunsaturated fatty acids in pregnant women: findings from the Avon Longitudinal Study of Parents and Children. American Journal of Clinical Nutrition. 2011;93(1):211–219. doi: 10.3945/ajcn.110.006189. [DOI] [PubMed] [Google Scholar]
- 28.Kwak JH, Paik JK, Kim OY, et al. FADS gene polymorphisms in Koreans: association with omega6 polyunsaturated fatty acids in serum phospholipids, lipid peroxides, and coronary artery disease. Atherosclerosis. 2011;214(1):94–100. doi: 10.1016/j.atherosclerosis.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka T, Shen J, Abecasis GR, et al. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PLoS Genetics. 2009;5(1):e1000338. doi: 10.1371/journal.pgen.1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lattka E, Illig T, Heinrich J, Koletzko B. FADS gene cluster polymorphisms: important modulators of fatty acid levels and their impact on atopic diseases. Journal of Nutrigenetics and Nutrigenomics. 2009;2(3):119–128. doi: 10.1159/000235559. [DOI] [PubMed] [Google Scholar]
- 31.Lattka E, Illig T, Heinrich J, Koletzko B. Do FADS genotypes enhance our knowledge about fatty acid related phenotypes? Clinical Nutrition. 2010;29(3):277–287. doi: 10.1016/j.clnu.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Martinelli N, Consoli L, Olivieri O. A ‘desaturase hypothesis’ for atherosclerosis: Janus-faced enzymes in omega-6 and omega-3 polyunsaturated fatty acid metabolism. Journal of Nutrigenetics and Nutrigenomics. 2009;2(3):129–139. doi: 10.1159/000238177. [DOI] [PubMed] [Google Scholar]
- 33.Lattka E, Illig T, Koletzko B, Heinrich J. Genetic variants of the FADS1 FADS2 gene cluster as related to essential fatty acid metabolism. Current Opinion in Lipidology. 2010;21(1):64–69. doi: 10.1097/MOL.0b013e3283327ca8. [DOI] [PubMed] [Google Scholar]
- 34.Merino DM, Ma DW, Mutch DM. Genetic variation in lipid desaturases and its impact on the development of human disease. Lipids in Health and Disease. 2010;9:63. doi: 10.1186/1476-511X-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merino DM, Johnston H, Clarke S, et al. Polymorphisms in FADS1 and FADS2 alter desaturase activity in young Caucasian and Asian adults. Molecular Genetics and Metabolism. 2011;103(2):171–178. doi: 10.1016/j.ymgme.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Research. 2006;66(12):5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 37.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature Reviews. Cancer. 2007;7(10):763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 38.Bandyopadhyay S, Pai SK, Watabe M, et al. FAS expression inversely correlates with PTEN level in prostate cancer and a PI 3-kinase inhibitor synergizes with FAS siRNA to induce apoptosis. Oncogene. 2005;24(34):5389–5395. doi: 10.1038/sj.onc.1208555. [DOI] [PubMed] [Google Scholar]
- 39.Milgraum LZ, Witters LA, Pasternack GR, Kuhajda FP. Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clinical Cancer Research. 1997;3(11):2115–2120. [PubMed] [Google Scholar]
- 40.Pflug BR, Pecher SM, Brink AW, Nelson JB, Foster BA. Increased fatty acid synthase expression and activity during progression of prostate cancer in the TRAMP model. Prostate. 2003;57(3):245–254. doi: 10.1002/pros.10297. [DOI] [PubMed] [Google Scholar]
- 41.Rossi S, Graner E, Febbo P, et al. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Molecular Cancer Research. 2003;1(10):707–715. [PubMed] [Google Scholar]
- 42.Shah US, Dhir R, Gollin SM, et al. Fatty acid synthase gene overexpression and copy number gain in prostate adenocarcinoma. Human Pathology. 2006;37(4):401–409. doi: 10.1016/j.humpath.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 43.Kridel SJ, Lowther WT, Pemble CWt. Fatty acid synthase inhibitors: new directions for oncology. Expert Opin Investig Drugs. 2007;16(11):1817–1829. doi: 10.1517/13543784.16.11.1817. [DOI] [PubMed] [Google Scholar]
- 44.Swinnen JV, Esquenet M, Goossens K, Heyns W, Verhoeven G. Androgens stimulate fatty acid synthase in the human prostate cancer cell line LNCaP. Cancer Research. 1997;57(6):1086–1090. [PubMed] [Google Scholar]
- 45.Swinnen JV, Ulrix W, Heyns W, Verhoeven G. Coordinate regulation of lipogenic gene expression by androgens: evidence for a cascade mechanism involving sterol regulatory element binding proteins. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(24):12975–12980. doi: 10.1073/pnas.94.24.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swinnen JV, Verhoeven G. Androgens and the control of lipid metabolism in human prostate cancer cells. The Journal of Steroid Biochemistry and Molecular Biology. 1998;65(1–6):191–198. doi: 10.1016/s0960-0760(97)00187-8. [DOI] [PubMed] [Google Scholar]
- 47.Swinnen JV, Roskams T, Joniau S, et al. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. International Journal of Cancer. 2002;98(1):19–22. doi: 10.1002/ijc.10127. [DOI] [PubMed] [Google Scholar]
- 48.Van de Sande T, De Schrijver E, Heyns W, Verhoeven G, Swinnen JV. Role of the phosphatidylinositol 3′-kinase/PTEN/Akt kinase pathway in the overexpression of fatty acid synthase in LNCaP prostate cancer cells. Cancer Research. 2002;62(3):642–646. [PubMed] [Google Scholar]
