Abstract
Purpose
Sorafenib is an oral Raf kinase inhibitor, approved for the treatment of advanced renal cancer. Clinical investigation of the safety and feasibility of sorafenib therapy in patients with impaired renal function was performed in this study.
Materials and Methods
The protocol was approved by the Human Investigation Committee of Wayne State University. Medical records of patients with metastatic renal cancer at Wayne State University started on sorafenib between November 2005 to January 2007 were reviewed. Patients with a calculated creatinine clearance (CrCl) of 60 mL/min or less (chronic kidney disease stage 3 or greater per Kidney Disease Outcomes Quality Initiative guidelines) were deemed to have renal insufficiency.
Results
A total of 32 patients who met the selection criteria were analyzed. Fourteen of 32 (44%) patients had renal insufficiency (range, 32–60 mL/min). Median age was 71 years in patients with CrCl ≤ 60 mL/min and 54 years in those with > 60 mL/min. Incidence of diarrhea (57% vs. 33%) and hand-foot syndrome (86% vs. 56%) were higher in the renal-dysfunction group. Dose interruptions and dose reductions were noted in 57% and 43% of patients with renal dysfunction versus 28% and 22% in those without. No significant differences were noted in response rate, progression-free survival or overall survival.
Conclusion
Renal insufficiency is frequently observed in patients with advanced renal cancer. Sorafenib therapy can be safely delivered in patients with mild and moderate renal dysfunction, and efficacy appears to be maintained.
Keywords: Hand-foot syndrome, Hypertension, Renal dysfunction
Introduction
Renal cancer is one of the most common malignancies; it is the seventh leading malignant condition among men and ninth among women. It is estimated that 54,390 new cases of renal cancer will be diagnosed in 2008, accounting for approximately 3.8% of all new cancers diagnosed in 2008,1 with estimated mortality of 13,010. The overall incidence of renal cancer has increased over the past 20 years. Many patients are being diagnosed at an earlier stage because of the incidental finding of a renal mass on radiologic examination.2 However, approximately one fourth of patients still present in an advanced stage, and one third of patients with resected localized disease will have a recurrence.3 Targeted therapy has revolutionized the management of metastatic kidney cancer. In the past few years, the identity of kidney cancer changed from a resistant malignancy with very few therapeutic options to a disease with a number of active treatment options. However, the question remains whether these therapies are widely applicable to patient populations with impaired organ function. Most clinical trials proving the safety and efficacy of these targeted therapies have been conducted in patients with optimal organ function and good performance status. Hence, there is a paucity of information regarding the expected toxicity profile of agents newly approved for use in advanced renal cancer, such as sorafenib, sunitinib, and temsirolimus, in patients with impaired creatinine clearance (CrCl). In this study, we evaluated the feasibility, safety, and efficacy of sorafenib in patients with metastatic renal cancer with impaired renal function. This will help guide the future clinical management of these patients.
Sorafenib is an orally administered multikinase inhibitor that has been tested in renal cancer. It is a multikinase inhibitor targeting the platelet-derived growth factor; vascular endothelial growth factor receptors -1, -2, and -3; FMS-like tryrosine kinase 3; c-Kit; RET; and Ras tyrosine kinases. Initial clinical evaluation was in a placebo-controlled, randomized phase II discontinuation trial of sorafenib in advanced renal cancer demonstrating improvement of progression-free survival (PFS) when compared with placebo.4 Sorafenib was further evaluated in a 903-patient randomized, placebo-controlled, double-blind phase III trial by Escudier et al.5 The results of this study confirmed the efficacy of sorafenib in metastatic renal cell carcinoma (RCC) pretreated with immunotherapy. Median PFS for sorafenib was 5.5 months versus 2.8 months for placebo. Though only 10% of patients had an objective partial response (PR), 74% of patients had stable disease (SD). The most common adverse events were diarrhea, rash, fatigue, hand-foot skin reactions, hypertension, alopecia, and nausea. The results of this trial led to the US Food and Drug Administration (FDA) approval of sorafenib in advanced renal cancer and commenced the nationwide use of the drug.
