Abstract
TASK-1 and TASK-3 tandem pore potassium channel subunits provide a constitutive acidic pH- and hypoxia-inhibited potassium conductance. TASK channels are expressed in a number of tissues in involved in regulation of breathing, and the TASK-1/TASK-3 heterodimer provides the predominant hypoxia-sensitive potassium conductance in carotid body Type I glomus chemosensing cells. The carotid bodies have an important role in regulation of breathing. Doxapram is a potent TASK-1 and TASK-3 potassium channel antagonist and a carotid body and breathing stimulant. PK-THPP and A1899 are potent and selective TASK-1 and TASK-3 antagonists. We hypothesized PK-THPP and A1899 are, like doxapram, breathing stimulants.
Methods
We studied rat TASK-3 (rTASK-3) potassium channel function by Ussing chamber using Fisher rat thyroid (FRT) monolayers. To quantify breathing effects, we studied male Sprague Dawley rats spontaneously breathing 1.5% isoflurane in room air by non-invasive plethysmography and by arterial blood gas analysis.
Results
PK-THPP, A1899, and doxapram inhibit rat TASK-3 potassium channel function with IC50s of 42 nM (33 to 52), 1.6 μM (0.8 to 3.3), and 22 μM (18 to 28) (n = 4 to 6; 95% confidence limits). Intravenous PK-THPP, A1899, and doxapram stimulated breathing by plethysmography with a peak change in minute ventilation relative to baseline of 84±19% and 226±56% (for PK-THPP at 0.5 and 5 mg/kg; mean±S.E.M.; n = 3 to 4; P<0.05 and P<0.001, respectively, relative to vehicle); 46±2% and 236±48% (for A1899 at 5 and 25 mg/kg; n=3 to 4; P>0.05 and P<0.001, respectively); 103±20% (for doxapram at 25 mg/kg; n = 4), and 33±9% (for DMSO vehicle at 1 ml/kg; n = 4). PK-THPP and A1899, unlike doxapram, induced a profound and lasting respiratory alkalosis by arterial blood gas analysis. Thirty minutes following intravenous drug administration, we observed an arterial pH and carbon dioxide partial pressure of 7.62±0.02 and 23±0.8 mmHg (for PK-THPP after 5 mg/kg; n = 4; P<0.001 for both relative to vehicle), 7.49±0.02 and 31±2 mHg (for A1899 at 25 mg/kg; n = 6; P<0.05 and 0.001, respectively), 7.43±0.03 and 39±4 mmHg (for doxapram after 25 mg/kg; n =4; P>0.05 for both), and 7.38±0.03 and 48±4 mmHg (for DMSO vehicle after 1 ml/kg; n = 3).
Conclusions
PK-THPP and A1899 are potent rTASK-3 antagonists and effective breathing stimulants. PK-THPP and A1899 effects on breathing were of greater magnitude and/or duration relative to that of doxapram. PK-THPP and A1899 or related compounds may have therapeutic potential for treating breathing disorders.
Introduction
Breathing is essential to life as it maintains blood oxygenation and eliminates carbon dioxide generated by metabolism. Many of the drugs required for anesthesia depress breathing, and significant effort is required by clinicians to minimize this adverse effect. Doxapram is a breathing stimulant drug that acts upon the carotid body to promote ventilation in patients during and recovering from anesthesia (Figure 1A) (1). Doxapram antagonizes opioid- and anesthetic-induced depression of breathing, expedites recovery from anesthesia, and decreases postoperative pulmonary complications (2–8).
Figure 1. PK-THPP and A1899 are potent rTASK-3 potassium channel antagonists.
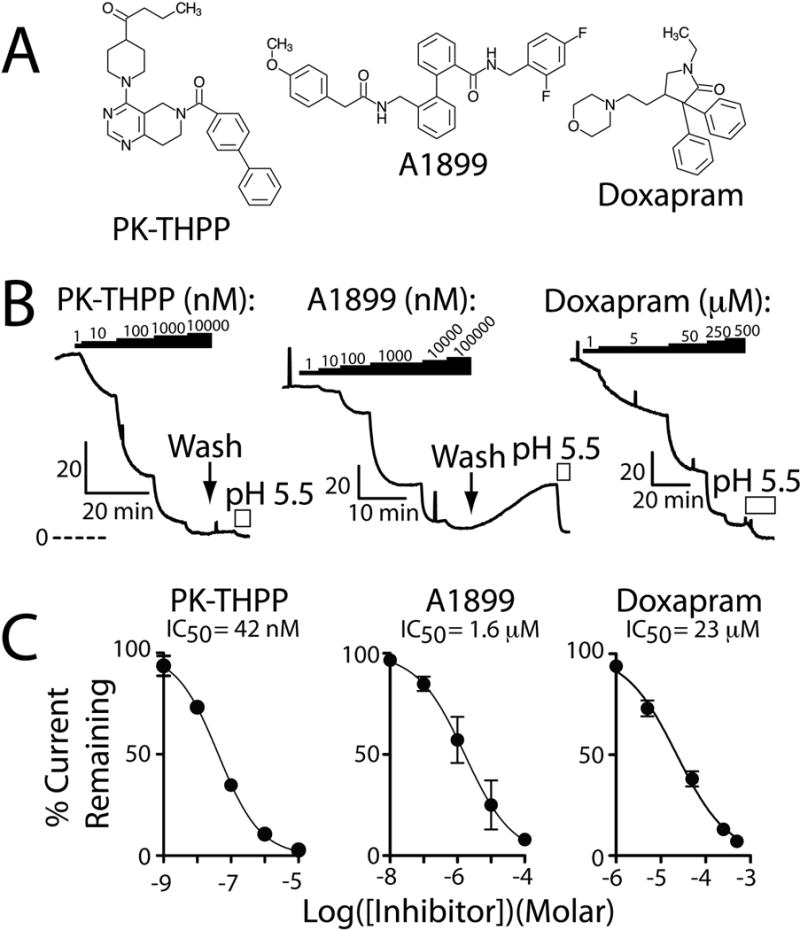
A, chemical structure of PK-THPP, A1899, and doxapram. B, Ussing chamber current records from FRT monolayers transiently expressing rTASK-3 and treated with PK-THPP, A1899, or doxapram. The black bars denote application of PK-THPP, A1899, or doxapram and the white bars indicate apical application of acidic pH. The perforated line indicates the zero current level, and the “L shaped” bars indicate current (μA/cm2) and time scaling. C, summary concentration-response data for PK-THPP, A1899, and doxapram. Each data point is n = 6 ± S.E.M.; error bars are not visible when smaller than data point. Data were fit with the following: I=100/(1+10ˆ((LogIC50−X)*HillSlope))). Hill Slope estimates were: PK-THPP −0.6953, A1899 −0.6124, and doxapram −0.7575.