- 49.Migita T, Ruiz S, Fornari A, et al. Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. Journal of the National Cancer Institute. 2009;101(7):519–532. doi: 10.1093/jnci/djp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24(41):6314–6322. doi: 10.1038/sj.onc.1208773. [DOI] [PubMed] [Google Scholar]
- 51.Hatzivassiliou G, Zhao F, Bauer DE, et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8(4):311–321. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 52.DeBerardinis RJ, Mancuso A, Daikhin E, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proceedings of the National Academy of Sciences. 2007;104(49):19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abu-Elheiga L, Matzuk MM, Kordari P, et al. Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically lethal. PNAS. 2005;102(34):12011–12016. doi: 10.1073/pnas.0505714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mao J, DeMayo FJ, Li H, et al. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(22):8552–8557. doi: 10.1073/pnas.0603115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chirala SS, Chang H, Matzuk M, et al. Fatty acid synthesis is essential in embryonic development: fatty acid synthase null mutants and most of the heterozygotes die in utero. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(11):6358–6363. doi: 10.1073/pnas.0931394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wakil SJ. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry. 1989;28(11):4523–4530. doi: 10.1021/bi00437a001. [DOI] [PubMed] [Google Scholar]
- 57.Wakil SJ, Stoops JK, Joshi VC. Fatty acid synthesis and its regulation. Annual Review of Biochemistry. 1983;52(1):537–579. doi: 10.1146/annurev.bi.52.070183.002541. [DOI] [PubMed] [Google Scholar]
- 58.Smith S. The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. The FASEB Journal. 1994;8(15):1248–1259. [PubMed] [Google Scholar]
- 59.Chajes V, Cambot M, Moreau K, Lenoir GM, Joulin V. Acetyl-CoA carboxylase alpha is essential to breast cancer cell survival. Cancer Research. 2006;66(10):5287–5294. doi: 10.1158/0008-5472.CAN-05-1489. [DOI] [PubMed] [Google Scholar]
- 60.Knowles LM, Axelrod F, Browne CD, Smith JW. A fatty acid synthase blockade induces tumor cell-cycle arrest by down-regulating Skp2. Journal of Biological Chemistry. 2004;279(29):30540–30545. doi: 10.1074/jbc.M405061200. [DOI] [PubMed] [Google Scholar]
- 61.Little JL, Wheeler FB, Fels DR, Koumenis C, Kridel SJ. Inhibition of fatty acid synthase induces endoplasmic reticulum stress in tumor cells. Cancer Research. 2007;67(3):1262–1269. doi: 10.1158/0008-5472.CAN-06-1794. [DOI] [PubMed] [Google Scholar]
- 62.Heiligtag SJ, Bredehorst R, David KA. Key role of mitochondria in cerulenin-mediated apoptosis. Cell Death and Differentiation. 2002;9(9):1017–1025. doi: 10.1038/sj.cdd.4401055. [DOI] [PubMed] [Google Scholar]
- 63.Fiorentino M, Zadra G, Palescandolo E, et al. Overexpression of fatty acid synthase is associated with palmitoylation of Wnt1 and cytoplasmic stabilization of beta-catenin in prostate cancer. Laboratory Investigation. 2008;88(12):1340–1348. doi: 10.1038/labinvest.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Migita T, Narita T, Nomura K, et al. ATP citrate lyase: activation and therapeutic implications in non-small cell lung cancer. Cancer Research. 2008;68(20):8547–8554. doi: 10.1158/0008-5472.CAN-08-1235. [DOI] [PubMed] [Google Scholar]
- 65.Brusselmans K, De Schrijver E, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the acetyl-CoA-carboxylase-alpha gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Research. 2005;65(15):6719–6725. doi: 10.1158/0008-5472.CAN-05-0571. [DOI] [PubMed] [Google Scholar]
- 66.Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Research. 2004;64(6):2070–2075. doi: 10.1158/0008-5472.can-03-3645. [DOI] [PubMed] [Google Scholar]
- 67.Alli PM, Pinn ML, Jaffee EM, McFadden JM, Kuhajda FP. Fatty acid synthase inhibitors are chemo-preventive for mammary cancer in neu-N transgenic mice. Oncogene. 2005;24(1):39–46. doi: 10.1038/sj.onc.1208174. [DOI] [PubMed] [Google Scholar]
- 68.Kuhajda FP, Jenner K, Wood FD, et al. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(14):6379–6383. doi: 10.1073/pnas.91.14.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Orita H, Coulter J, Tully E, Kuhajda FP, Gabrielson E. Inhibiting fatty acid synthase for chemoprevention of chemically induced lung tumors. Clinical Cancer Research. 2008;14(8):2458–2464. doi: 10.1158/1078-0432.CCR-07-4177. [DOI] [PubMed] [Google Scholar]
- 70.Swinnen JV, Van Veldhoven PP, Timmermans L, et al. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochemical and Biophysical Research Communications. 2003;302(4):898–903. doi: 10.1016/s0006-291x(03)00265-1. [DOI] [PubMed] [Google Scholar]
- 71.Rysman E, Brusselmans K, Scheys K, et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Research. 2010;70(20):8117–8126. doi: 10.1158/0008-5472.CAN-09-3871. [DOI] [PubMed] [Google Scholar]
- 72.Chakravarthy MV, Pan Z, Zhu Y, et al. “New” hepatic fat activates PPAR[alpha] to maintain glucose, lipid, and cholesterol homeostasis. Cell Metabolism. 2005;1(5):309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 73.Chakravarthy MV, Lodhi IJ, Yin L, et al. Identification of a physiologically relevant endogenous ligand for PPAR[alpha] in liver. Cell. 2009;138(3):476–488. doi: 10.1016/j.cell.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Schrijver E, Brusselmans K, Heyns W, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Research. 2003;63(13):3799–3804. [PubMed] [Google Scholar]