Pharmacokinetic studies of sorafenib demonstrate that, following oral administration, it reaches steady state in the plasma after 7 days at 400 mg twice daily, with a minimal increase in levels after that. The elimination half-life ranges between 20 and 27 hours after discontinuing the administration.6 Sorafenib is metabolized primarily in the liver, undergoing oxidative metabolism, mediated by CYP3A4, as well as glucuronidation, mediated by UGT1A9. Comparison of data across studies suggests that, in patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment, a 400-mg dose of sorafenib appears to be associated with area under the curve (AUC) values 23%–65% lower than those of patients without hepatic impairment.6 Sorafenib AUC was similar between patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment.7,8 The pharmacokinetics of sorafenib have not been studied in patients with severe (Child-Pugh C) hepatic impairment. No dosage reductions are recommended for patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment, and sorafenib has been found to be safe in this setting. A placebo-controlled, randomized phase III trial (SHARP) involving 602 patients with metastatic hepatocellular cancer showed that sorafenib improved overall survival (OS; median, 10.7 months vs. 7.9 months) when compared with placebo. In addition, the incidence of serious adverse events was similar for sorafenib versus placebo (52% vs. 54%).8 Sorafenib therapy has been extensively evaluated in mild and moderate hepatic dysfunction, however, not in patients with renal dysfunction.
Renal cancer is increasingly being diagnosed at a later age, with median age at diagnosis being 65 years.9 With the increasing incidence of diabetes and hypertension, a higher proportion of patients with renal cancer are likely to have underlying chronic kidney disease. In addition, a predominant proportion of patients with renal cancer (including those with metastasis) undergo nephrectomy.10 This also increases the risk of chronic kidney disease. Furthermore, the risk of renal cancer is increased in patients undergoing dialysis. One to two percent of patients on chronic hemodialysis or peritoneal dialysis develop renal cancer.11,12 Patients who undergo renal transplantations also are at an increased risk.13 Hence, a substantial proportion of patients with renal cancer have chronic renal insufficiency. Our study attempts to evaluate the safety and efficacy of sorafenib in patients with kidney cancer with impaired renal function.
Patients and Methods
The primary objective was to assess the safety and efficacy of sorafenib in patients with impaired renal function and contrast it with that noted in patients with normal renal function. The protocol was approved by the Human Investigation Committee of Wayne State University. Medical records of patients with metastatic renal cancer treated with sorafenib between November 2005 and January 2007 were reviewed. Patients with a calculated CrClof ≤ 60 mL/ min (chronic kidney disease stage 3, 4, or 5) per Kidney Disease Outcomes Quality Initiative guidelines) were deemed to have renal insufficiency.14 Data on safety, efficacy, and dosing of sorafenib therapy were collected and analyzed with respect to renal function. Toxicities were graded per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Dose modifications and interruptions of sorafenib administration were noted. Response was evaluated per Response Evaluation Criteria in Solid Tumors .15