TASK-1 and TASK-3 tandem pore potassium channel subunits provide a constitutive, acidic pH- and hypoxia-inhibited potassium conductance, which regulate cellular resting membrane potential and excitability (9–11). TASK-1 and TASK-3 subunits function as homodimers or co-associate and function as TASK-1/TASK-3 heterodimers (12–14). We had previously determined that doxapram inhibits TASK-1, TASK-3, and TASK-1/TASK-3 heterodimer function with IC50s of 410 nM, 37 μM, and 9 μM, respectively, which are near or within doxapram’s clinical concentration range (15). The TASK-1/TASK-3 heterodimer provides the predominant hypoxia-sensitive background potassium conductance in rat carotid body Type I glomus cells (14). TASK-1 knockout mice and TASK-1/TASK-3 double knockout mice have impaired carotid body function, suggesting these channels also contribute to carotid body function (16,17). Finally, doxapram inhibits calcium sensitive (BK) potassium channels (IC50 ~13 μM), which may also be important in carotid body function (18).
Several potent and selective TASK-1 and TASK-3 potassium channel antagonists have been identified recently. Brendel et al. made claims regarding a series of compounds, initially developed as Kv1.5 antagonists, to be potent TASK-1 and TASK-3 antagonists (19). Importantly, two of these compounds with IC50s of ~100 and ~500 nM for TASK-1, like doxapram, stimulated breathing in rabbits and rats and augmented upper airway genioglossus EMG activity. More recently, two additional antagonists, A1899 and PK-THPP, have been reported (20,21). A1899 is an open channel blocker of TASK-1 and TASK-3 channels with IC50s of 7 and 70 nM, respectively, in CHO cells (Figure 1A) (20). Like those studied by Brendel et al., A1899 was developed as a Kv1.5 potassium channel antagonist (22). PK-THPP is a propylketone (PK) derivative of tetrahydropyrido-pyrimidine (THPP) discovered using a high throughput strategy (Figure 1A) (21). PK-THPP inhibits TASK-1 and TASK-3 channels with IC50s of 300 and 35 nM, respectively, in HEK cells (21). The effects of PK-THPP and A1899 on breathing have not been reported.
Because doxapram and other TASK antagonists are ventilatory stimulants and because TASK channels are expressed in tissues involved in regulation of breathing, we hypothesized PK-THPP and A1899 may stimulate breathing. To test this hypothesis, we first confirmed that PK-THPP and A1899 are potent TASK-3 potassium channel antagonists. We then used plethysmography and arterial blood gas analysis to quantify the breathing response of isoflurane anesthetized rats following intravenous administration of PK-THPP or A1899.
Methods
Molecular Biology & Electrophysiology
Rat TASK-3 cDNA was expressed from a pcDNA3.1/V5-His-TOPO-TA vector (Invitrogen, Carlsbad, CA). Fischer rat thyroid (FRT) epithelial cells were cultured and transfected as previously described (23). FRT epithelial monolayers were studied 48 h after transfection in an Ussing chamber (Physiologic Instruments, San Diego, CA). Junction potentials were offset prior to each experiment. A potassium gradient was applied across the monolayer. The apical solution contained (in mM): 135 NaCl, 1.2 MgCl2, 1.2 CaCl2, 10 HEPES, 10 Dextrose, pH 7.4 with NaOH. The basolateral solution contained (in mM): 135 KCl, 1.2 MgCl2, 1.2 CaCl2, 10 HEPES, 10 Dextrose, pH 7.4 with KOH. MES was used in lieu of HEPES in pH 5.5 solutions. In all studies, the transepithelial voltage was clamped at 0 mV. A DVC-1000 amplifier (World Precision Instruments, Sarasota, FL) amplified the signal, which was digitized with no filtering at 10 Hz using a USB-6009 data acquisition board (National Instruments, Austin, TX) interfaced with an Apple Computer running Labview 8.5 software (National Instruments). Data were averaged every 1 sec for analysis. Positive current indicates positive charge flowing in the basolateral to apical direction. +5 mV (1 sec duration) transepithelial voltage pulses, referenced to the apical surface, were applied intermittently to assess transepithelial resistance and monolayer integrity. Air or air with isoflurane was continuously bubbled through apical and basolateral solutions. Isoflurane (Baxter Healthcare, Deerfield, IL) was applied and quantified as previously described (23). All studies were conducted at room temperature (22–24°C).
Animal Studies
Studies were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care, Boston, MA. Male Sprague Dawley rats weighing 350–600 gms were used and were obtained from Charles River Laboratories (Wilmington, MA) and housed in the MGH Center for Comparative Medicine Animal Facility. Femoral artery catheters were preimplanted by the vendor in some animals. In all studies, drugs were administered through a 24G lateral tail vein catheter and flushed in with 2 mls of saline. Rats in the PK-THPP, A1899 and DMSO vehicle only groups received 1 ml/kg intravenous DMSO.