- 75.Kuemmerle NB, Rysman E, Lombardo PS, et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Molecular Cancer Therapeutics. 2011;10(3):427–436. doi: 10.1158/1535-7163.MCT-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simopoulos AP. Essential fatty acids in health and chronic disease. American Journal of Clinical Nutrition. 1999;70(3 Suppl):560S–569S. doi: 10.1093/ajcn/70.3.560s. [DOI] [PubMed] [Google Scholar]
- 77.Crawford MA, Casperd NM, Sinclair AJ. The long chain metabolites of linoleic avid linolenic acids in liver and brain in herbivores and carnivores. Comparative Biochemistry and Physiology. B. 1976;54(3):395–401. doi: 10.1016/0305-0491(76)90264-9. [DOI] [PubMed] [Google Scholar]
- 78.Horrobin DF, Huang YS, Cunnane SC, Manku MS. Essential fatty acids in plasma, red blood cells and liver phospholipids in common laboratory animals as compared to humans. Lipids. 1984;19(10):806–811. doi: 10.1007/BF02534476. [DOI] [PubMed] [Google Scholar]
- 79.Fu Z, Sinclair AJ. Increased alpha-linolenic acid intake increases tissue alpha-linolenic acid content and apparent oxidation with little effect on tissue docosahexaenoic acid in the guinea pig. Lipids. 2000;35(4):395–400. doi: 10.1007/s11745-000-537-7. [DOI] [PubMed] [Google Scholar]
- 80.Leyton J, Drury PJ, Crawford MA. Differential oxidation of saturated and unsaturated fatty acids in vivo in the rat. British Journal of Nutrition. 1987;57(3):383–393. doi: 10.1079/bjn19870046. [DOI] [PubMed] [Google Scholar]
- 81.DeLany JP, Windhauser MM, Champagne CM, Bray GA. Differential oxidation of individual dietary fatty acids in humans. American Journal of Clinical Nutrition. 2000;72(4):905–911. doi: 10.1093/ajcn/72.4.905. [DOI] [PubMed] [Google Scholar]
- 82.Gavino VC, Cordeau S, Gavino G. Kinetic analysis of the selectivity of acylcarnitine synthesis in rat mitochondria. Lipids. 2003;38(4):485–490. doi: 10.1007/s11745-003-1088-7. [DOI] [PubMed] [Google Scholar]
- 83.Bryan DL, Hart P, Forsyth K, Gibson R. Incorporation of alpha-linolenic acid and linoleic acid into human respiratory epithelial cell lines. Lipids. 2001;36(7):713–717. doi: 10.1007/s11745-001-0776-7. [DOI] [PubMed] [Google Scholar]
- 84.Martin-Chouly CA, Menier V, Hichami A, et al. Modulation of PAF production by incorporation of arachidonic acid and eicosapentaenoic acid in phospholipids of human leukemic monocyte-like cells THP-1. Prostaglandins & Other Lipid Mediators. 2000;60(4–6):127–135. doi: 10.1016/s0090-6980(99)00058-1. [DOI] [PubMed] [Google Scholar]
- 85.Pickett WC, Ramesha CS. Ether phospholipids in control and 20:4-depleted rat PMN: additional evidence for a 1-O-alkyl-2-20:4-sn-glycerol-3-phosphocholine specific phospholipase A2. Agents and Actions. 1987;21(3–4):390–392. doi: 10.1007/BF01966525. [DOI] [PubMed] [Google Scholar]
- 86.Strokin M, Sergeeva M, Reiser G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+ British Journal of Pharmacology. 2003;139(5):1014–1022. doi: 10.1038/sj.bjp.0705326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakanishi M, Rosenberg DW. Roles of cPLA2alpha and arachidonic acid in cancer. Biochimica et Biophysica Acta. 2006;1761(11):1335–1343. doi: 10.1016/j.bbalip.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murakami M, Taketomi Y, Girard C, Yamamoto K, Lambeau G. Emerging roles of secreted phospholipase A2 enzymes: lessons from transgenic and knockout mice. Biochimie. 2010;92(6):561–582. doi: 10.1016/j.biochi.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 89.Scott KF, Sajinovic M, Hein J, et al. Emerging roles for phospholipase A2 enzymes in cancer. Biochimie. 2010;92(6):601–610. doi: 10.1016/j.biochi.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 90.Dong Q, Patel M, Scott KF, Graham GG, Russell PJ, Sved P. Oncogenic action of phospholipase A2 in prostate cancer. Cancer Letters. 2006;240(1):9–16. doi: 10.1016/j.canlet.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 91.Mirtti T, Laine VJ, Hiekkanen H, et al. Group IIA phospholipase A as a prognostic marker in prostate cancer: relevance to clinicopathological variables and disease-specific mortality. APMIS. 2009;117(3):151–161. doi: 10.1111/j.1600-0463.2008.00002.x. [DOI] [PubMed] [Google Scholar]
- 92.Wang D, Dubois RN. Eicosanoids and cancer. Nature Reviews Cancer. 2010;10(3):181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Panigrahy D, Kaipainen A, Greene ER, Huang S. Cytochrome P450-derived eicosanoids: the neglected pathway in cancer. Cancer Metastasis Reviews. 2010;29(4):723–735. doi: 10.1007/s10555-010-9264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2009;81(2–3):187–191. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dubois RN, Abramson SB, Crofford L, et al. Cyclooxygenase in biology and disease. The FASEB Journal. 1998;12(12):1063–1073. [PubMed] [Google Scholar]
- 96.Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol. 2010;2010:215158. doi: 10.1155/2010/215158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang MT, Honn KV, Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Reviews. 2007;26(3–4):525–534. doi: 10.1007/s10555-007-9096-5. [DOI] [PubMed] [Google Scholar]
