Abstract
Objective
Topotecan at a dose of 1.5 mg/m2 on days 1 to 5 of a 21-day cycle is an approved therapy for recurrent ovarian cancer. However, heavily pretreated patients may be predisposed to hematologic adverse events. This prospective study, therefore, investigates the safety and efficacy of an alternate weekly schedule of topotecan in patients with recurrent ovarian or peritoneal cancer.
Methods
Patients with potentially platinum-sensitive recurrent ovarian or peritoneal cancer were treated with 4.0 mg/m2 weekly topotecan as tolerated until disease progression. Antitumor response and safety were assessed. Dose reductions, delays, or omissions were implemented for grades 3–4 adverse events.
Results
Of the 41 enrolled patients (median age, 62 years; range, 42 to 82 years), 39 patients had ovarian cancer, and 2 patients had peritoneal cancer. The median platinum-free interval was 11.7 months. A median of 9 topotecan cycles (range, 1 to 45 doses) was administered. Weekly topotecan was well tolerated: 7 (17%) patients had grades 3–4 neutropenia, and 9 (22%) had grades 3–4 fatigue. No grade 4 thrombocytopenia or anemia was reported. Of 38 response-evaluable patients, 1 (3%) had a complete response, 8 (21%) had a partial response, 16 (42%) had stable disease, and 13 (34%) had progressive disease.
Conclusions
Weekly topotecan was well tolerated in patients with platinum-sensitive ovarian or peritoneal cancer at first relapse, with a hematologic profile that compared favorably with that of the 5-day topotecan regimen. Moreover, antitumor activity was similar to that reported for the 5-day regimen.
Keywords: Chemotherapy, Platinum-sensitive ovarian cancer, Relapsed ovarian cancer, Topotecan, Weekly topotecan
Introduction
Ovarian cancer will be diagnosed in an estimated 22,430 women in the United States in 2007, and an estimated 15,280 will succumb to this disease [1]. There are no effective screening tests for ovarian cancer, so it is often diagnosed after it has progressed to an advanced stage [2]. Less than 15% of patients who are diagnosed with advanced ovarian cancer achieve long-term remission after first-line therapy, and patients with relapsed disease often require multiple rounds of therapy to manage their disease and symptoms [3]. Therefore, ovarian cancer may be optimistically considered a chronic disease. Consequently, long-term treatment of patients with relapsed ovarian cancer requires that the possible antitumor effects of therapy be weighed against quality-of-life considerations. Moreover, the effects of various treatments on subsequent therapy options should be considered [3]. For example, recent clinical evidence suggests that platinum-based combination therapy is more efficacious than platinum monotherapy in patients with relapsed platinum-sensitive ovarian cancer [4,5]. However, platinum-based combination therapies are associated with cumulative and irreversible toxicities such as nephropathy, neuropathy, and thrombocytopenia [2,3]. Most nonplatinum therapies for relapsed ovarian cancer also are associated with cumulative nonhematologic toxicities such as neuropathy, palmar-plantar erythrodysesthesia, and increased risk of secondary myelodysplasia [2,3].
Topotecan (Hycamtin®; GlaxoSmithKline, Philadelphia, PA) is a topoisomerase I inhibitor that is approved in the relapsed ovarian cancer setting; topotecan has a manageable toxicity profile characterized by noncumulative neutropenia and generally low-grade nonhematologic toxicities [2]. The standard regimen of 1.5 mg/m2/day on days 1 to 5 of a 21-day cycle has demonstrated overall response rates (17.0% vs 19.7%, respectively; p=0.390) and survival (56.7 weeks vs 60.0 weeks, respectively; p=0.341) comparable with those of liposomal doxorubicin in patients with relapsed ovarian cancer [6]. This same regimen also resulted in similar median survival compared with paclitaxel (53.0 weeks vs 63.0 weeks, respectively; p=0.440) [7]. In a subgroup analysis of patients with platinum-sensitive ovarian cancer, liposomal doxorubicin demonstrated a survival advantage over topotecan at standard dosing. However, this may be due to the fact that patients in the doxorubicin arm may have received additional benefit from therapy with marrow toxic drugs upon completion of the study treatment [6]. Although topotecan is generally well tolerated, heavily pretreated patients may be especially susceptible to hematologic adverse events [8]. Dose reductions and alternate dosing regimens, including continuous infusions and weekly administration, have been investigated in the attempt to optimize efficacy and reduce toxicity [9–11].