Plethysmography
Rats were studied in a custom-built plethysmography chamber flushed with isoflurane 1.5% (Baxter Healthcare) in room air where they breathed spontaneously. All data were collected in the closed-chamber configuration, and solenoid valves (Cole Parmer, Vernon Hills, IL) (controlled via a Darlington power transistor array) were used to flush the chamber for 2 mins every 2 mins with isoflurane 1.5% in room air (at least 2 LPM flow) to remove rat generated CO2. Chamber outflow was continuously sampled and analyzed using a Capnomac Ultima medical gas analyzer (GE Healthcare, U.K.) to quantify oxygen, CO2, and isoflurane levels in the chamber. The highest single CO2 measurement in each study upon chamber flushing ranged from 1.1 to 1.9% (1.4 ± 0.1%, Mean ± S.E.M.; n = 24). All plethysmography data acquisition and valve control were performed using LabView 8.5 software (National Instruments) run on an Apple computer interfaced with a USB-6009 data acquisition board (National Instruments). Chamber temperature and humidity were recorded automatically (DHT22, Aosong Electronics, Guangzhou, China) and rat temperature was continuously measured via a rectal thermistor. LabView 8.5 software used the rectal thermistor data to control a heat lamp (Powerswitch Tail II, powerswitchtail.com) to maintain the rodent temperature at 37°C. A differential pressure transducer and demodulator (Models CD15 and MP45–14 – 871; Validyne Engineering, Northridge, CA) were used to convert the chamber pressure to an analog signal. The signal was high-pass filtered at 15 s, acquired at 100 Hz, and analyzed in 4-s epochs using LabView 8.5 software to determine signal amplitude and frequency. The chamber was calibrated by injecting 0.5 mls of air through a Luer port with a rat-sized object occupying the chamber, and tidal volumes during intermittent chamber closure were estimated using methods described by Drorbaugh and Fenn (24).
Blood Pressure and Blood Gas Analysis
Rats with femoral artery catheters were studied in a chamber continuously flushed with isoflurane 1.5% in room air where they breathed spontaneously. Mean arterial blood pressure was measured using a pressure transducer (TruWave, Edwards Life Sciences, Irvine, CA) interfaced with a custom-built amplifier (AD620 operational amplifier; Jameco Electronics, Belmont, CA). The signal was digitized at 1,000 Hz using a USB-6009 data acquisition board (National Instruments) and analyzed in 4-s epochs. An arterial blood sample (0.3 mls) was drawn after 30 min of equilibration in isoflurane 1.5%, and 15 and 30 min after administration of PK-THPP, A1899, Doxapram, or DMSO (vehicle). Samples were analyzed promptly using CG4+ cartridges in a Vetscan iStat 1 (Abaxis, Union City, CA) blood gas analyzer.
Reagents
PK-THPP and A1899 were synthesized by Abjerjona Laboratories (Beverly, MA) to 98% purity using published methods (21,22). Structures were validated by HPLC/Mass Spectrometry and by 1H-NMR. In all studies, PK-THPP and A1899 were solubilized in DMSO. Doxapram (Respiram) was from Modern Veterinary Therapeutics, LLC (Coral Gables, FL). All other reagents were from Fisher Scientific (Pittsburg, PA) or Sigma-Aldrich (St. Louis, MO), unless otherwise noted.
Statistical Analysis
Data were reported as mean ± S.E.M. Statistical analysis was done using Prism 5 software (GraphPad Software, Inc., La Jolla, CA). Multiple comparisons were performed using a one-way or repeated measures ANOVA followed by a Tukey-Kramer or Dunnett’s post test; a P-value less than 0.05 indicates statistical significance. This analysis assumes comparisons were made between normally distributed data populations of equal variance, and that all observations were independent of each other. Parameters derived from non-linear regression were compared using an extra sum-of-squares F test.
Results
PK-THPP and A1899 Effects on Rat TASK-3 Potassium Channel Function
Rat TASK-3 (rTASK-3) channels were transiently expressed in FRT monolayers, and their function studied by Ussing chamber. PK-THPP inhibited rTASK-3 function with an IC50 of 42 nM (33 to 52, 95% confidence interval; n = 6) and 38 nM (27 to 52; n =3) (P = 0.6 by extra sum-of-squares F test) in the absence and presence of isoflurane (2.37±0.1 mM), respectively (Figure 1B & 1C; isoflurane data not shown). A1899 inhibited rTASK-3 with an IC50 of 1.6 μM (0.8 to 3.3; n = 4) (Figure 1B & 1C). We have previously determined that DMSO (vehicle) up to 1% has minimal effect on rTASK-3 function (23). PK-THPP and A1899 inhibition were difficult to washout (Figure 1B). Doxapram inhibited rTASK-3 with an IC50 of 23 μM (18 to 28; n = 4) (Figure 1B & 1C).
Plethysmography Studies
PK-THPP, A1899 and doxapram increased minute ventilation by increasing tidal volume and breathing rate (Figure 2). PK-THPP effects, compared to that of A1899 and doxapram, occurred at lower doses. DMSO (1 ml/kg), the vehicle used for both agents caused a minor and short-lived increase in minute ventilation, as has been previously reported (25). The peak normalized response of PK-THPP (0.5 and 5 mg/kg; 247±17% and 326±56%) and A1899 (25 mg/kg; 336±48%) on minute ventilation were significantly different from that of doxapram (25 mg/kg; 203±20%), A1899 (5 mg/kg ; 146±2%), and DMSO (1 ml/kg; 133±9%) (Figure 2; P<0.05 by one-way ANOVA with a Tukey-Kramer post test).
Figure 2. Intravenous PK-THPP, A1899, and doxapram stimulate breathing in rats.