Time to progression (TTP) was measured from the day of starting sorafenib therapy until documented disease progression. Time to progression was censored as of the date of last tumor assessment for patients still free of progression. Time to progression was censored as of the date of death if a patient died of a cause other than RCC. Overall survival was measured from the day of starting sorafenib therapy until death from any cause. Overall survival was censored as of the date of last follow-up for patients still alive or as of the last date of contact for vital status determination. Follow-up data were complete for disease progression through June 30, 2007, and survival data were updated as of September 8, 2008.
Baseline patient characteristics and toxicity data were described with summary statistics. Standard 90% confidence limits for response rate (RR) were calculated with the Clopper-Pearson method. Response rates were compared with Fisher exact test (2-sided). Standard Kaplan-Meier estimates of the censored TTP and OS distributions were computed. Because of the small sample sizes, survival statistics (eg, median) were estimated more conservatively using linear interpolation among successive event times on the Kaplan-Meier curves.16
Results
Patient Characteristics
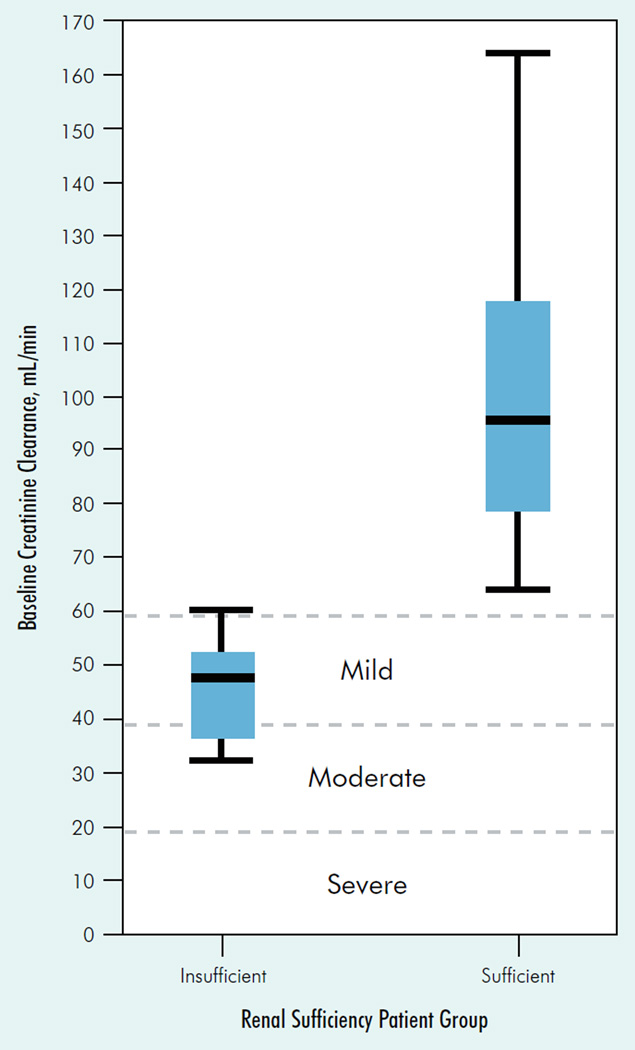
Thirty-two patients who met the selection criteria were analyzed. Demographic and tumor characteristics are summarized in Table 1. The median age was 62.5 years, with 22 men (69%) and 10 women (31%). Fourteen (44%) patients had renal insufficiency (CrCl range, 32–60 mL/min), and 18 patients had CrCl > 60 mL/min. None of the patients were dialysis dependent. The distribution of baseline CrCl of patients in both groups and the distribution across mild, moderate, and severe CrCl is shown in Figure 1. Both groups were well matched in performance status and Memorial Sloan-Kettering Cancer Center prognostic risk factors.17 Patients with impaired renal function tended to be older than those with normal renal function (median age, 71 years vs. 54 years). All patients were started with the initial FDA-approved dose of sorafenib in metastatic renal cancer of 400 mg (2 tablets of 200 mg each) orally twice daily.
Table 1.
Patient Characteristics
| Characteristic | CrCl ≤ 60 mL/min |
CrCl > 60 mL/min |
|---|---|---|
| Number of Patients | 14 | 18 |
| Median Age, Years (Minimum-Maximum) | 71.(57–80) | 54 (40–70) |
| KPS≥ 80 | 12/14 (86%) | 17/18 (94%) |
| MSKCC 0-1 | 10/14 (71%) | 14/18 (77%) |
| Clear Cell Variant | 6/14 (43%) | 14/18 (77%) |
| Nephrectomy | 14/14 (100%) | 15/18 (83%) |
| Median CrCl in mL/min (Minimum-Maximum) | 47.5 (32–59) | 95.5 (64–164) |
| Median Cr in mg/dL (Minimum-Maximum) | 1.6 (1.1–3.2) | 1.1 (0.9–1.5) |
| Median SBP in mm Hg (Minimum-Maximum) | 143 (104–180) | 145 (94–163) |
| Median DBP in mm Hg (Minimum-Maximum) | 71 (60–100) | 75.5 (38–99) |
| Number of Patients Still Medications at Last Visita | 0/14 | 3/18 |
| Number of Patients Progression Free at Last Visita | 4/14 | 6/18 |
Last visit to physician or 06/30/07.
Abbreviations: CrCl = creatinine clearance; DBP = diastolic blood pressure; KPS = Karnofsky performance score; MSKCC = Memorial Sloan-Kettering Cancer Center; SBP = systolic blood pressure
Figure 1. Ceatinine Clearance Distributions for Normal Renal Function and Renally Insufficient Patients.
On the x-axis, our study definition of renal insufficiency is CrCl ≤ 60 mL/min, and renal sufficiency is CrCl > 60 mL/min. On the y-axis, the 3 dashed reference lines indicate the upper limit of the successively poorer categories of renal dysfunction as defined by Miller et al19: CrCl ≤59 mL/min for mild; CrCl ≤ 39 mL/min for moderate; and CrCl ≤ 19 mL/min for severe renal dysfunction. For each box plot, the minimum and maximum baseline CrCl values in our study are marked at the end of the lower and upper vertical “whiskers,” respectively. The top and bottom of each box mark the 75th and 25th percentiles, respectively. The horizontal line within each box marks the median CrCl value.
