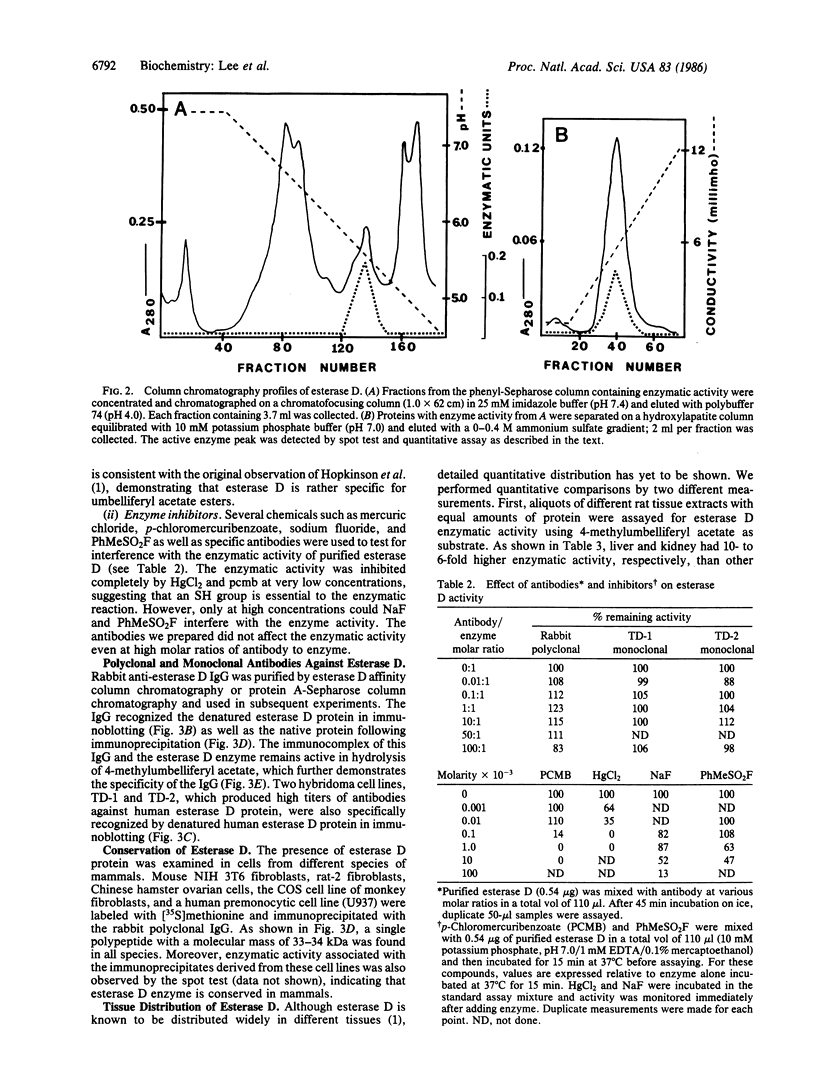
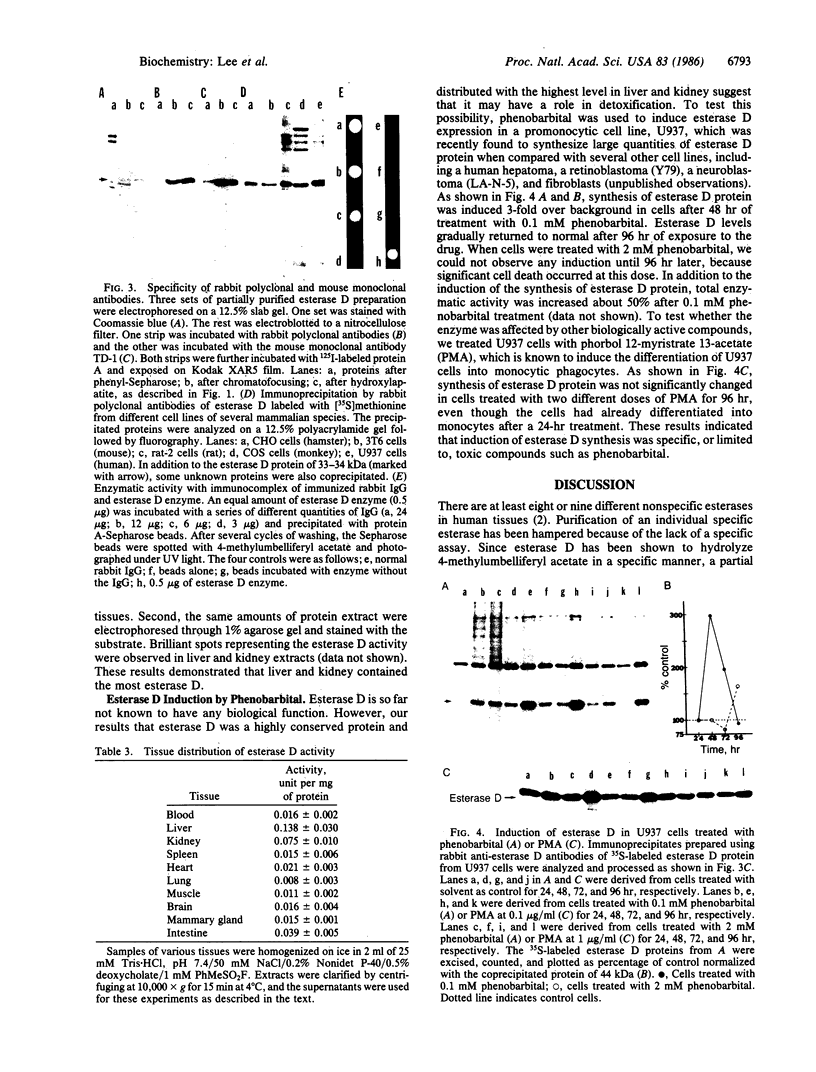
Abstract
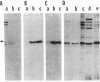
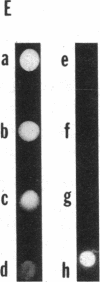
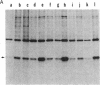
Human esterase D (carboxylesterase; carboxylic-ester hydrolase, EC 3.1.1.1), a genetic marker of retinoblastoma, was purified to biochemical homogeneity from erythrocytes. The purification scheme including carboxymethylcellulose, phenyl-Sepharose, chromatofocusing, and hydroxylapatite chromatographies resulted in a 10,000-fold purification of the enzyme with 15% recovery of total activity. The Km of esterase D was estimated to be 10 X 10(-6) M using 4-methylumbelliferyl acetate as substrate. The enzymatic activity was inhibited by p-chloromercuribenzoate and HgCl2, suggesting an important role of SH group(s) in enzyme function. Specific rabbit polyclonal and mouse monoclonal antibodies against esterase D were prepared and recognized either denatured or native human esterase D protein. Moreover, the polyclonal antibodies immunoprecipitated a polypeptide with a molecular mass of about 33-34 kDa from various cell lines of different mammalian species, indicating that the esterase D protein is highly conserved. The highest levels of this enzyme were found in liver and kidney. Furthermore, the expression of esterase D was enhanced 3-fold in a promonocytic cell line treated with phenobarbital but not with phorbol myristate acetate, suggesting that esterase D may have a role in detoxification. The availability of the homogeneous protein and its specific antibodies allows for cloning of the esterase D gene and facilitates studies of retinoblastomas.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen S., Creagan R. P., Nichols E. A., Ruddle F. H. Assignment of human esterase-D gene to chromosome 13. Birth Defects Orig Artic Ser. 1975;11(3):99–102. [PubMed] [Google Scholar]
- Coates P. M., Edwards Y. H., Hopkinson D. A. Purification and properties of an esterase B4 from human liver. Eur J Biochem. 1976 Jan 15;61(2):331–335. doi: 10.1111/j.1432-1033.1976.tb10026.x. [DOI] [PubMed] [Google Scholar]