- 98.Reese AC, Fradet V, Witte JS. Omega-3 fatty acids, genetic variants in COX-2 and prostate cancer. Journal of Nutrigenetics and Nutrigenomics. 2009;2(3):149–158. doi: 10.1159/000235565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Menter DG, Schilsky RL, DuBois RN. Cyclooxygenase-2 and cancer treatment: understanding the risk should be worth the reward. Clinical Cancer Research. 2010;16(5):1384–1390. doi: 10.1158/1078-0432.CCR-09-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. Journal of Biological Chemistry. 1999;274(34):23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 101.Pidgeon GP, Lysaght J, Krishnamoorthy S, et al. Lipoxygenase metabolism: roles in tumor progression and survival. Cancer Metastasis Reviews. 2007;26(3–4):503–524. doi: 10.1007/s10555-007-9098-3. [DOI] [PubMed] [Google Scholar]
- 102.Avis IM, Jett M, Boyle T, et al. Growth control of lung cancer by interruption of 5-lipoxygenase-mediated growth factor signaling. The Journal of Clinical Investigation. 1996;97(3):806–813. doi: 10.1172/JCI118480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Soumaoro LT, Iida S, Uetake H, et al. Expression of 5-lipoxygenase in human colorectal cancer. World Journal of Gastroenterology. 2006;12(39):6355–6360. doi: 10.3748/wjg.v12.i39.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ye YN, Wu WK, Shin VY, Bruce IC, Wong BC, Cho CH. Dual inhibition of 5-LOX and COX-2 suppresses colon cancer formation promoted by cigarette smoke. Carcinogenesis. 2005;26(4):827–834. doi: 10.1093/carcin/bgi012. [DOI] [PubMed] [Google Scholar]
- 105.Faronato M, Muzzonigro G, Milanese G, et al. Increased expression of 5-lipoxygenase is common in clear cell renal cell carcinoma. Histology and Histopathology. 2007;22(10):1109–1118. doi: 10.14670/HH-22.1109. [DOI] [PubMed] [Google Scholar]
- 106.Hayashi T, Nishiyama K, Shirahama T. Inhibition of 5-lipoxygenase pathway suppresses the growth of bladder cancer cells. International Journal of Urology. 2006;13(8):1086–1091. doi: 10.1111/j.1442-2042.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- 107.Ghosh J. Inhibition of arachidonate 5-lipoxygenase triggers prostate cancer cell death through rapid activation of c-Jun N-terminal kinase. Biochemical and Biophysical Research Communications. 2003;307(2):342–349. doi: 10.1016/s0006-291x(03)01201-4. [DOI] [PubMed] [Google Scholar]
- 108.Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(22):13182–13187. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sharma BK, Pilania P, Singh P. Modeling of cyclooxygenase-2 and 5-lipooxygenase inhibitory activity of apoptosis-inducing agents potentially useful in prostate cancer chemotherapy: derivatives of diarylpyrazole. Journal of Enzyme Inhibition and Medicinal Chemistry. 2009;24(2):607–615. doi: 10.1080/14756360802318878. [DOI] [PubMed] [Google Scholar]
- 110.Sundaram S, Ghosh J. Expression of 5-oxoETE receptor in prostate cancer cells: critical role in survival. Biochemical and Biophysical Research Communications. 2006;339(1):93–98. doi: 10.1016/j.bbrc.2005.10.189. [DOI] [PubMed] [Google Scholar]
- 111.Koh WP, Yuan JM, van den Berg D, Lee HP, Yu MC. Interaction between cyclooxygenase-2 gene polymorphism and dietary n-6 polyunsaturated fatty acids on colon cancer risk: the Singapore Chinese Health Study. British Journal of Cancer. 2004;90(9):1760–1764. doi: 10.1038/sj.bjc.6601797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Siezen CL, van Leeuwen AI, Kram NR, Luken ME, van Kranen HJ, Kampman E. Colorectal adenoma risk is modified by the interplay between polymorphisms in arachidonic acid pathway genes and fish consumption. Carcinogenesis. 2005;26(2):449–457. doi: 10.1093/carcin/bgh336. [DOI] [PubMed] [Google Scholar]
- 113.Hedelin M, Chang ET, Wiklund F, et al. Association of frequent consumption of fatty fish with prostate cancer risk is modified by COX-2 polymorphism. International Journal of Cancer. 2007;120(2):398–405. doi: 10.1002/ijc.22319. [DOI] [PubMed] [Google Scholar]
- 114.Fradet V, Cheng I, Casey G, Witte JS. Dietary omega-3 fatty acids, cyclooxygenase-2 genetic variation, and aggressive prostate cancer risk. Clinical Cancer Research. 2009;15(7):2559–2566. doi: 10.1158/1078-0432.CCR-08-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. American Journal of Clinical Nutrition. 2004;79(6):935–945. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 116.Haeggstrom JZ, Rinaldo-Matthis A, Wheelock CE, Wetterholm A. Advances in eicosanoid research, novel therapeutic implications. Biochemical and Biophysical Research Communications. 2010;396(1):135–139. doi: 10.1016/j.bbrc.2010.03.140. [DOI] [PubMed] [Google Scholar]
- 117.Radmark O, Samuelsson B. Microsomal prostaglandin E synthase-1 and 5-lipoxygenase: potential drug targets in cancer. Journal of Internal Medicine. 2010;268(1):5–14. doi: 10.1111/j.1365-2796.2010.02246.x. [DOI] [PubMed] [Google Scholar]
- 118.Tanioka T, Nakatani Y, Semmyo N, Murakami M, Kudo I. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. Journal of Biological Chemistry. 2000;275(42):32775–32782. doi: 10.1074/jbc.M003504200. [DOI] [PubMed] [Google Scholar]