Toxicity
Overall incidence of toxicities was not statistically different in both of the groups; however, the duration of toxicities was longer, and more patients seemed to develop grade 2 and 3 toxicities in the renal-insufficiency group (Table 2). This resulted in more frequent dose modifications and prolonged dose interruptions. Overall, sorafenib was well tolerated in both subgroups. Predominant toxicities noted were hand-foot syndrome (HFS), diarrhea, fatigue, and hypertension. Increased incidence of grade 2 and 3 HFS was noted in the patients group with CrCl 60 mL/min or less (9 of 14 patients) as compared with that in the group with CrCl above 60 mL/min (4 of 18 patients). Minor differences were noted between the 2 groups in the incidence and severity of other side effects such as diarrhea, fatigue, and bleeding as depicted in Table 2.
Table 2.
Toxicities
| Adverse Event |
CrCl ≤ 60 mL/min (n = 14) |
CrCl > 60 mL/min (n = 18) |
|---|---|---|
| Skin Rash/HFS | ||
| Grade 1 | 3 | 6 |
| Grade 2 | 8 | 6 |
| Grade 3 | 1 | 2 |
| Grade 4 | 0 | 0 |
| Diarrhea | ||
| Grade 1 | 3 | 2 |
| Grade 2 | 5 | 4 |
| Grade 3 | 0 | 0 |
| Grade 4 | 0 | 0 |
| Fatigue | ||
| Grade 1 | 2 | 1 |
| Grade 2 | 9 | 11 |
| Grade 3 | 2 | 2 |
| Grade 4 | 0 | 0 |
| Bleeding, Number of Episodes | ||
| 0 | 13 | 16 |
| 1 | 0 | 2 |
| 2 | 1 | 0 |
| > 2 | 0 | 0 |
| Hospitalization, Number of Episodes | ||
| 0 | 11 | 14 |
| 1 | 2 | 3 |
| 2 | 0 | 0 |
| > 2 | 1 | 0 |
Abbreviations: CrCl = creatinine clearance; HFS = hand-foot syndrome
Hypertension
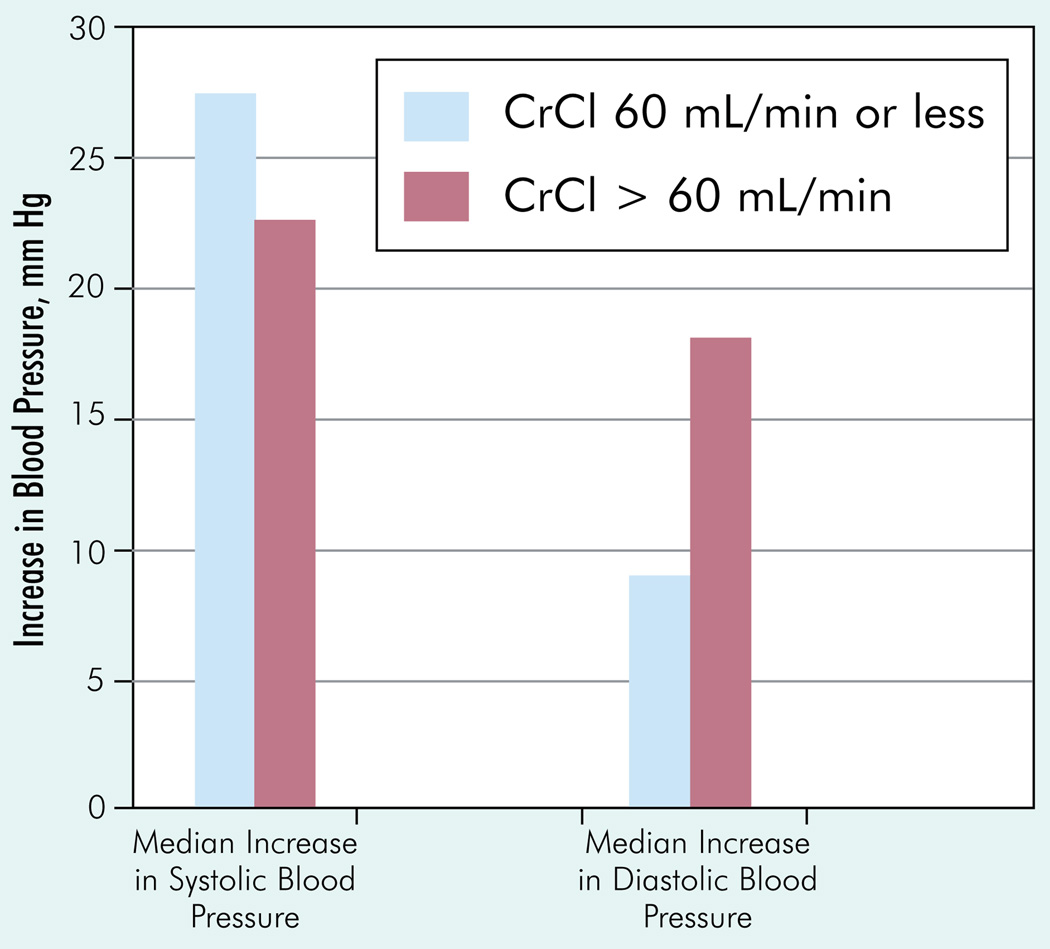
Hypertension was another common toxicity related to sorafenib. The changes in blood pressure readings on sorafenib therapy in each of the groups are shown in Figure 1. In the renal-insufficiency group, 7 out of 14 patients had baseline hypertension, and all of them had increasing blood pressure readings on therapy. The other 7 patients who started normotensive also had worsening blood pressure readings on sorafenib therapy. Five of the 7 patients with normal blood pressure pretherapy had readings > 140/90 mm Hg, thus being diagnosed as new-onset