In vivo data support the use of repeated bolus topotecan dosing to avoid possible down-regulation of the target enzyme, topoisomerase [12]. Intermittent dosing schedules in murine models, which simulate weekly dosing in humans, appear to maintain potential therapeutic benefit [8], and recent clinical data have shown that weekly topotecan is not only clinically active but also has a hematologic toxicity profile that compares favorably with that reported for the standard 5-day regimen in patients with relapsed ovarian cancer [13–17]. Additionally, like other agents used in the recurrent setting, topotecan is especially efficacious in platinum-sensitive disease [18]. This prospective phase II clinical trial investigated the safety and efficacy of 4 mg/m2 weekly topotecan in patients with potentially platinum-sensitive relapsed ovarian or peritoneal cancer.
Patients and methods
Patients
Women with recurrent ovarian cancer or primary peritoneal carcinoma who had received 1 prior platinum-based regimen were eligible. Patients who received maintenance chemotherapy, excluding topotecan, as front-line treatment were included. Patients who had received prior treatment with topotecan or radiotherapy were not eligible. Patients were required to have bidimensional measurable disease assessed by physical exam or radiologic exam; serologic recurrence measured by cancer antigen-125 level alone was not sufficient for study entry. Additional eligibility criteria include treatment-free interval of at least 6 months after an initial response to platinum-based therapy, a Gynecologic Oncology Group (GOG) performance status of ≤2, adequate hematologic cell counts (absolute neutrophil count ≥1500/μL and platelets ≥100,000/μL), and adequate liver (total bilirubin ≤1.5 mg/dL and ALT, AST, and alkaline phosphatase <3 times the institutional upper limit of normal) and renal function (creatinine clearance ≥40 cm3/min). The institutional review boards of participating centers approved the study, and all patients provided signed informed consent.
Study design and treatment
This was a prospective, multicenter, phase II evaluation of weekly topotecan. Treatment was given as a 30-minute intravenous bolus, with a starting dose of 4.0 mg/m2/week. Patients with a creatinine clearance <40 cm3/ min were treated with 3.0 mg/m2/week followed by dose escalation in the subsequent cycle to 4.0 mg/m2/week in the absence of any grade 3 toxicity. One cycle was defined as 3 weekly treatments. Patients were treated weekly with continuous therapy until documented disease progression, confirmed complete response (CR), or treatment-limiting toxicity. There was no planned break week. Treatment was continued for 2 additional cycles for patients with CR. Hematopoietic growth-factor support was not permitted. A weekly infusion was omitted if the absolute neutrophil count was <1500/μL or platelet count was <100,000/μL. Patients who failed to achieve hematologic recovery for 2 consecutive weeks were removed from the study at the discretion of the investigator. Based on weekly laboratory data, dose reductions of topotecan were implemented for grades 3–4 thrombocytopenia, neutropenia, or anemia persisting for longer than 7 days; the weekly dose was reduced by increments of 0.5 mg/m2/week to 2.0 mg/m2/week. Following any dose interruption, the dose/ doses were omitted. Patients who experienced grades 3–4 hematologic toxicity at the 2.0 mg/m2/week dose were removed from the study.
On-study evaluation
Tumor response was assessed with standard methods before each cycle. Patients with radiographically measurable disease were assessed every second cycle (6 weeks). A CR was defined as the disappearance of all measurable disease. A partial response (PR) was defined as a ≥50% reduction in the sum of the products of the 2 largest perpendicular diameters of each lesion. All responses required confirmation by a second assessment at least 4 weeks later. Progressive disease (PD) was defined as a ≥25% increase in the sum of the products of the 2 largest perpendicular diameters of each lesion or the appearance of any new lesion within 8 weeks of study entry. Stable disease (SD) was defined as any disease that did not meet the criteria for CR, PR, or PD. Response duration (RD) was measured from the start date of the best response (date of first scan or examination showing response by World Health Organization criteria) until the date of relapse (based on scan or examination). Time to treatment failure (TTF) was measured from study registration to the date of first documented PD or date off treatment because of toxicity, patient refusal of additional treatment, or death, whichever occurred first. Time to progression (TTP) was measured from study registration to the date of first documented disease progression. Overall survival (OS) was measured from study registration to last follow-up or death.