- 119.Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clinical Immunology. 2006;119(3):229–240. doi: 10.1016/j.clim.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 120.Lovgren AK, Kovarova M, Koller BH. cPGES/p23 is required for glucocorticoid receptor function and embryonic growth but not prostaglandin E2 synthesis. Molecular and Cellular Biology. 2007;27(12):4416–4430. doi: 10.1128/MCB.02314-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hanaka H, Pawelzik SC, Johnsen JI, et al. Microsomal prostaglandin E synthase 1 determines tumor growth in vivo of prostate and lung cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(44):18757–18762. doi: 10.1073/pnas.0910218106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Amirian ES, Ittmann MM, Scheurer ME. Associations between arachidonic acid metabolism gene polymorphisms and prostate cancer risk. Prostate. 2011;71(13):1382–1389. doi: 10.1002/pros.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cathcart MC, Reynolds JV, O’Byrne KJ, Pidgeon GP. The role of prostacyclin synthase and thromboxane synthase signaling in the development and progression of cancer. Biochimica et Biophysica Acta. 2010;1805(2):153–166. doi: 10.1016/j.bbcan.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 124.Frigola J, Munoz M, Clark SJ, Moreno V, Capella G, Peinado MA. Hypermethylation of the prostacyclin synthase (PTGIS) promoter is a frequent event in colorectal cancer and associated with aneuploidy. Oncogene. 2005;24(49):7320–7326. doi: 10.1038/sj.onc.1208883. [DOI] [PubMed] [Google Scholar]
- 125.Poole EM, Bigler J, Whitton J, Sibert JG, Potter JD, Ulrich CM. Prostacyclin synthase and arachidonate 5-lipoxygenase polymorphisms and risk of colorectal polyps. Cancer Epidemiology, Biomarkers & Prevention. 2006;15(3):502–508. doi: 10.1158/1055-9965.EPI-05-0804. [DOI] [PubMed] [Google Scholar]
- 126.Ermert L, Dierkes C, Ermert M. Immunohistochemical expression of cyclooxygenase isoenzymes and downstream enzymes in human lung tumors. Clinical Cancer Research. 2003;9(5):1604–1610. [PubMed] [Google Scholar]
- 127.Nana-Sinkam P, Golpon H, Keith RL, et al. Prostacyclin in human non-small cell lung cancers. Chest. 2004;125(5 Suppl):141S. doi: 10.1378/chest.125.5_suppl.141s. [DOI] [PubMed] [Google Scholar]
- 128.Niknami M, Vignarajan S, Yao M, et al. Decrease in expression or activity of cytosolic phospholipase A2alpha increases cyclooxygenase-1 action: a cross-talk between key enzymes in arachidonic acid pathway in prostate cancer cells. Biochimica et Biophysica Acta. 2010;1801(7):731–737. doi: 10.1016/j.bbalip.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 129.Nie D, Che M, Zacharek A, et al. Differential expression of thromboxane synthase in prostate carcinoma: role in tumor cell motility. American Journal of Pathology. 2004;164(2):429–439. doi: 10.1016/S0002-9440(10)63133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiological Reviews. 1999;79(4):1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 131.Chen Y, Hughes-Fulford M. Prostaglandin E2 and the protein kinase A pathway mediate arachidonic acid induction of c-fos in human prostate cancer cells. British Journal of Cancer. 2000;82(12):2000–2006. doi: 10.1054/bjoc.2000.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang X, Klein RD. Prostaglandin E2 induces vascular endothelial growth factor secretion in prostate cancer cells through EP2 receptor-mediated cAMP pathway. Molecular Carcinogenesis. 2007;46(11):912–923. doi: 10.1002/mc.20320. [DOI] [PubMed] [Google Scholar]
- 133.Dassesse T, de Leval X, de Leval L, Pirotte B, Castronovo V, Waltregny D. Activation of the thromboxane A2 pathway in human prostate cancer correlates with tumor Gleason score and pathologic stage. Eur Urol. 2006;50(5):1021–1031. doi: 10.1016/j.eururo.2006.01.036. discussion 31. [DOI] [PubMed] [Google Scholar]
- 134.Nie D, Guo Y, Yang D, et al. Thromboxane A2 receptors in prostate carcinoma: expression and its role in regulating cell motility via small GTPase rho. Cancer Research. 2008;68(1):115–121. doi: 10.1158/0008-5472.CAN-07-1018. [DOI] [PubMed] [Google Scholar]
- 135.Mahmud S, Franco E, Aprikian A. Prostate cancer and use of nonsteroidal anti-inflammatory drugs: systematic review and meta-analysis. British Journal of Cancer. 2004;90(1):93–99. doi: 10.1038/sj.bjc.6601416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Platz EA, Rohrmann S, Pearson JD, et al. Nonsteroidal anti-inflammatory drugs and risk of prostate cancer in the Baltimore Longitudinal Study of Aging. Cancer Epidemiology, Biomarkers & Prevention. 2005;14(2):390–396. doi: 10.1158/1055-9965.EPI-04-0532. [DOI] [PubMed] [Google Scholar]
- 137.Chan JM, Feraco A, Shuman M, Hernandez-Diaz S. The epidemiology of prostate cancer—with a focus on nonsteroidal anti-inflammatory drugs. Hematology/Oncology Clinics of North America. 2006;20(4):797–809. doi: 10.1016/j.hoc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 138.Salinas CA, Kwon EM, FitzGerald LM, et al. Use of aspirin and other nonsteroidal antiinflammatory medications in relation to prostate cancer risk. American Journal of Epidemiology. 2010;172(5):578–590. doi: 10.1093/aje/kwq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29(6):781–788. doi: 10.1038/onc.2009.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chapkin RS, Seo J, McMurray DN, Lupton JR. Mechanisms by which docosahexaenoic acid and related fatty acids reduce colon cancer risk and inflammatory disorders of the intestine. Chemistry and Physics of Lipids. 