hypertension. In the patients with CrCl > 60 mL/min, 11 of 18 had hypertension before therapy. In 2 patients, blood pressure remained within normal range, whereas it worsened in 9 patients. Seven of 18 patients had normal blood pressure before therapy, and all of these demonstrated an elevated blood pressure, with 5 patients developing new-onset hypertension. Median increase in systolic blood pressure was 27.5 mmHg in the group with CrCl ≤60 mL/min, whereas it was 22.5 mmHg in the group with CrCl > 60 mL/min (Figure 2). The median increases in diastolic blood pressure were 9 mm and 18 mm, respectively, in patients with impaired versus normal kidney function. In the renal-insufficiency group, one patient developed a hypertensive emergency and needed to be hospitalized, and 3 patients developed a systolic blood pressure > 200 mm Hg. In the normal-renal-function group, none of the patients developed a systolic blood pressure > 200 mm Hg.
Figure 2. Increase in Blood Pressure in Patient Subgroups With or Without Renal Dysfunction.
Abbreviation: CrCl = creatinine clearance
Dose Modifications, Interruptions, and Duration of Therapy
The dose adjustments consisted of decreasing sorafenib to 400 mg daily, then to 200 mg daily, and finally to 200 mg every alternate day. The group with decreased CrCl had a higher incidence of dose reductions and dose interruptions. Dose was interrupted in 57% of the patients with renal insufficiency, versus 28% of patients in the other group. Dose was reduced in 43% of the patients with renal dysfunction but only in 22% of those with normal renal function. The number of dose reductions was higher in the presence of impaired renal function. The main reasons for dose reduction were HFS and hypertension. Four patients in each group had 1 dose reduction. One patient in the renal insufficiency group had 4 dose reductions (because of worsening hypertension), whereas another had 2 dose reductions (because of HFS). Details of dose modifications, interruptions, and durations of therapy by renal function subgroup are summarized in Table 3.
Table 3.
Characteristics of Sorafenib Therapy