The safety and tolerability of weekly topotecan were evaluated by clinical laboratory assessments, physical examination, and the frequency and severity of adverse events. Complete blood counts were performed each week, and serum biochemical assessments were performed every 3 weeks throughout the study. The severity of adverse events was graded according to the Common Toxicity Criteria of the National Cancer Institute. Serious adverse events included grades 3–4 hematologic and nonhematologic toxicities. Concurrent illnesses, infections, blood product support, and antimicrobial therapies were monitored.
Statistical methods
This trial was planned with a Simon 2-stage minimax design [19]. The primary endpoint was CR or PR. We wished to distinguish these regions of the true, unknown response rate: at most 10% vs at least 30%. The 2-stage design called for a maximum of 37 response-evaluable patients (25 patients in stage 1 and 12 patients in stage 2). Patients were considered response-evaluable if they either completed at least 1 therapy cycle and returned 4 weeks later for tumor measurement (if by physical exam) or completed 2 therapy cycles and response was measured by imaging. The design had a type I error of 0.027 and power of 0.901. The probability of early termination was 0.537, if the true response was 10%. Response rates are reported in both the response-evaluable patients (N=38) and in the intent-to-treat population (all registered patients, N=41).
Exact, minimum-width 90% confidence intervals (CIs) for response and toxicity rates were calculated using the Casella method as implemented in StatXact software [20,21]. Standard Kaplan–Meier estimates of the censored RD, TTF, TTP, and OS distributions were determined. Because of the small sample size, survival statistics (eg, median and 1-year rate) were estimated more conservatively using linear interpolation among successive event times on the Kaplan–Meier curves [22].
Results
Patient demographics and disposition
Forty-one patients were enrolled between October 2000 and September 2004. Median age was 62 years (range, 42 to 82 years). Three (7%) patients were originally diagnosed with stage II, and 36 (88%) patients had stage III/IV ovarian cancer, whereas 2 (5%) patients had peritoneal cancer (Table 1). Twenty-seven (66%) patients had papillary serous histology, 5 (12%) had endometrioid carcinoma, and 9 had unspecified adenocarcinoma (mucinous, 1; clear cell, 1; mixed histologies, 3; unspecified, 4). Grade 2 lesions were found in 6 (15%) patients and grade 3 in 35 (85%) patients. The median time from completion of first-line therapy was 11.7 months (range, 6 to 61 months). A total of 541 weekly topotecan doses were administered, with a median of 9 doses (range, 1 to 45 doses).
Table 1. Patient demographics and baseline disease characteristics.
| Characteristic | Patients (N=41) |
|---|---|
| Age, years | |
| Median | 62 |
| Range | 42–82 |
| Ovarian cancer disease stage at original diagnosis, patients, N (%) | |
| II | 3 (7) |
| III/IV | 36 (88) |
| Peritoneal cancer, patients, N (%) | 2 (5) |
| Histology | |
| Serous | 27 (66) |
| Endometrioid | 5 (12) |
| Mixed | 3 (8) |
| Clear | 1 (2) |
| Mucinous | 1 (2) |
| Adenocarcinoma (NOS) | 4 (10) |
| Grade, patients, N (%) | |
| 2 | 6 (15) |
| 3 | 35 (85) |
| GOG PS, patients, N (%) | |
| 0 | 18 (44) |
| 1 | 22 (54) |
| 2 | 1 (2) |
| Platinum-free interval, months | |
| Median | 11.7 |
| Range | 6–61 |
Abbreviation: GOG PS, Gynecologic Oncology Group performance status.