- Coates P. M., Mestriner M. A., Hopkinson D. A. A preliminary genetic interpretation of the esterase isozymes of human tissues. Ann Hum Genet. 1975 Jul;39(1):1–20. doi: 10.1111/j.1469-1809.1975.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Frydman M., Bonné-Tamir B., Farrer L. A., Conneally P. M., Magazanik A., Ashbel S., Goldwitch Z. Assignment of the gene for Wilson disease to chromosome 13: linkage to the esterase D locus. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1819–1821. doi: 10.1073/pnas.82.6.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout R., Dryja T. P., Squire J., Gallie B. L., Phillips R. A. Somatic inactivation of genes on chromosome 13 is a common event in retinoblastoma. Nature. 1983 Aug 4;304(5925):451–453. doi: 10.1038/304451a0. [DOI] [PubMed] [Google Scholar]
- Hopkinson D. A., Mestriner M. A., Cortner J., Harris H. Esterase D: a new human polymorphism. Ann Hum Genet. 1973 Oct;37(2):119–137. doi: 10.1111/j.1469-1809.1973.tb01820.x. [DOI] [PubMed] [Google Scholar]
- Huang C. M., Parsons M., Oi V. T., Huang H. J., Herzenberg L. A. Genetic characterization of mouse immunoglobulin allotypic determinants (allotopes) defined by monoclonal antibodies. Immunogenetics. 1983;18(4):311–321. doi: 10.1007/BF00372464. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Yoshida Y., Enomoto K., Kaku T., Hirata K., Onoé T. Induction of a microsomal butyrylesterase in rat liver by phenobarbital treatment. Biochim Biophys Acta. 1979 Jan 18;582(2):185–195. doi: 10.1016/0304-4165(79)90383-0. [DOI] [PubMed] [Google Scholar]
- Köster B., Leupold H., Mauff G. Esterase D polymorphism: high-voltage agarose-gel electrophoresis and distribution of phenotypes in different European populations. Humangenetik. 1975 May 26;28(1):75–78. doi: 10.1007/BF00272486. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Lee W. H. Molecular cloning of the human esterase D gene, a genetic marker of retinoblastoma. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6337–6341. doi: 10.1073/pnas.83.17.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. H., Bister K., Moscovici C., Duesberg P. H. Temperature-sensitive mutants of Fujinami sarcoma virus: tumorigenicity and reversible phosphorylation of the transforming p140 protein. J Virol. 