| Characteristic | CrCl ≤ 60 mL/min (n = 14) |
CrCl > 60 mL/min (n = 18) |
|---|---|---|
| Duration, Months | ||
| Median | 2.9 | 3.4 |
| Mean | 3.9 | 4.6 |
| Minimum | 0.7 | 0.9 |
| Maximum | 15.1 | 17.4 |
| Dose Interruptions, Number of Interruptions | ||
| 0 | 6 | 13 |
| 1 | 4 | 2 |
| 2 | 3 | 1 |
| 3 | 0 | 2 |
| 6 | 1 | 0 |
| Dose Reductions, Number of Reductions | ||
| 0 | 8 | 14 |
| 1 | 4 | 4 |
| 2 | 1 | 0 |
| 4 | 1 | 0 |
| Reason for Discontinuation | ||
| Disease progression, n | 10 | 12 |
| Toxicity, n | 2 | 2 |
| Other, n | 1 | 1 |
| Still on sorafenib, n | 1 | 3 |
Abbreviation: CrCl = creatinine clearance
Response and Survival
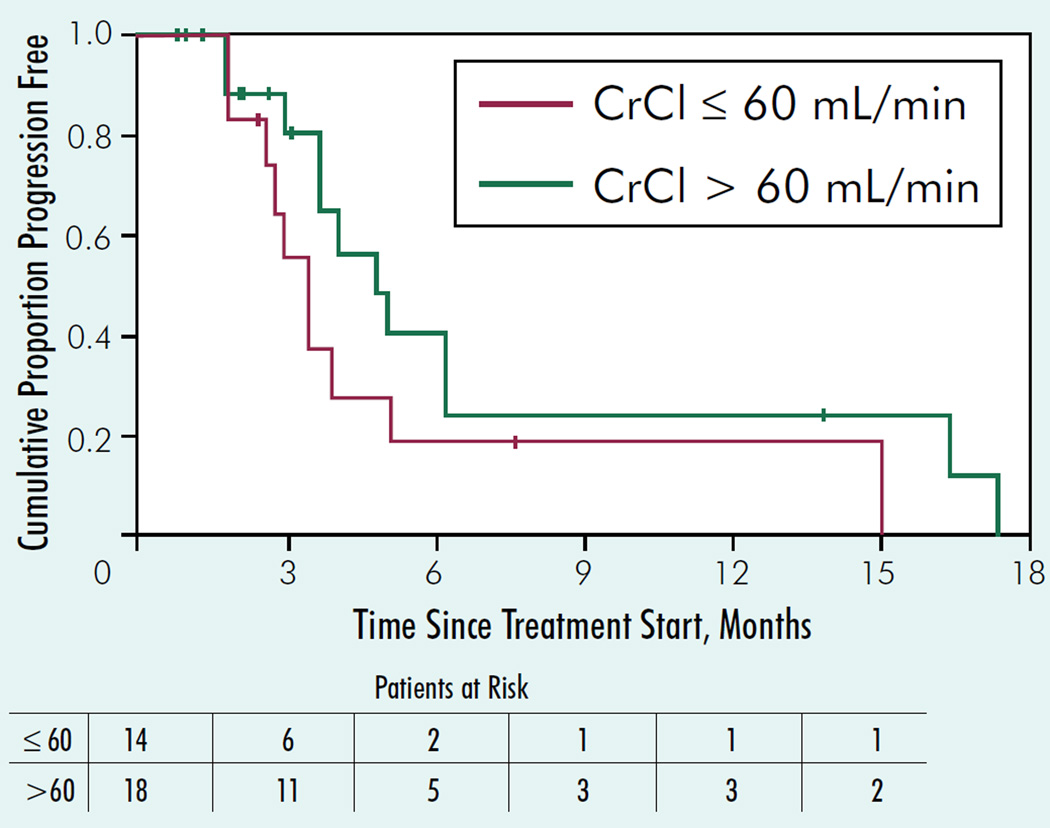
The RR was higher in the group with normal renal function (6/18 = 33%; 90% CI, 16%–54% vs. 2/14 = 14%; 90% CI, 4%- 37%; Table 4). However, the difference was not statistically significant. Median TTP in the group with CrCl > 60 mL/min was 4.7 months (90% CI, 3.6–6.1 months), whereas median TTP in the group with CrCl ≤ 60 mL/min was 3.2 months (90% CI, 2.6–4.9 months; Figure 3). The median duration of TTP follow-up for the 10 patients still progression free was 2.2 months. Median OS was 12.3 months in the CrCl ≤ 60–mL/min group and 11.1 months in the CrCl > 60–mL/min group. The median duration of OS followup for the 7 patients still alive was 31.4 months. Additional TTP and OS statistics are given in Table 4.
Table 4.
Response Rates and Survival
| Statistic | CrCl ≤ 60 mL/min (n = 14) |
CrCl > 60 mL/min (n = 18) |
|---|---|---|
| Response Rate, % | 14 (90% CI, 4–37) | 33 (90% CI, 16–54) |
| Median TTP, Months | 3.2 (90% CI, 2.6–4.9) | 4.7 (90% CI, 3.6–6.1) |
| 6-Month TTP Rate, % | 17 (90% CI, 0- 36) | 34 (90% CI, 11- 56) |
| Median Survival, Months | 12.3 (90% CI, 6.8–16.9) | 11.1 (90% CI, 5.2–18.2) |
| 12-Month OS Rate, % | 51 (90% CI, 28–73) | 48 (90% CI, 29–67) |
| 24-Month OS Rate, % | 16 (90% CI, 0–36) | 25 (90% CI, 8–43) |
Abbreviations: CrCl = creatinine clearance; OS = overall survival; TTP = time to progression
Figure 3. Kaplan-Meier Graph of Time to Progression in Patient Subgroups With or Without Renal Dysfunction.
