Abstract
Background
The ever-expanding range of dermal filler products for aesthetic soft tissue augmentation is of benefit for patients and physicians, but as indications and the number of procedures performed increase, the number of complications will likely also increase.
Objective
To describe potential adverse events associated with dermal fillers and to provide structured and clear guidance on their treatment and avoidance.
Methods
Reports of dermal filler complications in the medical literature were reviewed and, based on the publications retrieved and the authors’ extensive experience, recommendations for avoiding and managing complications are provided.
Results
Different dermal fillers have widely varying properties, associated risks, and injection requirements. All dermal fillers have the potential to cause complications. Most are related to volume and technique, though some are associated with the material itself. The majority of adverse reactions are mild and transient, such as bruising and trauma-related edema. Serious adverse events are rare, and most are avoidable with proper planning and technique.
Conclusion
For optimum outcomes, aesthetic physicians should have a detailed understanding of facial anatomy; the individual characteristics of available fillers; their indications, contraindications, benefits, and drawbacks; and ways to prevent and avoid potential complications.
Keywords: aesthetic medicine, complications
Introduction
The popularity of dermal fillers has grown rapidly in recent years because they offer the rejuvenative and enhancing aesthetic improvements previously only achievable with surgery, but at lower cost and with limited-to-no recovery time. According to data from the American Society for Aesthetic Plastic Surgery (ASAPS), more than 1.6 million dermal filler treatments were performed in 2011, making them the second most popular nonsurgical cosmetic procedure performed in the USA after neuromodulators; the latter procedure is frequently performed in concert with dermal filler injections.1
As public awareness and acceptance of dermal fillers grows, so does the size of the market, with an estimated 160 products currently available worldwide from more than 50 companies. Their main indications are the filling of rhytides and folds, and correction of soft tissue loss due to disease or age.2 Increasingly, fillers are used for volume replacement and enhancement procedures,3 including cheek and chin augmentation, tear trough correction, nose reshaping, midfacial volumization, lip enhancement, hand rejuvenation, and the correction of facial asymmetry. As the indications and the number of procedures performed increase, the number of complications will likely also increase.
Understanding the different characteristics, capabilities, injection techniques, risks, and limitations of available fillers is essential for injectors to reduce the risk of complications, improve patient outcomes, and care for patients who have experienced adverse events. This requires expert familiarity with the properties and potential complications of a wide range of products, including those that are not available in the injector’s country of practice, as patients may present with adverse reactions to fillers that were injected abroad. Particularly important is how the incidence of local adverse events following treatment is related to the injection technique versus the chemical composition of the dermal filler.4 This review will provide physicians with a background to the etiology of filler-related complications, and structured and clear guidance on their treatment and avoidance.
Categories of dermal filler
Several methods of categorizing dermal fillers exist, but for a discussion of dermal filler complications it is perhaps most useful to categorize in terms of biodegradable (moderate and long duration) versus nonbiodegradable fillers, and in terms of particulate versus nonparticulate fillers (Table 1). Moderate duration biodegradable fillers, such as collagen and the hyaluronic acid (HA) fillers, are reabsorbed by the body quite quickly, so their cosmetic effects are relatively short-lived. HA derivatives are the most widely used biodegradable fillers in both Europe and the USA,1,5 and generally have effects lasting 6–18 months depending on the source and extent of cross-linking and concentration and particle size of each product.6 HAs are linear polymeric dimers of N-acetyl glucosamine and glucuronic acid, which differ in the proprietary methods used to cross-link their dimers, their degree and method of chain cross-linking, the uniformity and size of their particles, and their concentration. These characteristics all have a significant impact on the clinical effect of these products. Increased cross-linking and concentration increase the viscosity and elasticity as well as the resistance to degradation by native hyaluronidase. The hydrophilic nature of HA means that the more concentrated and/or large particle products will tend to absorb more water, and thus produce more tissue swelling after injection. HA products are also characterized by the size of their microspheres. Biphasic fillers, such as Restylane®, Perlane®, and Macrolane® (all Q-MED, Uppsala, Sweden), contain a range of microsphere sizes. Monophasic HA products, such as Juvederm® (Allergan, Irvine, CA, USA), Belotero® (Merz Pharmaceuticals GmbH, Frankfurt, Germany), Teosyal® (Clarion Medical Technologies, Cambridge, ON, Canada), Prevelle Silk® (Mentor Worldwide LLC, Santa Barbara, CA, USA), and Varioderm™ (Adoderm, Langenfeld, Germany), contain homogeneous microspheres and are the preferred HA of most other companies. The different HAs have varying degrees of hardness (G’), which will influence their suitability for a particular procedure. In general, the greater the G’ of the product, the deeper it should be injected. It should be noted that while more concentrated products with a greater degree of cross-linking have a longer duration of effect, they also increase reactivity in the body and thus the risk of inflammation and granuloma formation.