1981 Jun;38(3):1064–1076. doi: 10.1128/jvi.38.3.1064-1076.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftell M., Berzins K., Blomberg F. Immunochemical studies on a phenobarbital-inducible esterase in rat liver microsomes. Arch Biochem Biophys. 1977 Jun;181(2):534–541. doi: 10.1016/0003-9861(77)90260-0. [DOI] [PubMed] [Google Scholar]
- Scott E. M. Purification of red cell enzymes by treatment with n-butanol and chloroform. Prep Biochem. 1976;6(2-3):147–152. doi: 10.1080/00327487608061609. [DOI] [PubMed] [Google Scholar]
- Scott E. M., Wright R. C. Purification and substrate specificity of polymorphic forms of esterase D from human erythrocytes. Am J Hum Genet. 1978 Jan;30(1):14–18. [PMC free article] [PubMed] [Google Scholar]
- Sparkes R. S., Murphree A. L., Lingua R. W., Sparkes M. C., Field L. L., Funderburk S. J., Benedict W. F. Gene for hereditary retinoblastoma assigned to human chromosome 13 by linkage to esterase D. Science. 1983 Feb 25;219(4587):971–973. doi: 10.1126/science.6823558. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Sparkes M. C., Wilson M. G., Towner J. W., Benedict W., Murphree A. L., Yunis J. J. Regional assignment of genes for human esterase D and retinoblastoma to chromosome band 13q14. Science. 1980 May 30;208(4447):1042–1044. doi: 10.1126/science.7375916. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A., Muchmore E., Diaz S. A sialic acid-specific O-acetylesterase in human erythrocytes: possible identity with esterase D, the genetic marker of retinoblastomas and Wilson disease. Proc Natl Acad Sci U S A. 1986 Feb;83(4):882–886. doi: 10.1073/pnas.83.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P., Packman S., Loughman W., Sparkes M., Sparkes R., McMahon A., Gregory T., Ablin A. Location of the retinoblastoma susceptibility gene(s) and the human esterase D locus. J Med Genet. 1984 Apr;21(2):92–95. doi: 10.1136/jmg.21.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis J. J., Ramsay N. Retinoblastoma and subband deletion of chromosome 13. Am J Dis Child. 1978 Feb;132(2):161–163. doi: 10.1001/archpedi.1978.02120270059012. [DOI] [PubMed] [Google Scholar]