2008;153(1):14–23. doi: 10.1016/j.chemphyslip.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutrition Reviews. 2010;68(5):280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 142.Wang D, DuBois RN. Pro-inflammatory prostaglandins and progression of colorectal cancer. Cancer Letters. 2008;267(2):197–203. doi: 10.1016/j.canlet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wymann MP, Schneiter R. Lipid signalling in disease. Nature Reviews Molecular Cell Biology. 2008;9(2):162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 144.Folkman J, Cole P, Zimmerman S. Tumor behavior in isolated perfused organs: in vitro growth and metastases of biopsy material in rabbit thyroid and canine intestinal segment. Annals of Surgery. 1966;164(3):491–502. doi: 10.1097/00000658-196609000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gimbrone MA, Jr, Leapman SB, Cotran RS, Folkman J. Tumor dormancy in vivo by prevention of neovascularization. The Journal of Experimental Medicine. 1972;136(2):261–276. doi: 10.1084/jem.136.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Borre M, Offersen BV, Nerstrom B, Overgaard J. Microvessel density predicts survival in prostate cancer patients subjected to watchful waiting. British Journal of Cancer. 1998;78(7):940–944. doi: 10.1038/bjc.1998.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bono AV, Celato N, Cova V, Salvadore M, Chinetti S, Novario R. Microvessel density in prostate carcinoma. Prostate Cancer and Prostatic Diseases. 2002;5(2):123–127. doi: 10.1038/sj.pcan.4500572. [DOI] [PubMed] [Google Scholar]
- 148.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocrine Reviews. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 149.Tsuzuki T, Shibata A, Kawakami Y, Nakagawa K, Miyazawa T. Conjugated eicosapentaenoic acid inhibits vascular endothelial growth factor-induced angiogenesis by suppressing the migration of human umbilical vein endothelial cells. Journal of Nutrition. 2007;137(3):641–646. doi: 10.1093/jn/137.3.641. [DOI] [PubMed] [Google Scholar]
- 150.Tsuji M, Murota SI, Morita I. Docosapentaenoic acid (22:5, n-3) suppressed tube-forming activity in endothelial cells induced by vascular endothelial growth factor. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2003;68(5):337–342. doi: 10.1016/s0952-3278(03)00025-5. [DOI] [PubMed] [Google Scholar]
- 151.Yang SP, Morita I, Murota SI. Eicosapentaenoic acid attenuates vascular endothelial growth factor-induced proliferation via inhibiting Flk-1 receptor expression in bovine carotid artery endothelial cells. Journal of Cellular Physiology. 1998;176(2):342–349. doi: 10.1002/(SICI)1097-4652(199808)176:2<342::AID-JCP12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 152.Calviello G, Di Nicuolo F, Gragnoli S, et al. n-3 PUFAs reduce VEGF expression in human colon cancer cells modulating the COX-2/PGE2 induced ERK-1 and-2 and HIF-1alpha induction pathway. Carcinogenesis. 2004;25(12):2303–2310. doi: 10.1093/carcin/bgh265. [DOI] [PubMed] [Google Scholar]
- 153.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nature Medicine. 2007;13(7):868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stahl A, Sapieha P, Connor KM, et al. Short communication: PPAR gamma mediates a direct antiangiogenic effect of omega 3-PUFAs in proliferative retinopathy. Circulation Research. 2010;107(4):495–500. doi: 10.1161/CIRCRESAHA.110.221317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sapieha P, Stahl A, Chen J, et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of {omega}-3 polyunsaturated fatty acids. Science Translational Medicine. 2011;3(69):69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rose DP, Connolly JM. Antiangiogenicity of docosahexaenoic acid and its role in the suppression of breast cancer cell growth in nude mice. International Journal of Oncology. 1999;15(5):1011–1015. doi: 10.3892/ijo.15.5.1011. [DOI] [PubMed] [Google Scholar]
- 157.Ambring A, Johansson M, Axelsen M, Gan L, Strandvik B, Friberg P. Mediterranean-inspired diet lowers the ratio of serum phospholipid n-6 to n-3 fatty acids, the number of leukocytes and platelets, and vascular endothelial growth factor in healthy subjects. American Journal of Clinical Nutrition. 2006;83(3):575–581. doi: 10.1093/ajcn.83.3.575. [DOI] [PubMed] [Google Scholar]
- 158.Fox PL, DiCorleto PE. Fish oils inhibit endothelial cell production of platelet-derived growth factor-like protein. Science. 1988;241(4864):453–456. doi: 10.1126/science.3393911. [DOI] [PubMed] [Google Scholar]
- 159.Kaminski WE, Jendraschak E, Kiefl R, von Schacky C. Dietary omega-3 fatty acids lower levels of platelet-derived growth factor mRNA in human mononuclear cells. Blood. 1993;81(7):1871–1879. [PubMed] [Google Scholar]
- 160.Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocrine-Related Cancer. 2000;7(3):165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 161.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biology. 2001;2(3) doi: 10.1186/gb-2001-2-3-reviews3005. REVIEWS3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kwabi-Addo B, Ozen M, Ittmann M. The role of fibroblast growth factors and their receptors in prostate cancer. Endocrine-Related Cancer. 2004;11(4):709–724. doi: 10.1677/erc.1.00535. [DOI] [PubMed] [Google Scholar]