Abbreviation: CrCl = creatinine clearance
Discussion
A sizeable proportion of patients with renal cancer have renal dysfunction; in our study, 44% of the patients with renal cancer treated with sorafenib had significant impairment of kidney function. There is a lack of data regarding the safety of sorafenib and other targeted agents in patients with renal insufficiency. With the advent of tolerable targeted therapies, a significant proportion of patients with advanced renal cancer with renal dysfunction are being treated. The randomized clinical trials evaluating sorafenib did not include patients with impaired CrCl. Hence, studies assessing the safety and efficacy of these agents in renal dysfunction patients are warranted.
There is some preliminary information on the pharmacokinetics of sorafenib in patients with renal dysfunction. Smith and Marbury conducted a study on 32 patients with varying degrees of renal dysfunction. 18 Patients were given a single 400-mg dose of sorafenib, and urine and blood samples were collected at various intervals to calculate drug clearance. No differences in pharmacokinetics were noted after that first dose. However, clinical parameters such as dose modifications, toxicities, response, and survival were not evaluated. Also, cumulative toxicities after prolonged administration frequently require dose modifications and might have an effect on safety and efficacy of the drug, especially in patients with suboptimal CrCl.
Miller et al conducted a phase I study of sorafenib in 146 patients with hepatic and renal dysfunction.19 This was conducted in patients with solid tumors and hematologic malignancies. For the purposes of the study, renal dysfunction was graded as mild (CrCl, 40–59 mL/min), moderate (CrCl, 20–39 mL/min), severe (CrCl < 20 mL/min), and very severe (hemodialysis, any CrCl). Initially, a single test dose of sorafenib 400 mg was given orally for pharmacokinetics, followed by a 1-week rest. Starting on day 8, sorafenib was given continuously for 3 months. The number of patients enrolled on the study was 8 in the mild group, 14 in the moderate group, 5 in the severe group, and 17 in the very severe dysfunction. The maximum tolerated dose was 400 mg twice daily in the mild-dysfunction group, with dose-limiting toxicity (DLT) of grade 3 fatigue, 200 mg twice daily in the moderate dysfunction group (DLT of grade 3 HFS and grade 3 nausea/vomiting despite optimal supportive care), and 200 mg once daily in the very severe dysfunction group (DLT of grade 3 hypertension). The severe-dysfunction group had a grade 4 hemorrhage into brain metastases at a dose of 200 mg on alternate days. That DLTs were reached at lower dose levels in the groups with progressively more severe dysfunction suggests that there is an association between renal function and maximum tolerated dose. This suggests that prolonged treatment would lead to dose reductions if one starts at a full dose.
If one were to believe that DLT of sorafenib in patients with renal impairment is seen at lower-than-standard doses, then it would be worthwhile to evaluate if dose increments of sorafenib are associated with improved efficacy. The 2 trials conducted to investigate dose escalation of sorafenib have reported conflicting results. Amato et al conducted a phase II trial of intrapatient dose escalation of sorafenib.20 Of 46 treated patients, 44 were evaluable. The distributions of response were 8 patients with a complete response (CR), 14 with a PR, and 14 with SD. Median duration of therapy was 6 months (range, 0.2+ to 12+). Ninety-one percent of patients were escalated to 1200 mg or 1600 mg per day. In this trial, sorafenib dose escalation revealed extremely promising antitumor activity as demonstrated by a 52% CR or PR rate. On the contrary, another trial by George and Garcia revealed no correlation between sorafenib dose and response.21 The study attempted to determine if increasing the dose of sorafenib above standard doses (400 mg twice daily) would lead to an increase in antitumor activity in patients with progressing advanced renal cancer, started on sorafenib at 400 mg twice daily. Of the 14 patients enrolled, 10 tolerated dose escalation to 600 mg twice daily. Four patients tolerated subsequent dose escalation to 800 mg twice daily. One individual was dose escalated to 1000 mg twice daily but could not tolerate this because of multiple toxicities of abdominal pain, diarrhea, and metabolic acidosis. The predominant severe toxicities on the higher doses were HFS, diarrhea, and hypertension (n = 1). Best responses with standard-dose sorafenib therapy were 1 PR and 11 SDs, whereas on higher-dose sorafenib there were no CRs/PRs and 8 SD. The median PFS on the standard and escalated doses of sorafenib were identical at 3.4 months. This trial suggested that in patients who do not respond to standard-dose sorafenib, dose escalation offers no additional advantage and increases toxicity.