Hematologic toxicity
Weekly topotecan was generally well tolerated (Table 2). There were no treatment-related deaths. Neutropenia was the main toxicity: 1 (2%) patient had grade 4 neutropenia, and 7 (17%) patients had grade 3 neutropenia. Grade 3 thrombocytopenia and leukopenia each occurred in 3 (7%) patients, and grade 3 anemia occurred in 4 (10%) patients. No patients had febrile neutropenia or grade 4 thrombocytopenia, leukopenia, or anemia.
Table 2. Summary of all grades 3–4 adverse events with incidence ≥5%.
| No. of patients (%) (N=41) | ||
|---|---|---|
|
|
||
| Adverse event | Grade 3 | Grade 4 |
| Hematologic | ||
| Neutropenia | 7 (17) | 1 (2) |
| Thrombocytopenia | 3 (7) | 0 |
| Leukopenia | 3 (7) | 0 |
| Anemia | 4 (10) | 0 |
| Nonhematologic | ||
| Fatigue | 7 (17) | 2 (5) |
| Nausea/vomiting | 3 (7) | 0 |
| Pain | 2 (5) | 0 |
| Dyspnea | 3 (7) | 0 |
Thirty-one (76%) patients received all treatments without a dose reduction. Treatment delays or omissions were implemented in 22 patients because of insufficient hematologic recovery. Of the intended doses, 8% were delayed or omitted because of leukopenia, 8% were delayed or omitted because of thrombocytopenia, and <1% were delayed or omitted because of anemia. The majority of the thrombocytopenia episodes that led to delayed or omitted treatments occurred in 1 patient who experienced 25 (63%) of the 40 reported incidents. The proportion of patients who required dose delays or omissions did not increase during later treatments. Three patients withdrew from the study after experiencing neutropenia of >2 weeks' duration.
Nonhematologic toxicity
Grades 1–2 nonhematologic adverse events were more common than grades 3–4 events, with grades 1–2 gastrointestinal events reported in 31 (76%) patients and infections without neutropenia reported in 8 (20%) patients. The most common grades 3–4 nonhematologic adverse event was fatigue, which occurred in 9 (22%) patients, 2 of whom withdrew from the study because of these events (Table 2). Grade 3 dyspnea occurred in 3 (7%) patients. Dyspnea was not associated with anemia, fatigue, cardiac performance, or changes on chest imaging. All cases of dyspnea resolved after treatment discontinuation. Two (5%) patients experienced grade 3 pain. There were no reports of grades 3–4 neuropathy, other grade 4 nonhematologic toxicities, or renal toxicity, and no treatment-related deaths occurred. Nonhematologic adverse events that caused missed or delayed treatments were reported in 11 patients.
Response
Of the first 25 response-evaluable patients, 8 were responders at the interim analysis, and patient accrual continued based on study design requirements (≥3 responses required to begin stage 2 of accrual). Nine patients were responders at the end of stage 2 (≥8 responses required to suggest efficacy), which led to the statistical conclusion that the sample response rate among the response-evaluable patients better supported the alternative hypothesis that the true unknown response rate was at least 0.30.
Of the 38 response-evaluable patients, 25 (66%) experienced clinical benefit (SD or disease response). Eight (21%) patients achieved PR, 1 (3%) achieved CR, and the overall response rate (ORR) was 24% (90% CI, 14 to 36; Table 3). In an intent-to-treat analysis of all 41 patients enrolled, the ORR was 22% (90% CI, 13 to 35). Sixteen (42%) patients had SD, and 13 (34%) had PD. The median RD for the 9 responding patients was 4.3 months (90% CI, 3.7 to 7.2).
Table 3. Tumor response summary.
| Patient subset | ||||
|---|---|---|---|---|
|
|
||||
| Response category | All patients (N=41) | Response-evaluable (N=38) | ||
| Overall response, N | 9 | 9 | ||
| Response rate, % | 22 | 24 | ||
| 90% CI | 13 to 35 | 14 to 36 | ||
| Complete response, N | 1 | 1 | ||
| Response rate, % | 2 | 3 | ||
| 90% CI | 0 to 10 | 0 to 11 | ||
| Partial response, N | 8 | 8 | ||
| Response rate, % | 20 | 21 | ||
| 90% CI | 10 to 31 | 11 to 33 | ||
| Stable disease, N | 16 | 16 | ||
| Response rate, % | 39 | 42 | ||
| 90% CI | 26 to 53 | 28 to 57 | ||
| Progressive disease, N | 13 | 13 | ||
| Response rate, % | 32 | 34 | ||
| 90% CI | 20 to 45 | 22 to 49 | ||
Abbreviation: CI, confidence interval.