- 163.Li ZG, Mathew P, Yang J, et al. Androgen receptor-negative human prostate cancer cells induce osteogenesis in mice through FGF9-mediated mechanisms. The Journal of Clinical Investigation. 2008;118(8):2697–2710. doi: 10.1172/JCI33093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Heer R, Douglas D, Mathers ME, Robson CN, Leung HY. Fibroblast growth factor 17 is over-expressed in human prostate cancer. The Journal of Pathology. 2004;204(5):578–586. doi: 10.1002/path.1668. [DOI] [PubMed] [Google Scholar]
- 165.Folkman J, Shing Y. Angiogenesis. Journal of Biological Chemistry. 1992;267(16):10931–10934. [PubMed] [Google Scholar]
- 166.Dorkin TJ, Robinson MC, Marsh C, Neal DE, Leung HY. aFGF immunoreactivity in prostate cancer and its co-localization with bFGF and FGF8. The Journal of Pathology. 1999;189(4):564–569. doi: 10.1002/(SICI)1096-9896(199912)189:4<564::AID-PATH480>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 167.Polnaszek N, Kwabi-Addo B, Peterson LE, et al. Fibroblast growth factor 2 promotes tumor progression in an autochthonous mouse model of prostate cancer. Cancer Research. 2003;63(18):5754–5760. [PubMed] [Google Scholar]
- 168.Gnanapragasam VJ, Robinson MC, Marsh C, Robson CN, Hamdy FC, Leung HY. FGF8 isoform b expression in human prostate cancer. British Journal of Cancer. 2003;88(9):1432–1438. doi: 10.1038/sj.bjc.6600875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Valta MP, Tuomela J, Vuorikoski H, et al. FGF-8b induces growth and rich vascularization in an orthotopic PC-3 model of prostate cancer. Journal of Cellular Biochemistry. 2009;107(4):769–784. doi: 10.1002/jcb.22175. [DOI] [PubMed] [Google Scholar]
- 170.Elo TD, Valve EM, Seppanen JA, et al. Stromal activation associated with development of prostate cancer in prostate-targeted fibroblast growth factor 8b transgenic mice. Neoplasia. 2010;12(11):915–927. doi: 10.1593/neo.10776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kasayama S, Koga M, Kouhara H, et al. Unsaturated fatty acids are required for continuous proliferation of transformed androgen-dependent cells by fibroblast growth factor family proteins. Cancer Research. 1994;54(24):6441–6445. [PubMed] [Google Scholar]
- 172.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature Immunology. 2004;5(10):987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 173.Huang B, Zhao J, Li H, et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Research. 2005;65(12):5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 174.Kelly MG, Alvero AB, Chen R, et al. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Research. 2006;66(7):3859–3868. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- 175.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nature Reviews Cancer. 2009;9(1):57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 176.Zheng SL, Augustsson-Balter K, Chang B, et al. Sequence variants of toll-like receptor 4 are associated with prostate cancer risk: results from the Cancer Prostate in Sweden Study. Cancer Research. 2004;64(8):2918–2922. doi: 10.1158/0008-5472.can-03-3280. [DOI] [PubMed] [Google Scholar]
- 177.Sun J, Wiklund F, Zheng SL, et al. Sequence variants in toll-like receptor gene cluster (TLR6-TLR1-TLR10) and prostate cancer risk. Journal of the National Cancer Institute. 2005;97(7):525–532. doi: 10.1093/jnci/dji070. [DOI] [PubMed] [Google Scholar]
- 178.Lee JY, Plakidas A, Lee WH, et al. Differential modulation of toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. Journal of Lipid Research. 2003;44(3):479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 179.Lee JY, Zhao L, Youn HS, et al. Saturated fatty acid activates but polyunsaturated fatty acid inhibits toll-like receptor 2 dimerized with toll-like receptor 6 or 1. Journal of Biological Chemistry. 2004;279(17):16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 180.Paone A, Galli R, Gabellini C, et al. Toll-like receptor 3 regulates angiogenesis and apoptosis in prostate cancer cell lines through hypoxia-inducible factor 1 alpha. Neoplasia. 2010;12(7):539–549. doi: 10.1593/neo.92106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rapraeger A, Jalkanen M, Endo E, Koda J, Bernfield M. The cell surface proteoglycan from mouse mammary epithelial cells bears chondroitin sulfate and heparan sulfate glycosaminoglycans. Journal of Biological Chemistry. 1985;260(20):11046–11052. [PubMed] [Google Scholar]
- 182.Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. The FEBS Journal. 2010;277(19):3876–3889. doi: 10.1111/j.1742-4658.2010.07798.x. [DOI] [PubMed] [Google Scholar]
- 183.Inki P, Jalkanen M. The role of syndecan-1 in malignancies. Annali Medici. 1996;28(1):63–67. doi: 10.3109/07853899608999076. [DOI] [PubMed] [Google Scholar]