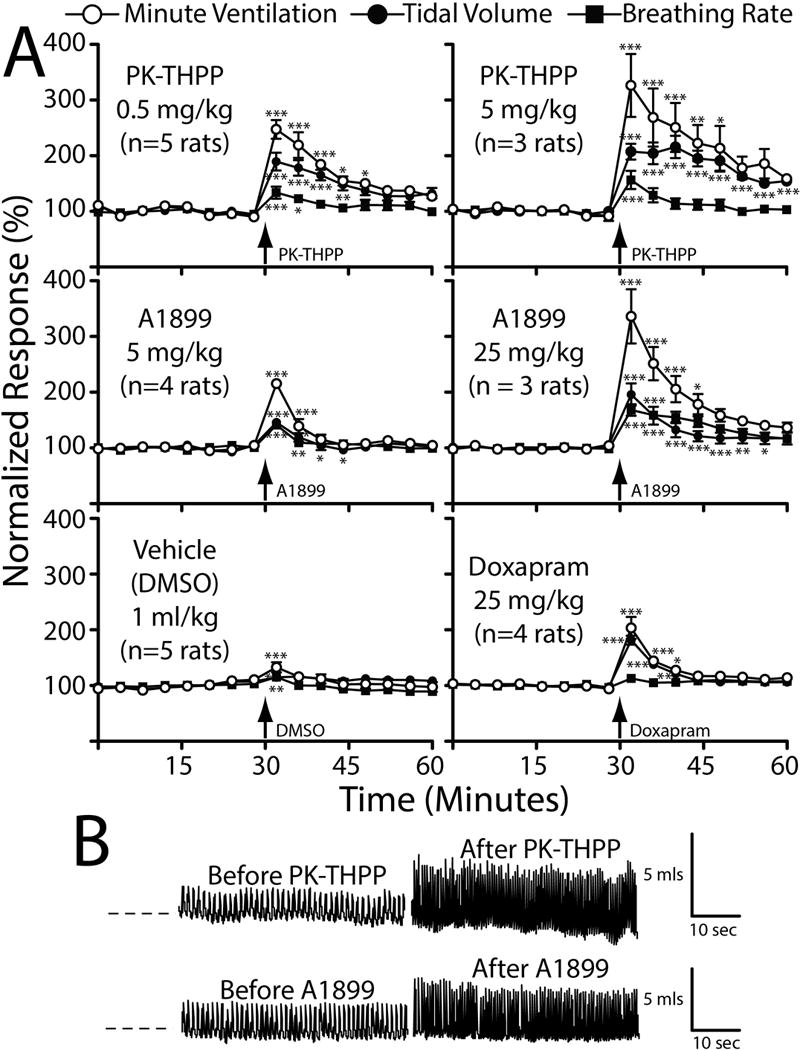
A, normalized minute ventilation (open circles), tidal volume (filled squares), and breathing rate (filled circles) before and after intravenous administration of PK-THPP (0.5 and 5 mg/kg), A1899 (5 and 25 mg/kg), doxapram (25 mg/kg), and vehicle (DMSO; 1 ml/kg) measured by plethysmography. Data were collected from spontaneously breathing rats inhaling 1.5% isoflurane in air. Data were normalized to the average minute ventilation, tidal volume, or breathing rate prior to study drug administration. n = 3 to 5 ± S.E.M. for each; error bars are not visible when smaller than data point. Baseline minute ventilation, tidal volume, and breathing rate were 48±3 mls/100 gms/min, 0.7±1 mls, and 67±2 breathes/min, respectively (n = 24). B, plethysmography signal tracings from a rat immediately before and immediately after receiving 5 mg/kg PK-THPP or 25 mg/kg A1899. Asterisks (*, **, and ***) indicate statistical significance (P < 0.05, P < 0.01, and P < 0.001, respectively) relative to the initial data point using repeated measures ANOVA with a Dunnett’s post test. The perforated line indicates zero relative pressure, with upward deflections indicating inspiration. The “L shaped” bars indicate time and calculated volume scaling.
Arterial Blood Gas and Arterial Blood Pressure Analysis
Both PK-THPP and A1899 induced a respiratory alkalosis and improved oxygenation 15 and 30 minutes following intravenous administration (Figure 3). Doxapram data merely trended towards alkalosis, and DMSO trended towards acidosis (Figure 3). A small increase in lactate levels was detected in A1899 and doxapram treated rats (Figure 3). Neither PK-THPP nor DMSO had significant effects on normalized mean blood pressure (Figure 4). A1899 had one data point that was elevated, and doxapram caused an approximately 10% sustained increase in normalized mean blood pressure (Figure 4).
Figure 3. Intravenous PK-THPP and A1899 induce a respiratory alkalosis in spontaneously breathing rats.
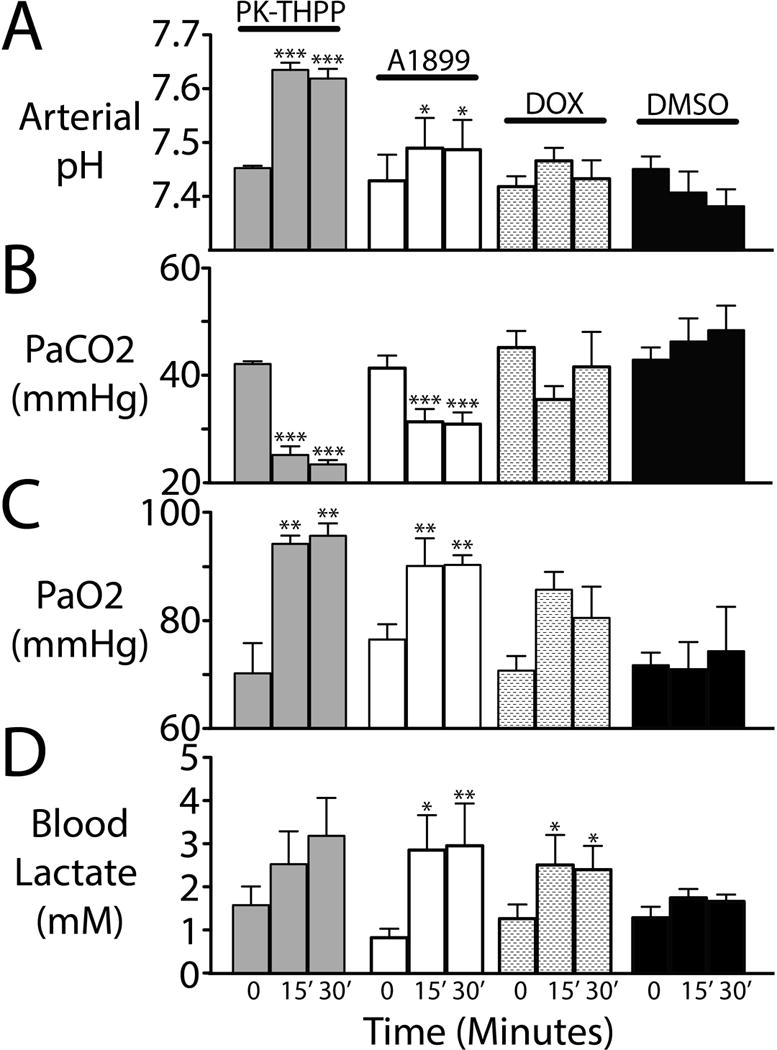
Arterial blood pH, carbon dioxide, oxygen, and lactate levels (A–D) from rats at baseline and 15 and 30 minutes after receiving intravenous PK-THPP (5 mg/kg), A1899 (25 mg/kg), doxapram (25 mg/kg), and DMSO (vehicle only; 1 ml/kg). n = 3 to 4 ± S.E.M. for each. Rats were breathing 1.5% isoflurane in room air. Asterisks (*, **, and ***) indicate statistical significance (P < 0.05, P < 0.01, and P < 0.001, respectively) by one-way ANOVA with a Dunnett’s post test relative to the baseline values within each group.