Progression and overall survival
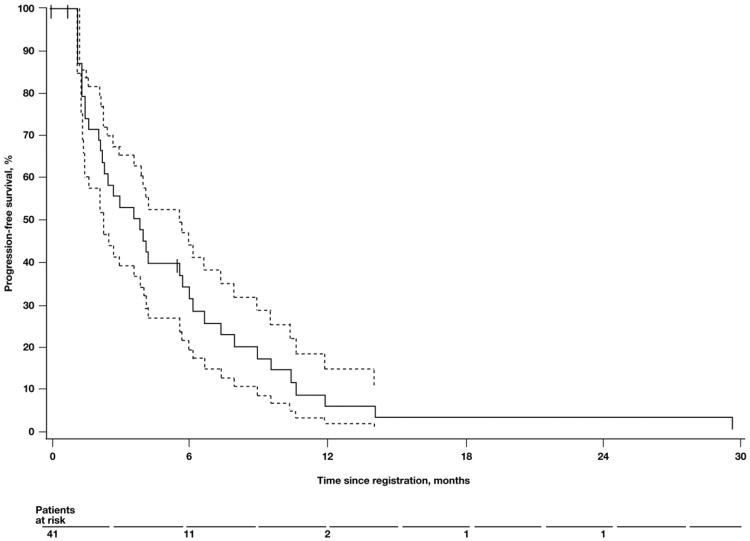
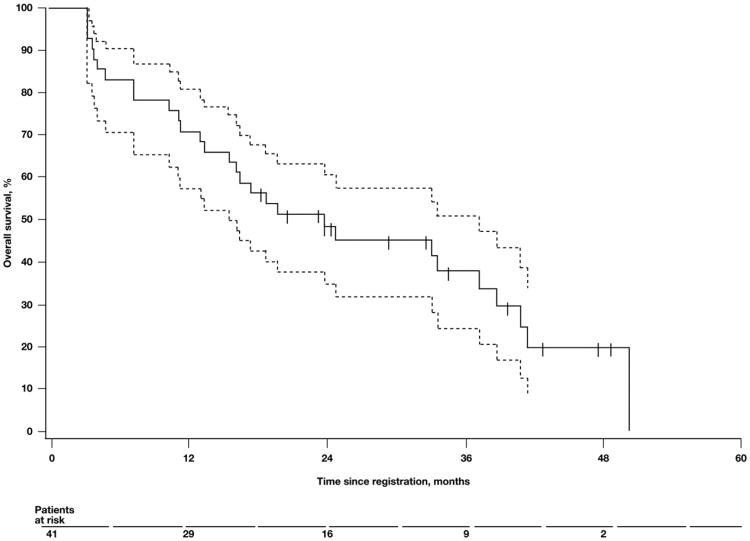
Follow-up data were current for all patients as of March 2007. The median TTP was 3.6 months (90% CI, 2.1 to 5.4; Fig. 1), and the median TTF was 2.4 months (90% CI, 1.5 to 3.9). The median OS was 21.2 months (90% CI, 13.6 to 34.7; Fig. 2). The 2-year and 3-year survival rates were 48% (90% CI, 34 to 61) and 35% (90% CI, 21 to 48), respectively.
Fig. 1.
Median time to progression. Kaplan–Meier plot of time to progression in patients treated with weekly topotecan. Dashed lines represent the 90% confidence interval. Tick marks on the Kaplan–Meier curve represent censored patients.
Fig. 2.
Median overall survival. Kaplan–Meier plot of overall survival in patients treated with weekly topotecan. Dashed lines represent the 90% confidence interval. Tick marks on the Kaplan–Meier curve represent censored patients.