- 184.Matsumoto A, Ono M, Fujimoto Y, Gallo RL, Bernfield M, Kohgo Y. Reduced expression of syndecan-1 in human hepatocellular carcinoma with high metastatic potential. International Journal of Cancer. 1997;74(5):482–491. doi: 10.1002/(sici)1097-0215(19971021)74:5<482::aid-ijc2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 185.Kumar-Singh S, Jacobs W, Dhaene K, et al. Syndecan-1 expression in malignant mesothelioma: correlation with cell differentiation, WT1 expression, and clinical outcome. The Journal of Pathology. 1998;186(3):300–305. doi: 10.1002/(SICI)1096-9896(1998110)186:3<300::AID-PATH180>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 186.Loussouarn D, Campion L, Sagan C, et al. Prognostic impact of syndecan-1 expression in invasive ductal breast carcinomas. British Journal of Cancer. 2008;98(12):1993–1998. doi: 10.1038/sj.bjc.6604400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Barbareschi M, Maisonneuve P, Aldovini D, et al. High syndecan-1 expression in breast carcinoma is related to an aggressive phenotype and to poorer prognosis. Cancer. 2003;98(3):474–483. doi: 10.1002/cncr.11515. [DOI] [PubMed] [Google Scholar]
- 188.Davies EJ, Blackhall FH, Shanks JH, et al. Distribution and clinical significance of heparan sulfate proteoglycans in ovarian cancer. Clinical Cancer Research. 2004;10(15):5178–5186. doi: 10.1158/1078-0432.CCR-03-0103. [DOI] [PubMed] [Google Scholar]
- 189.Choi DS, Kim JH, Ryu HS, et al. Syndecan-1, a key regulator of cell viability in endometrial cancer. International Journal of Cancer. 2007;121(4):741–750. doi: 10.1002/ijc.22713. [DOI] [PubMed] [Google Scholar]
- 190.Stanley MJ, Stanley MW, Sanderson RD, Zera R. Syndecan-1 expression is induced in the stroma of infiltrating breast carcinoma. American Journal of Clinical Pathology. 1999;112(3):377–383. doi: 10.1093/ajcp/112.3.377. [DOI] [PubMed] [Google Scholar]
- 191.Mennerich D, Vogel A, Klaman I, et al. Shift of syndecan-1 expression from epithelial to stromal cells during progression of solid tumours. European Journal of Cancer. 2004;40(9):1373–1382. doi: 10.1016/j.ejca.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 192.Wiksten JP, Lundin J, Nordling S, et al. Epithelial and stromal syndecan-1 expression as predictor of outcome in patients with gastric cancer. International Journal of Cancer. 2001;95(1):1–6. doi: 10.1002/1097-0215(20010120)95:1<1::aid-ijc1000>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 193.Kiviniemi J, Kallajoki M, Kujala I, et al. Altered expression of syndecan-1 in prostate cancer. APMIS. 2004;112(2):89–97. doi: 10.1111/j.1600-0463.2004.apm1120202.x. [DOI] [PubMed] [Google Scholar]
- 194.Chen D, Adenekan B, Chen L, et al. Syndecan-1 expression in locally invasive and metastatic prostate cancer. Urology. 2004;63(2):402–407. doi: 10.1016/j.urology.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 195.Zellweger T, Ninck C, Mirlacher M, et al. Tissue microarray analysis reveals prognostic significance of syndecan-1 expression in prostate cancer. Prostate. 2003;55(1):20–29. doi: 10.1002/pros.10209. [DOI] [PubMed] [Google Scholar]
- 196.Hu Y, Sun H, Owens RT, et al. Syndecan-1-dependent suppression of PDK1/Akt/bad signaling by docosahexaenoic acid induces apoptosis in prostate cancer. Neoplasia. 2010;12(10):826–836. doi: 10.1593/neo.10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Edwards IJ, Sun H, Hu Y, et al. In vivo and in vitro regulation of syndecan 1 in prostate cells by N-3 polyunsaturated fatty acids. Journal of Biological Chemistry. 2008;283(26):18441–18449. doi: 10.1074/jbc.M802107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Edwards IJ, Berquin IM, Sun H, et al. Differential effects of delivery of omega-3 fatty acids to human cancer cells by low-density lipoproteins versus albumin. Clinical Cancer Research. 2004;10(24):8275–8283. doi: 10.1158/1078-0432.CCR-04-1357. [DOI] [PubMed] [Google Scholar]
- 199.Sun H, Berquin IM, Edwards IJ. Omega-3 polyunsaturated fatty acids regulate syndecan-1 expression in human breast cancer cells. Cancer Research. 2005;65(10):4442–4447. doi: 10.1158/0008-5472.CAN-04-4200. [DOI] [PubMed] [Google Scholar]
- 200.Park PW, Pier GB, Hinkes MT, Bernfield M. Exploitation of syndecan-1 shedding by Pseudomonas aeruginosa enhances virulence. Nature. 2001;411(6833):98–102. doi: 10.1038/35075100. [DOI] [PubMed] [Google Scholar]
- 201.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111(5):635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 202.Xu J, Park PW, Kheradmand F, Corry DB. Endogenous attenuation of allergic lung inflammation by syndecan-1. Journal of Immunology. 2005;174(9):5758–5765. doi: 10.4049/jimmunol.174.9.5758. [DOI] [PubMed] [Google Scholar]
- 203.Gotte M, Joussen AM, Klein C, et al. Role of syndecan-1 in leukocyte-endothelial interactions in the ocular vasculature. Investigative Ophthalmology & Visual Science. 2002;43(4):1135–1141. [PubMed] [Google Scholar]
- 204.Gotte M, Bernfield M, Joussen AM. Increased leukocyte-endothelial interactions in syndecan-1-deficient mice involve heparan sulfate-dependent and -independent steps. Current Eye Research. 2005;30(6):417–422. doi: 10.1080/02713680590956289. [DOI] [PubMed] [Google Scholar]
- 205.Gardiner TA, Gibson DS, de Gooyer TE, de la Cruz VF, McDonald DM, Stitt AW. Inhibition of tumor necrosis factor-alpha improves physiological angiogenesis and reduces pathological neovascularization in ischemic retinopathy. American Journal of Pathology. 2005;166(2):637–644. doi: 10.1016/s0002-9440(10)62284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kainulainen V, Nelimarkka L, Jarvelainen H, Laato M, Jalkanen M, Elenius K. Suppression of syndecan-1 expression in endothelial cells by tumor necrosis factor-alpha. Journal of Biological Chemistry. 1996;271(31):18759–18766. doi: 10.1074/jbc.271.31.18759. [DOI] [PubMed] [Google Scholar]