Figure 4. Intravenous PK-THPP and A1899 have minimal effect on arterial blood pressure in spontaneously breathing rats.
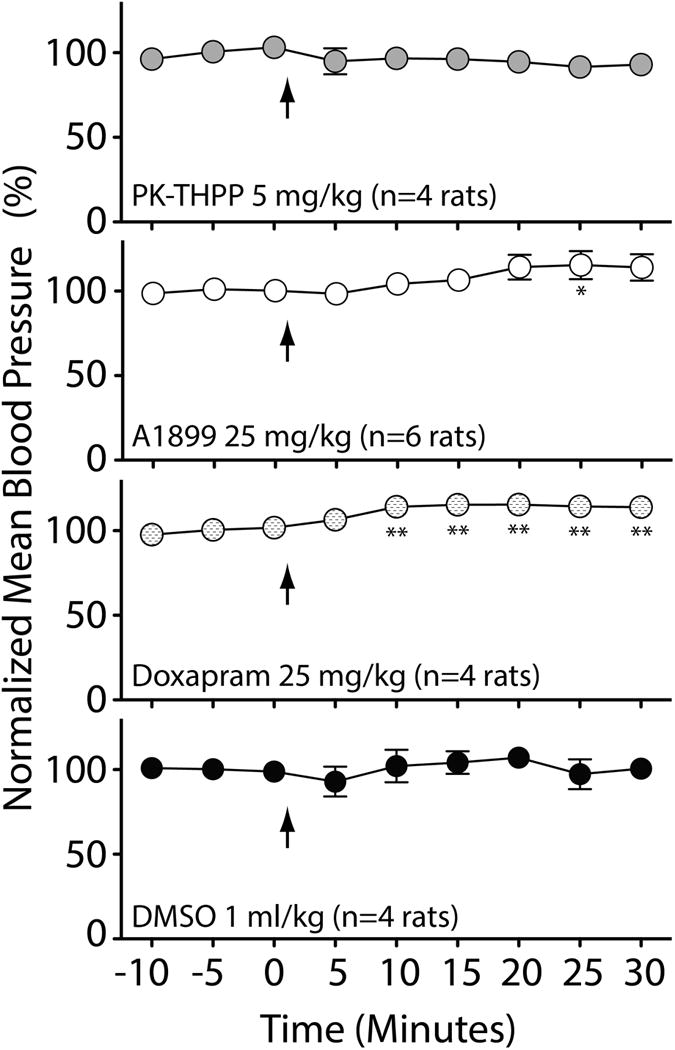
Mean blood pressure determined via femoral artery catheter at baseline (for 15 min) and for 30 minutes after receiving intravenous PK-THPP (5 mg/kg), A1899 (25 mg/kg), doxapram (25 mg/kg), and DMSO (vehicle; 1 ml/kg) are shown. Each data point is the average pressure data over a 5 min time window normalized to average baseline mean pressure prior to study drug administration. Average mean blood pressure for rats in these studies were 88±1 mmHg. n = 4 rats ± S.E.M. for each data point. Asterisk (* and **) indicates significance (P < 0.05 and P < 0.01, respectively) relative to the three baseline data points by one-way ANOVA with a Dunnett’s post test.
Discussion
In this study we tested the hypothesis that two recently identified TASK potassium channel antagonists, PK-THPP and A1899, are breathing stimulants. We compared their effects to that of doxapram, a known breathing stimulant and TASK potassium channel antagonist. We confirmed that PK-THPP, A1899, and doxapram were potent rTASK3 antagonists with IC50s of 42 nM, 1.6 μM, and 23 μM, respectively (Figure 1). Isoflurane had no effect on PK-THPP potency. Plethysmography studies demonstrated that PK-THPP, A1899, and doxapram stimulate breathing by increasing tidal volume and breathing rate (Figure 2). PK-THPP and A1899 induced a respiratory alkalosis and increased oxygenation for over 30 minutes (Figure 3). The magnitude of PK-THPP and A1899 breathing effects exceeded that of doxapram. A1899 and doxapram caused a modest increase in lactate levels (Figure 3D). Unlike doxapram, which caused hypertension, PK-THPP and A1899 had limited effects on mean arterial blood pressure (Figure 4).
Breathing effects by plethysmography analysis were transient, yet arterial blood gas data showed a sustained effect (i.e., greater than 30 minutes). We speculate the respiratory alkalosis, which evolves following drug administration, opposes the drug-induced increases in ventilation and likely explains this discrepancy (26). The drug-induced increase in arterial oxygen pressure is likely due to increased alveolar oxygen pressure secondary to hypocapnia as predicted by the alveolar gas equation and/or due to diminished intrapulmonary shunting secondary to increased lung expansion/recruitment during hyperventilation (27). The origin of the lactic acidosis is unclear. Since the acidosis was not present in DMSO only treated rats, it is unlikely from experimental artifact such as hypovolemia from repeated blood draws. It may be due to altered tissue perfusion from hypocapnia-related vasoconstriction, impaired oxygen delivery by hemoglobin (i.e., the Bohr effect), the metabolic demands of breathing-related muscle activity, and/or some other unknown direct drug effect.