Discussion
As duration of survival in patients with relapsed ovarian cancer continues to lengthen, maintaining quality-of-life during therapy and maximizing subsequent treatment options are important therapeutic goals [3]. Unfortunately, many approved treatments for ovarian cancer have cumulative toxicities that leave patients more susceptible to adverse events during subsequent lines of therapy [2]. In addition to developing treatment strategies that continue to lengthen survival, additional treatment options addressing quality-of-life for patients with relapsed ovarian cancer are needed.
Topotecan at 1.5 mg/m2 on days 1 to 5 of a 21-day cycle is generally well tolerated and is an important treatment option in patients with relapsed ovarian cancer [23–27]. However, because heavily pretreated patients may be predisposed to myelotoxicity, these patients often require dose delays, reductions, or the use of hematopoietic growth-factor support during treatment with the standard 5-day regimen of topotecan [24– 26]. In the relapsed setting, grades 3–4 hematologic toxicity appears to be noncumulative but is problematic because of a recurrence rate of 85% to 90% [24–26]. Therefore, alternate dosing regimens of topotecan, including weekly dosing and prolonged infusions, have been examined [6,18,9]. Down-regulation of the target enzyme, topoisomerase I, has been demonstrated after 24 to 48 h of exposure to topotecan in tissue culture [12]. This closely correlates with the diminished cell-kill kinetics of topotecan demonstrated after the same time interval [12]. Topoisomerase I levels returned to baseline 5 to 7 days after removal of topotecan exposure, therefore supporting weekly bolus dosing of topotecan [12].
In the current study, weekly topotecan was active and well tolerated in patients with relapsed platinum-sensitive ovarian or peritoneal cancer. The ORR of 24% and SD rate of 42% are generally consistent with the rates reported in retrospective studies of weekly topotecan in relapsed ovarian cancer [11,13,16]. Response rates associated with the standard topotecan regimen in patients with platinum-sensitive ovarian cancer range from 19% to 33%, and SD rates range from 17% to 48% [18,23,24]. Response rates were similar to those reported in a GOG phase II study evaluating topotecan with standard dosing in patients with platinum-sensitive disease (32.6%; 90% CI, 18 to 48) [18,23,24]. Response and SD rates in this trial were substantially higher than those reported in an early trial by Hoskins et al. [25] using a lower dose of weekly topotecan (1.75 mg/m2) in which the ORR was 3%. However, the low dose of weekly topotecan and continuous infusion schedule used in that trial may have affected the efficacy of topotecan [25]. Similar to results in the current trial, retrospective studies of 2.5 mg/m2 to 3.7 mg/m2 weekly topotecan in heavily pretreated patients with ovarian cancer, approximately 50% of whom had potentially platinum-sensitive disease, demonstrated an ORR of 18% to 31%; additionally, 38% to 43% of patients achieved SD [13,16]. Moreover, in a small phase II trial, 4 mg/m2 weekly topotecan was associated with anORR of almost 50%, with similar response rates for platinum-sensitive and platinum-resistant disease [15].
An update of this same study reported ORR and median survival similar to those in the current study, 24% and 22.3 months, respectively [11]. These data are also similar to those reported in the ICON4/AGO-OVAR-2.2 trial, in which patients with platinum-sensitive ovarian cancer treated with either platinum-based chemotherapy or in combination with paclitaxel achieved median survival of 24 and 29 months, respectively [4]. Although the median RD appears inferior in the current study compared with the study conducted by GOG (4.3 months vs 11.2 months), the median OS was similar in these 2 trials (21.2 months vs 20.2 months) [18]. These data are also comparable to a phase III study of patients with platinum-sensitive ovarian cancer treated with standard dosing topotecan (median OS, 71 weeks) [6]. In the current trial, 26 of 41 (63%) of patients were re-treated with a platinum-containing regimen. Subsequent therapies may affect survival rates and therefore, these studies may not be directly compared. Nonetheless, survival remains a critical measure of efficacy and appears to be comparable between the weekly and standard dose regimens.
