Abstract
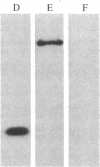
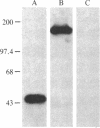
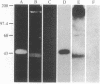
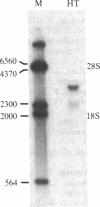
A lambda gt11 expression library containing cDNA inserts prepared from human placental mRNA was screened immunologically using an antibody probe developed against the beta-migrating plasminogen activator inhibitor (beta-PAI) purified from cultured bovine aortic endothelial cells. Thirty-four positive clones were isolated after screening 7 X 10(5) phages. Three clones (lambda 1.2, lambda 3, and lambda 9.2) were randomly picked and further characterized. These contained inserts 1.9, 3.0, and 1.9 kilobases (kb) long, respectively. Escherichia coli lysogenic for lambda 9.2, but not for lambda gt11, produced a fusion protein of 180 kDa that was recognized by affinity-purified antibodies against the bovine aortic endothelial cell beta-PAI and had beta-PAI activity when analyzed by reverse fibrin autography. The largest cDNA insert was sequenced and shown to be 2944 base pairs (bp) long. It has a large 3' untranslated region [1788 bp, excluding the poly(A) tail] and contains the entire coding region of the mature protein but lacks the initiation codon and part of the signal peptide coding region at the 5' terminus. The two clones carrying the 1.9-kb cDNA inserts were partially sequenced and shown to be identical to the 3.0-kb cDNA except that they were truncated, lacking much of the 3' untranslated region. Blot hybridization analysis of electrophoretically fractionated RNA from the human fibrosarcoma cell line HT-1080 was performed using the 3.0-kb cDNA as hybridization probe. Two distinct transcripts, 2.2 and 3.0 kb, were detected, suggesting that the 1.9-kb cDNA may have been copied from the shorter RNA transcript. The amino acid sequence deduced from the cDNA was aligned with the NH2-terminal sequence of the human beta-PAI. Based on this alignment, the mature human beta-PAI is 379 amino acids long and contains an NH2-terminal valine. The deduced amino acid sequence has extensive (30%) homology with alpha 1-antitrypsin and antithrombin III, indicating that the beta-PAI is a member of the serine proteinase inhibitor (serpin) superfamily.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astedt B., Lecander I., Brodin T., Lundblad A., Löw K. Purification of a specific placental plasminogen activator inhibitor by monoclonal antibody and its complex formation with plasminogen activator. Thromb Haemost. 1985 Feb 18;53(1):122–125. [PubMed] [Google Scholar]
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Collen D. On the regulation and control of fibrinolysis. Edward Kowalski Memorial Lecture. Thromb Haemost. 1980 Jun 18;43(2):77–89. [PubMed] [Google Scholar]
- Crabtree G. R., Kant J. A. Organization of the rat gamma-fibrinogen gene: alternative mRNA splice patterns produce the gamma A and gamma B (gamma ') chains of fibrinogen. Cell. 1982 Nov;31(1):159–166. doi: 10.1016/0092-8674(82)90415-9. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Insulin-sepharose: immunoreactivity and use in the purification of antibody. Biochem Biophys Res Commun. 1969 May 22;35(4):531–537. doi: 10.1016/0006-291x(69)90379-9. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Angiotensinogen is related to the antitrypsin-antithrombin-ovalbumin family. Science. 1983 Oct 28;222(4622):417–419. doi: 10.1126/science.6604942. [DOI] [PubMed] [Google Scholar]
- Emeis J. J., van Hinsbergh V. W., Verheijen J. H., Wijngaards G. Inhibition of tissue-type plasminogen activator by conditioned medium from cultured human and porcine vascular endothelial cells. Biochem Biophys Res Commun. 1983 Jan 27;110(2):392–398. doi: 10.1016/0006-291x(83)91161-0. [DOI] [PubMed] [Google Scholar]
- Erickson L. A., Hekman C. M., Loskutoff D. J. The primary plasminogen-activator inhibitors in endothelial cells, platelets, serum, and plasma are immunologically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8710–8714. doi: 10.1073/pnas.82.24.8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson L. A., Lawrence D. A., Loskutoff D. J. Reverse fibrin autography: a method to detect and partially characterize protease inhibitors after sodium dodecyl sulfate--polyacrylamide gel electrophoresis. Anal Biochem. 1984 Mar;137(2):454–463. doi: 10.1016/0003-2697(84)90113-1. [DOI] [PubMed] [Google Scholar]