Anatomic Site(s) of Action
PK-THPP and A1899 directly stimulate breathing as demonstrated by the respiratory alkalosis on arterial blood gas analysis. Furthermore, blood pressure and blood gas data demonstrate these compounds do not stimulate breathing through marked changes in blood pressure, blood pH, metabolism, or oxygenation. PK-THPP, A1899, and doxapram are structurally different molecules (Figure 1A). Therefore, they may or may not share a common site(s) or mechanism(s) of action. Since potassium permeability via potassium channel activity has a hyperpolarizing effect on neurons, a potassium channel antagonist will cause neuronal depolarization. This depolarization may decrease the threshold for neuronal activation and/or may be sufficient to cause direct neuronal activation. There are at least four general anatomic areas upon which PK-THPP and A1899 may act: 1) the peripheral chemosensing cells of the carotid body, which stimulate breathing in response to hypoxia and acute acidemia; 2) the central chemosensing cells of the ventrolateral medulla, which stimulate breathing in response to CSF acidification; 3) the central pattern generating brainstem neurons, which receive and integrate input from the chemosensing processes and which in summation provide the neuronal output to respiratory motor neurons; and/or 4) the motor neurons and muscles involved in breathing, which contract and relax in response to the brainstem neuronal output. TASK-1 and/or TASK-3 channels are expressed in each of these areas including motor neurons; only small levels of TASK-3 mRNA are present in rodent skeletal muscle (10,11,14,28–34). The carotid body is a likely target since TASK-1 and TASK-3 potassium channel function is prominent in carotid body chemosensing cells. Additionally, the carotid body is targeted by at least two breathing stimulants, doxapram and almitrine, and both drugs are known to inhibit potassium channels (1,35–38).
Molecular Site of Action
PK-THPP and A1899 were chosen for study because of their potent and selective inhibition of TASK-1 and TASK-3 potassium channels. Some or all of the effects on breathing may occur through TASK-1 and/or TASK-3 inhibition. However, we do not know the concentration of either compound at its site of action; and both PK-THPP and A1899 inhibit other potassium channels, albeit at markedly higher concentrations. Also, no one has reported the effects of PK-THPP and A1899 on the TASK-1/TASK-3 heterodimer. PK-THPP inhibits TREK-1, Kv1.5, hERG and KATP potassium channels with IC50s (in μM) of >10, ~5, >15, and >10, respectively (21). A1899 inhibits TASK-2, TASK-4, TREK-1, TREK-2, TRAAK, THIK-1, TRESK, Kv1.1, and Kv1.5 potassium channels with IC50s (in μM) of 12.0, 8.1, 23.8, 8.4, >20, 2.2, 0.9, 2.7, and 1.2, respectively (20,22). Of these potassium channels, modulations of at least two are known to alter breathing. Inhibition of THIK-1 function by isoflurane within brainstem chemosensing neurons may augment breathing during inhaled anesthesia (39). TASK-2 activation during hypoxia may mediate central hypoxic ventilatory depression (40). Other potassium channels relevant to breathing, but not specifically addressed in these panels, include the calcium sensitive (BK) and rabbit Kv channels, which are inhibited by hypoxia to cause carotid body Type I chemosensing cell activation (41,42). Of note, PK-THPP at 10 μM showed no activity against 100 different receptors in a PanLabs screen (21).
PK-THPP, A1899, and doxapram, though structurally different (Figure 1A), all share at least two properties 1) potent TASK inhibition and 2) stimulation of breathing. Therefore, it is notable that the in vitro rank order potency for TASK-3 inhibition (PK-THPP > A1899 > doxapram) (Figure 1) is preserved during in vivo breathing studies. PK-THPP is the most potent breathing stimulant and doxapram the least (Figures 2 and 3). Though our observations are consistent with TASK-3 as a molecular site of action, pharmacokinetic differences, which include differences in protein binding in the blood, cannot be excluded. We also did not study the effects on TASK-1, in vitro, which provide relatively small currents in our expression system.
The TASK-3 IC50 for PK-THPP determined in this study (42 nM) agrees well with that published by Coburn et al. (35 nM) (21). Similarly, the IC50 for doxapram (23 μM) agrees well with our prior study (37 μM) (15). However, there was significant discrepancy between our A1899 IC50 (1.6 μM) and that published by Streit et al. (70 nM in CHO cells and 318 nM in Xenopus oocytes) (20). It may be due to differences in expression system or method of application, since Streit et al. found large differences between CHO cells studied by the whole cell patch clamp method (70 nM) and Xenopus oocytes studied by the two-electrode voltage clamp method (318 nM). Also, A1899, which acts deep in the intracellular open pore, has the added constraint of gaining access to this site after extracellular application.
Streit et al. identified the specific amino acids lining the intracellular pore vestibule of the TASK-1 open pore involved in A1899 blockade (20). These amino acids are highly conserved in TASK-3. Nothing, however, is known about the mechanism by which PK-THPP inhibits TASK-1 or TASK-3.
Effects of Isoflurane Anesthesia
All breathing studies were conducted in the presence of 1.5% (1 MAC) inhaled isoflurane. Isoflurane was used since we were uncertain if these compounds would induce convulsions or extreme agitation, particularly at higher dosages. In fact, no convulsions and no agitation were observed in any study subjects, even upon recovery from isoflurane. Future studies will need to clarify if PK-THPP and A1899 stimulate breathing in the absence of isoflurane. PK-THPP inhibitory potency for TASK-3 is unaffected by isoflurane.
TASK-1 and TASK-3 potassium channels are activated by halogenated volatile anesthetics, including isoflurane, and may contribute to volatile anesthetic effects including immobility and unconsciousness (43–45). However, other than some transient movement upon injection, which was also observed in the DMSO control group, we observed no overt signs of anesthesia reversal at 1.5% isoflurane.
Potential Clinical Utility
Doxapram has been useful in managing opioid and anesthetic depression of breathing and may shorten anesthetic recovery and minimize pulmonary complications, particularly in the obese (5–8). Doxapram is administered by continuous intravenous infusion due to rapid redistribution after injection, and this necessity likely limits its utility. PK-THPP and A1899 as breathing stimulants, relative to doxapram, are more potent and/or of longer duration. A more potent breathing stimulant requires administration of less drug, and therefore provides at least the potential to cause fewer undesired side effects (e.g., panic, agitation, hypertension, or fever as can be caused by doxapram). A longer acting agent, which does not require administration by continuous infusion, might find greater utility in treating drug-induced ventilatory depression beyond the perioperative environment and in treating chronic breathing disorders such as sleep apnea, obesity hypoventilation, or apnea of prematurity.
Acknowledgments
We thank our laboratory colleagues including Drs. Stuart Forman, Keith Miller, Doug Raines, and Ken Solt for many helpful discussions.
Financial Support: NIH/NIGMS GM083216; Massachusetts General Hospital Department of Anesthesia, Critical Care, and Pain Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None.