In our current study, weekly topotecan treatment was associated with few grades 3–4 hematologic toxicities, and the proportion of patients experiencing grades 3–4 neutropenia (19%) was much lower than the proportion reported with the standard topotecan regimen (53% to 100%) [25–27]. Moreover, our results are comparable with those presented in recent studies evaluating 4.0 mg/m2 of weekly topotecan in which grades 3–4 neutropenia was reported in 18% to 22% of participating patients [6,23,13,14]. One study evaluating weekly topotecan administered on days 1, 8, and 15 of a 28-day cycle that allowed hematopoietic growth-factor support reported grades 3–4 neutropenia in just 4% of patients [15]. Treatment-related nonhematologic toxicities were usually mild to moderate, with fatigue and gastrointestinal manifestations being the most common. The rate of grades 3–4 fatigue in this study (22%) was somewhat higher than in the standard regimen trials (0 to 6%). However, similar to the study conducted by GOG and reported by McGuire et al. [18], fatigue was the most common reason for patients with SD or responding disease to withdraw from the study. The rates of grades 3–4 nausea and vomiting in the current study (7% and 5%, respectively) were lower or comparable with those reported in trials using standard topotecan regimen (grades 3–4 nausea, 7% to 12%; grades 3–4 vomiting, 3% to 10%) [23,25,27].
This prospective, multicenter trial in patients with measurable disease provides further insight into the activity of weekly topotecan and adds to the growing body of evidence that weekly topotecan is well tolerated and efficacious in patients with relapsed ovarian or primary peritoneal cancer. Further investigations into the role of weekly topotecan are anticipated. The GOG is currently evaluating weekly and daily ×5 topotecan in a randomized phase II study. Furthermore, the highly favorable toxicity profile of topotecan has prompted investigations of combination therapy with other cytotoxic agents and targeted therapies.
Footnotes
This study was sponsored by GlaxoSmithKline, Philadelphia, PA, and by NIH Cancer Center Support Grant CA-22453.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Dunton CJ. Management of treatment-related toxicity in advanced ovarian cancer. Oncologist. 2002;7(suppl 5):11–9. doi: 10.1634/theoncologist.7-suppl_5-11. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DK. Relapsed ovarian cancer: challenges and management strategies for a chronic disease. Oncologist. 2002;7(suppl 5):20–8. doi: 10.1634/theoncologist.7-suppl_5-20. [DOI] [PubMed] [Google Scholar]
- 4.Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 5.Pfisterer J, Plante M, Vergote I, et al. Gemcitabine/carboplatin (GC) vs. carboplatin (C) in platinum sensitive recurrent ovarian cancer (OVCA). Results of a Gynecologic Cancer Intergroup randomized phase III trial of the AGO OVAR, the NCIC CTG and the EORTC GCG (abstr 5005) Proc Am Assoc Cancer Res. 2004;23:449s. [Google Scholar]
- 6.Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–22. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 7.ten Bokkel Huinink W, Lane SR, Ross GA. Long-term survival in a phase III, randomised study of topotecan versus paclitaxel in advanced epithelial ovarian carcinoma. Ann Oncol. 2004;15:100–3. doi: 10.1093/annonc/mdh025. [DOI] [PubMed] [Google Scholar]
- 8.Rowinsky EK. Weekly topotecan: an alternative to topotecan's standard daily × 5 schedule? Oncologist. 2002;7:324–30. doi: 10.1634/theoncologist.7-4-324. [DOI] [PubMed] [Google Scholar]
- 9.Morris R, Munkarah A. Alternate dosing schedules for topotecan in the treatment of recurrent ovarian cancer. Oncologist. 2002;7(suppl 5):29–35. doi: 10.1634/theoncologist.7-suppl_5-29. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez M, Rose PG. Improved therapeutic index of lower dose topotecan chemotherapy in recurrent ovarian cancer. Gynecol Oncol. 2001;83:257–62. doi: 10.1006/gyno.2001.6365. [DOI] [PubMed] [Google Scholar]