- Fellous M., Nir U., Wallach D., Merlin G., Rubinstein M., Revel M. Interferon-dependent induction of mRNA for the major histocompatibility antigens in human fibroblasts and lymphoblastoid cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3082–3086. doi: 10.1073/pnas.79.10.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok N. J., Seppänen P. J., Kontula K. K., Jänne P. A., Bardin C. W., Jänne O. A. Two ornithine decarboxylase mRNA species in mouse kidney arise from size heterogeneity at their 3' termini. Proc Natl Acad Sci U S A. 1986 Feb;83(3):594–598. doi: 10.1073/pnas.83.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoal E. G., Wilson E. L., Dowdle E. B. The regulation of tissue plasminogen activator activity by human fibroblasts. Cell. 1983 Aug;34(1):273–279. doi: 10.1016/0092-8674(83)90158-7. [DOI] [PubMed] [Google Scholar]
- Hoylaerts M., Rijken D. C., Lijnen H. R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- King C. R., Piatigorsky J. Alternative RNA splicing of the murine alpha A-crystallin gene: protein-coding information within an intron. Cell. 1983 Mar;32(3):707–712. doi: 10.1016/0092-8674(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Loskutoff D. J., Roegner K., Erickson L. A., Schleef R. R., Huttenlocher A., Coleman P. L., Gelehrter T. D. The dexamethasone-induced inhibitor of plasminogen activator in hepatoma cells is antigenically-related to an inhibitor produced by bovine aortic endothelial cells. Thromb Haemost. 1986 Feb 28;55(1):8–11. [PubMed] [Google Scholar]
- Loskutoff D. J., van Mourik J. A., Erickson L. A., Lawrence D. Detection of an unusually stable fibrinolytic inhibitor produced by bovine endothelial cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2956–2960. doi: 10.1073/pnas.80.10.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lämmle B., Berrettini M., Schwarz H. P., Heeb M. J., Griffin J. H. Quantitative immunoblotting assay of blood coagulation factor XII. Thromb Res. 1986 Mar 15;41(6):747–759. doi: 10.1016/0049-3848(86)90373-7. [DOI] [PubMed] [Google Scholar]
- Marshall R. D. The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem Soc Symp. 1974;(40):17–26. [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Millán J. L. Molecular cloning and sequence analysis of human placental alkaline phosphatase. J Biol Chem. 1986 Mar 5;261(7):3112–3115. [PubMed] [Google Scholar]
- Ny T., Bjersing L., Hsueh A. J., Loskutoff D. J. Cultured granulosa cells produce two plasminogen activators and an antiactivator, each regulated differently by gonadotropins. Endocrinology. 1985 Apr;116(4):1666–1668. doi: 10.1210/endo-116-4-1666. [DOI] [PubMed] [Google Scholar]
- Ohkubo H., Kageyama R., Ujihara M., Hirose T., Inayama S., Nakanishi S. Cloning and sequence analysis of cDNA for rat angiotensinogen. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2196–2200. doi: 10.1073/pnas.80.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawdey M., Ny T., Loskutoff D. J. Messenger RNA for plasminogen activator inhibitor. Thromb Res. 1986 Jan 15;41(2):151–160. doi: 10.1016/0049-3848(86)90225-2. [DOI] [PubMed] [Google Scholar]
- Schleef R. R., Sinha M., Loskutoff D. J. Immunoradiometric assay to measure the binding of a specific inhibitor to tissue-type plasminogen activator. J Lab Clin Med. 1985 Oct;106(4):408–415. [PubMed] [Google Scholar]
- Scott R. W., Bergman B. L., Bajpai A., Hersh R. T., Rodriguez H., Jones B. N., Barreda C., Watts S., Baker J. B. Protease nexin. Properties and a modified purification procedure. J Biol Chem. 1985 Jun 10;260(11):7029–7034. [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen S., Philips M. Isolation of tissue-type plasminogen activator-inhibitor complexes from human plasma. Evidence for a rapid plasminogen activator inhibitor. Biochim Biophys Acta. 1984 Nov 6;802(1):111–118. doi: 10.1016/0304-4165(84)90040-0. [DOI] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Baccino D., Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J Cell Biol. 1985 Jan;100(1):86–92. doi: 10.1083/jcb.100.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- van Mourik J. A., Lawrence D. A., Loskutoff D. J. Purification of an inhibitor of plasminogen activator (antiactivator) synthesized by endothelial cells. J Biol Chem. 1984 Dec 10;259(23):14914–14921. [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]