Attestation: Study design, Conduct of study, Data analysis, and Manuscript preparation
Reprints: Will not be made available.
References
- 1.Mitchell RA, Herbert DA. Potencies of doxapram and hypoxia in stimulating carotid-body chemoreceptors and ventilation in anesthetized cats. Anesthesiology. 1975;42:559–66. doi: 10.1097/00000542-197505000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Sajjad T. Comparison of the effects of doxapram or carbon dioxide on ventilatory frequency and tidal volume during induction of anaesthesia with propofol. Br J Anaesth. 1994;73:266. [Google Scholar]
- 3.O’Connor B, Levy DM, Peacock JE. The influence of alfentanil pre-treatment on ventilatory effects of doxapram following induction of anaesthesia with propofol. Acta Anaesthesiol Scand. 1996;40:156–9. doi: 10.1111/j.1399-6576.1996.tb04413.x. [DOI] [PubMed] [Google Scholar]
- 4.Sun KO. Doxapram in tubeless anaesthesia for microlaryngeal surgery. Anaesthesia and Intensive Care. 1993;21:250–1. [PubMed] [Google Scholar]
- 5.Bamgbade OA. Advantages of doxapram for post-anaesthesia recovery and outcomes in bariatric surgery patients with obstructive sleep apnoea. Eur J Anaesthesiol. 2011;28:387–8. doi: 10.1097/EJA.0b013e328342956b. [DOI] [PubMed] [Google Scholar]
- 6.Bjork L, Arborelius M, Jr, Renck H, Rosberg B. Doxapram improves pulmonary function after upper abdominal surgery. Acta Anaesthesiol Scand. 1993;37:181–8. doi: 10.1111/j.1399-6576.1993.tb03697.x. [DOI] [PubMed] [Google Scholar]
- 7.Jansen JE, Sorensen AI, Naesh O, Erichsen CJ, Pedersen A. Effect of doxapram on postoperative pulmonary complications after upper abdominal surgery in high-risk patients. Lancet. 1990;335:936–8. doi: 10.1016/0140-6736(90)90998-k. [DOI] [PubMed] [Google Scholar]
- 8.Riddell PL, Robertson GS. Use of doxapram as an arousal agent in outpatient general anaesthesia. Br J Anaesth. 1978;50:921–4. doi: 10.1093/bja/50.9.921. [DOI] [PubMed] [Google Scholar]
- 9.Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. Embo J. 1997;16:5464–71. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K(+) channel family. J Biol Chem. 2000;275:9340–7. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- 11.Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525(Pt 1):135–42. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang D, Han J, Talley EM, Bayliss DA, Kim D. Functional Expression of TASK-1/TASK-3 Heteromers in Cerebellar Granule Cells. J Physiol. 2003 doi: 10.1113/jphysiol.2003.054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czirjak G, Enyedi P. Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J Biol Chem. 2002;277:5426–32. doi: 10.1074/jbc.M107138200. [DOI] [PubMed] [Google Scholar]
- 14.Kim D, Cavanaugh EJ, Kim I, Carroll JL. Heteromeric TASK-1/TASK-3 is the major oxygen-sensitive background K+ channel in rat carotid body glomus cells. J Physiol. 2009;587:2963–75. doi: 10.1113/jphysiol.2009.171181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotten JF, Keshavaprasad B, Laster MJ, Eger EI, 2nd, Yost CS. The ventilatory stimulant doxapram inhibits TASK tandem pore (K2P) potassium channel function but does not affect minimum alveolar anesthetic concentration. Anesth Analg. 2006;102:779–85. doi: 10.1213/01.ane.0000194289.34345.63. [DOI] [PubMed] [Google Scholar]
- 16.Ortega-Saenz P, Levitsky KL, Marcos-Almaraz MT, Bonilla-Henao V, Pascual A, Lopez-Barneo J. Carotid body chemosensory responses in mice deficient of TASK channels. J Gen Physiol. 2010;135:379–92. doi: 10.1085/jgp.200910302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trapp S, Aller MI, Wisden W, Gourine AV. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci. 2008;28:8844–50. doi: 10.1523/JNEUROSCI.1810-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peers C. Actions of doxapram on K+ currents in isolated type I cells of the neonatal rat carotid body. Adv Exp Med Biol. 1993;337:421–7. doi: 10.1007/978-1-4615-2966-8_59. [DOI] [PubMed] [Google Scholar]
- 19.Brendel J, Goegelein H, Wirth K, Kamm W. Inhibitors of the TASK-1 and TASK-3 Ion Channel (Patent Application) USA: Sanofi-Aventis Deutschland GMBH; 2008. [Google Scholar]
- 20.Streit AK, Netter MF, Kempf F, Walecki M, Rinne S, Bollepalli MK, Preisig-Muller R, Renigunta V, Daut J, Baukrowitz T, Sansom MS, Stansfeld PJ, Decher N. A specific two-pore domain potassium channel blocker defines the structure of the TASK-1 open pore. J Biol Chem. 2011;286:13977–84. doi: 10.1074/jbc.M111.227884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coburn CA, Luo Y, Cui M, Wang J, Soll R, Dong J, Hu B, Lyon MA, Santarelli VP, Kraus RL, Gregan Y, Wang Y, Fox SV, Binns J, Doran SM, Reiss DR, Tannenbaum PL, Gotter AL, Meinke PT, Renger JJ. Discovery of a pharmacologically active antagonist of the two-pore-domain potassium channel K2P9.1 (TASK-3) Chem Med Chem. 2012;7:123–33. doi: 10.1002/cmdc.201100351. [DOI] [PubMed] [Google Scholar]
- 22.Peukert S, Brendel J, Pirard B, Bruggemann A, Below P, Kleemann HW, Hemmerle H, Schmidt W. Identification, synthesis, and activity of novel blockers of the voltage-gated potassium channel Kv1.5. J Med Chem. 2003;46:486–98. doi: 10.1021/jm0210461. [DOI] [PubMed] [Google Scholar]
- 23.Conway KE, Cotten JF. Covalent modification of a volatile anesthetic regulatory site activates TASK-3 (KCNK9) tandem-pore potassium channels. Mol Pharmacol. 2012;81:393–400. doi: 10.1124/mol.111.076281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–7. [PubMed] [Google Scholar]