In our above reported study, 14/32 (44%) had renal dysfunction as defined by a CrCl of ≤ 60 mL/min. Our case series is the largest to our knowledge reporting on the safety and efficacy of sorafenib in patients with kidney cancer with renal dysfunction over a prolonged duration. It demonstrates that mild or moderate renal insufficiency is not a contraindication to initiation of treatment with sorafenib. However, a higher proportion of patients with renal insufficiency need dose interruptions and dose reductions. Furthermore, though the incidence of toxicities was similar in both arms, the patients in the impaired renal function group experienced higher-grade and prolonged duration of side effects. Subsequently, patients with renal insufficiency required sorafenib dose modifications with increasing frequency. This could be either because of longer half-life of excretion or cumulative accumulation of sorafenib and metabolites in patients with renal dysfunction. It is also likely that renal dysfunction being associated with other comorbidities such as hypertension would lead to rapid onset and increased severity of toxicities. Hence, close monitoring of patients with renal dysfunction is warranted, and frequent dose reductions might be necessary. Because no severe or very severe renal insufficiency patients were included in our review, the safety of sorafenib in this patient population remains unknown and warrants investigation.
Conclusion
Renal insufficiency is a frequent occurrence in patients with advanced renal cancer. Sorafenib therapy can be safely delivered, and efficacy is maintained in patients with mild or moderate renal impairment. However, close monitoring and frequent dose modifications are advised.
Acknowledgments
Supported in part by NIH Cancer Center Support Grant CA-22453 and the Julia Wilson Cancer Foundation.
Footnotes
Disclosures
The authors report no relevant financial conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics’ 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51:203–205. doi: 10.1016/s0090-4295(97)00506-2. [DOI] [PubMed] [Google Scholar]
- 3.Lam JS, Belldegrun AS. Long term outcomes of the surgical management of renal cell carcinoma. World J Urol. 2006;24:255–266. doi: 10.1007/s00345-006-0055-5. [DOI] [PubMed] [Google Scholar]
- 4.Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–2512. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Eisen T, Stadler WM, et al. TARGET Study Group. Sorafenib in advanced clear-cell renal cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 6.Strumberg D, Clark JW, Awada A, et al. Safety’ pharmacokinetics’ and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–437. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 7.Furuse J, Ishii H. Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci. 2008;99:159–165. doi: 10.1111/j.1349-7006.2007.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llovet JM, Ricci S, Mazzaferro V, et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 9.NCCN practice guidelines in oncology. V.1. 2008 [Google Scholar]
- 10.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 11.Savaj S, Liakopoulos V, et al. Renal cell carcinoma in peritoenal dialysis patients. Int Urol Nephrol. 2003;35:263–265. doi: 10.1023/b:urol.0000020355.30870.3a. [DOI] [PubMed] [Google Scholar]
- 12.Kojima Y, Takahara S, Miyake O, et al. Renal cell carcinoma in dialysis patients: a single center experience. Int J Urol. 2006;13:1045–1048. doi: 10.1111/j.1442-2042.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- 13.Moudouni SM, Lakmichi A, Tliqui M, et al. Renal cell carcinoma of native kidney in renal transplant recipients. BJU Int. 2006;98:298–302. doi: 10.1111/j.1464-410X.2006.06267.x. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer’ National Cancer Institute of the United States’ National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Lee E, et al. Statistical Methods for Survival Data Analysis. 3rd ed. Wiley & Sons, Inc.; 2003. pp. 76–91. [Google Scholar]
- 17.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–463. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 18.Smith W, Marbury T. Effects of renal impairment on the pharmacokinetics of sorafenib and it’s metabolites. Proc Am Assoc Clin Res. 2007 Abstract 934. [Google Scholar]
- 19.Miller AA, Murray DJ, Owzar K, et al. Pharmacokinetics (PK) and phase I study of Sorafenib(S) for solid tumors and hematologic malignancies in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol. 2007;25(suppl):147s. (Abstract 3538). [Google Scholar]
- 20.Amato RJ, Harris P, Dalton M, et al. A phase II trial of intra-patient doseescalalted sorafenib in patients with metastatic renal cell cancer. J Clin Oncol. 2007;25(suppl):241s. (Abstract 5026). [Google Scholar]
- 21.George S, Garcia JA. Dose escalation of sorafenib in metastatic renal cell carcinoma (mRCC) patients; Presented at: the American Society of Clinical Oncology: 2008 Gastrointestinal Cancers Symposium; January 25–27; Orlando, FL; 2008. Abstract 360. [Google Scholar]