- 11.Safra T, Menczer J, Bernstein R, et al. Efficacy and toxicity of weekly topotecan in recurrent epithelial ovarian and primary peritoneal cancer. Gynecol Oncol. 2007;105:205–10. doi: 10.1016/j.ygyno.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Bence AK, Mattingly CA, Desimone PA. Evaluation of topotecan cytotoxicity and topoisomerase I levels in non-small cell lung cancer cells (poster) Proc Am Assoc Cancer Res. 2002;43:247. [Google Scholar]
- 13.Bhoola SM, Coleman RL, Herzog T, et al. Retrospective analysis of weekly topotecan as salvage therapy in relapsed ovarian cancer. Gynecol Oncol. 2004;95:564–9. doi: 10.1016/j.ygyno.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Homesley HD, Hall DJ, Martin DA, et al. A dose-escalating study of weekly bolus topotecan in previously treated ovarian cancer patients. Gynecol Oncol. 2001;83:394–9. doi: 10.1006/gyno.2001.6435. [DOI] [PubMed] [Google Scholar]
- 15.Levy T, Inbar M, Menczer J, Grisaru D, Glezerman M, Safra T. Phase II study of weekly topotecan in patients with recurrent or persistent epithelial ovarian cancer. Gynecol Oncol. 2004;95:686–90. doi: 10.1016/j.ygyno.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 16.O'Malley DM, Azodi M, Makkenchery A, et al. Weekly topotecan in heavily pretreated patients with recurrent epithelial ovarian carcinoma. Gynecol Oncol. 2005;98:242–8. doi: 10.1016/j.ygyno.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 17.Tan BR, Picus J, Fracasso PM, Clark R. Weekly bolus topotecan (T): update on a phase I dose escalation trial (abstr 2147) Proc Am Soc Clin Oncol. 2002;21:84b. [Google Scholar]
- 18.McGuire WP, Blessing JA, Bookman MA, Lentz SS, Dunton CJ. Topotecan has substantial antitumor activity as first-line salvage therapy in platinum-sensitive epithelial ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2000;18:1062–7. doi: 10.1200/JCO.2000.18.5.1062. [DOI] [PubMed] [Google Scholar]
- 19.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 20.Casella G. Refining binomial confidence intervals. Can J Stat. 1987;14:113–29. [Google Scholar]
- 21.Mehta C, Patel N. User manual. Cambridge, MA: Cytel Software Corp; 2003. StatXact-6: statistical software for exact nonparametric inference. [Google Scholar]
- 22.Lee ET, Wang JW. Statistical methods for survival data analysis. 3rd. Hoboken, NJ: Wiley & Sons; 2003. [Google Scholar]
- 23.Bookman MA, Malmstrom H, Bolis G, et al. Topotecan for the treatment of advanced epithelial ovarian cancer: an open-label phase II study in patients treated after prior chemotherapy that contained cisplatin or carboplatin and paclitaxel. J Clin Oncol. 1998;16:3345–52. doi: 10.1200/JCO.1998.16.10.3345. [DOI] [PubMed] [Google Scholar]
- 24.Creemers GJ, Bolis G, Gore M, et al. Topotecan, an active drug in the second-line treatment of epithelial ovarian cancer: results of a large European phase II study. J Clin Oncol. 1996;14:3056–61. doi: 10.1200/JCO.1996.14.12.3056. [DOI] [PubMed] [Google Scholar]
- 25.Hoskins P, Eisenhauer E, Beare S, et al. Randomized phase II study of two schedules of topotecan in previously treated patients with ovarian cancer: a National Cancer Institute of Canada Clinical Trials Group study. J Clin Oncol. 1998;16:2233–7. doi: 10.1200/JCO.1998.16.6.2233. [DOI] [PubMed] [Google Scholar]
- 26.Kudelka AP, Tresukosol D, Edwards CL, et al. Phase II study of intravenous topotecan as a 5-day infusion for refractory epithelial ovarian carcinoma. J Clin Oncol. 1996;14:1552–7. doi: 10.1200/JCO.1996.14.5.1552. [DOI] [PubMed] [Google Scholar]
- 27.ten Bokkel Huinink W, Gore M, Carmichael J, et al. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol. 1997;15:2183–93. doi: 10.1200/JCO.1997.15.6.2183. [DOI] [PubMed] [Google Scholar]