- 25.De la Torre JC, Rowed DW. DMSO: a new respiratory stimulant? J Clin Pharmacol. 1974;14:345–53. doi: 10.1002/j.1552-4604.1974.tb01410.x. [DOI] [PubMed] [Google Scholar]
- 26.Middendorf T, Loeschcke HH. Cooperation of peripheral and central chemosensitive mechanisms in the control of the extracellular pH in brain in non-respiratory acidosis. Pflugers Arch. 1978;375:257–60. doi: 10.1007/BF00582439. [DOI] [PubMed] [Google Scholar]
- 27.Lumb AB. Nunn’s Applied Respiratory Physiology. 5. Boston: Butterworth-Heinemann; 2000. [Google Scholar]
- 28.Yamamoto Y, Kummer W, Atoji Y, Suzuki Y. TASK-1, TASK-2, TASK-3 and TRAAK immunoreactivities in the rat carotid body. Brain Res. 2002;950:304–7. doi: 10.1016/s0006-8993(02)03181-5. [DOI] [PubMed] [Google Scholar]
- 29.Fagerlund MJ, Kahlin J, Ebberyd A, Schulte G, Mkrtchian S, Eriksson LI. The human carotid body: expression of oxygen sensing and signaling genes of relevance for anesthesia. Anesthesiology. 2010;113:1270–9. doi: 10.1097/ALN.0b013e3181fac061. [DOI] [PubMed] [Google Scholar]
- 30.Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27:14049–58. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koizumi H, Smerin SE, Yamanishi T, Moorjani BR, Zhang R, Smith JC. TASK channels contribute to the K+-dominated leak current regulating respiratory rhythm generation in vitro. J Neurosci. 2010;30:4273–84. doi: 10.1523/JNEUROSCI.4017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Washburn CP, Bayliss DA, Guyenet PG. Cardiorespiratory neurons of the rat ventrolateral medulla contain TASK-1 and TASK-3 channel mRNA. Respir Physiol Neurobiol. 2003;138:19–35. doi: 10.1016/s1569-9048(03)00185-x. [DOI] [PubMed] [Google Scholar]
- 33.Bayliss DA, Talley EM, Sirois JE, Lei Q. TASK-1 is a highly modulated pH-sensitive ‘leak’ K(+) channel expressed in brainstem respiratory neurons. Respir Physiol. 2001;129:159–74. doi: 10.1016/s0034-5687(01)00288-2. [DOI] [PubMed] [Google Scholar]
- 34.Leonoudakis D, Gray AT, Winegar BD, Kindler CH, Harada M, Taylor DM, Chavez RA, Forsayeth JR, Yost CS. An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum. J Neurosci. 1998;18:868–77. doi: 10.1523/JNEUROSCI.18-03-00868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sapru HN, Krieger AJ. Carotid and aortic chemoreceptor function in the rat. J Appl Physiol. 1977;42:344–8. doi: 10.1152/jappl.1977.42.3.344. [DOI] [PubMed] [Google Scholar]
- 36.Severinghaus J, Ozanne G, Massuda Y. Measurement of the ventilatory response to hypoxia. A step hypoxia three-minute test. Chest. 1976;70:121–4. [PubMed] [Google Scholar]
- 37.Laubie M, Schmitt H. Long-lasting hyperventilation induced by almitrine: evidence for a specific effect on carotid and thoracic chemoreceptors. Eur J Pharmacol. 1980;61:125–36. doi: 10.1016/0014-2999(80)90155-7. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Lopez JR, Perez-Garcia MT, Canet E, Gonzalez C. Effects of almitrine bismesylate on the ionic currents of chemoreceptor cells from the carotid body. Mol Pharmacol. 1998;53:330–9. doi: 10.1124/mol.53.2.330. [DOI] [PubMed] [Google Scholar]
- 39.Lazarenko RM, Fortuna MG, Shi Y, Mulkey DK, Takakura AC, Moreira TS, Guyenet PG, Bayliss DA. Anesthetic activation of central respiratory chemoreceptor neurons involves inhibition of a THIK-1-like background K(+) current. J Neurosci. 2010;30:9324–34. doi: 10.1523/JNEUROSCI.1956-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gestreau C, Heitzmann D, Thomas J, Dubreuil V, Bandulik S, Reichold M, Bendahhou S, Pierson P, Sterner C, Peyronnet-Roux J, Benfriha C, Tegtmeier I, Ehnes H, Georgieff M, Lesage F, Brunet JF, Goridis C, Warth R, Barhanin J. Task2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc Natl Acad Sci U S A. 2010;107:2325–30. doi: 10.1073/pnas.0910059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez-Barneo J, Lopez-Lopez JR, Urena J, Gonzalez C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988;241:580–2. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- 42.Peers C. Hypoxic suppression of K+ currents in type I carotid body cells: selective effect on the Ca2(+)-activated K+ current. Neurosci Lett. 1990;119:253–6. doi: 10.1016/0304-3940(90)90846-2. [DOI] [PubMed] [Google Scholar]
- 43.Linden AM, Aller MI, Leppa E, Vekovischeva O, Aitta-Aho T, Veale EL, Mathie A, Rosenberg P, Wisden W, Korpi ER. The in vivo contributions of TASK-1-containing channels to the actions of inhalation anesthetics, the alpha(2) adrenergic sedative dexmedetomidine, and cannabinoid agonists. J Pharmacol Exp Ther. 2006;317:615–26. doi: 10.1124/jpet.105.098525. [DOI] [PubMed] [Google Scholar]
- 44.Pang DS, Robledo CJ, Carr DR, Gent TC, Vyssotski AL, Caley A, Zecharia AY, Wisden W, Brickley SG, Franks NP. An unexpected role for TASK-3 potassium channels in network oscillations with implications for sleep mechanisms and anesthetic action. Proc Natl Acad Sci U S A. 2009;106:17546–51. doi: 10.1073/pnas.0907228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lazarenko RM, Willcox SC, Shu S, Berg AP, Jevtovic-Todorovic V, Talley EM, Chen X, Bayliss DA. Motoneuronal TASK channels contribute to immobilizing effects of inhalational general anesthetics. J Neurosci. 2010;30:7691–704. doi: 10.1523/JNEUROSCI.1655-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