Table 1.
Characteristics of commonly used fillers and their indications
| Brand name | Manufacturer | Key component | Specific uses | Duration of results |
|---|---|---|---|---|
| Biodegradable products | ||||
| Restylane | Q-Med/Medicis | HA gel (nonanimal source) | Creases, wrinkles, scars and lip enhancement; 100,000 particles per mL. | 6 months on average, with retreatment every 6–9 months |
| Restylane Fine lines/Restylane Touch |
Q-Med/Medicis | HA gel (nonanimal source) | Smaller gel particle size (vs Restylane) used to correct thin superficial lines; 500,000 particles per mL. | As above |
| Restylane Perlane | Q-Med/Medicis | HA gel (nonanimal source) | Deeper dermal filler with a larger gel particle size (compared to Restylane) used to fill deeper creases and more prominent facial lines; 10,000 particles per mL. | As above |
| Captique | Genzyme/Inamed/AllerganMentor | HA gel (nonanimal source) | Medium-depth facial lines. | Average treatment lasts about 4 months |
| Juvederm Ultra (2, 3, and 4) and Juvederm Ultra XC | Inamed/Allergan | HA gel (nonanimal source) | Contouring and volumizing facial wrinkles and folds. Juvederm Ultra 2 erases moderate lines (around lips, eyes). Juvederm Ultra 3 smooths wrinkles between nose and corner of mouth. Juvederm Ultra 4 is for severe folds and lines and for facial contouring. Juvederm Ultra XC is formulated with lidocaine. | Lasts 1 year |
| Juvederm Ultra Plus and Juvederm Ultra Plus XC |
Inamed/Allergan | HA gel (nonanimal source) | More highly cross-linked formulation for generalized facial volume enhancement and correcting deeper folds and wrinkles. | Lasts up to 18 months |
| Juvederm voluma | Inamed/Allergan | HA gel (nonanimal source) | Very high viscosity gel for restoring lost facial volume; eg, in cheeks. | Lasts 12–18 months |
| Belotero Soft | Merz Pharmaceuticals | HA gel (nonanimal source) | For fine superficial folds, including crow’s feet and perioral lines. | Lasts up to 12 months |
| Belotero Basic | Merz Pharmaceuticals | HA gel (nonanimal source) | For moderate to deep folds and lip contouring. | Lasts up to 12 months |
| Belotero Intense | Merz Pharmaceuticals | HA gel (nonanimal source) | For deep folds and lip and volume augmentation. | Lasts up to 12 months |
| Varioderm | Adoderm GmbH/Medical Aesthetics group | HA gel (nonanimal source) | Range of products for superficial lines to deep creases. Highly cross-linked. | Lasts 6–16 months |
| Emervel Touch | Galderma | HA gel (nonanimal source) | For superficial wrinkles such as perioral lines and rhytides. | Lasts 6–12 months |
| Emervel Classic | Galderma | HA gel (nonanimal source) | For moderate to deep wrinkles; for example, moderate nasolabial folds. | Lasts 6–12 months |
| Emervel Deep | Galderma | HA gel (nonanimal source) | For moderate to deep wrinkles (eg, deep nasolabial folds). | Lasts 6–12 months |
| Emervel Volume | Galderma | HA gel (nonanimal source) | For facial contouring (eg, cheeks and chin). | Lasts 6–12 months |
| Teosyal range (Deep Lines, Global Action, Ultra Deep, First Lines, Meso, Touch Up, Ultimate) | Lifestyle Aesthetics | HA gel (nonanimal source) | Teosyal® range consists of different formulations which restore face volume and repair cutaneous depressions. | Lasts 6–9 months |
| Cosmoderm | Inamed corporation | Human collagen | Fine lines (around the nose, mouth, and frown area) and acne scars. | Lasts 3 months |
| Cosmoplast | Inamed corporation | Human collagen | Deeper lines and furrows. | Lasts 3 months |
| Zyderm | Inamed corporation | Purified bovine collagen with 0.3% lidocaine | Zyderm 1 used for fine lines, wrinkles, and shallow scars. Zyderm 2 used for moderate lines, wrinkles, and scars. |
Lasts 3 months |
| Zyplast | Inamed corporation | Purified bovine collagen with 0.3% lidocaine | Deeper lines, wrinkles, and scars. | Lasts 3 months. The collagen fillers are not available in the United States |
| Biodegradable collagen stimulator products | ||||
| Sculptra | Dermik aesthetic | Poly-L-lactic acid | Addresses lines and wrinkles and adds volume. | Lasts 1–2 years, effects develop gradually |
| Radiesse | Merz Pharmaceuticals | Calcium hydroxyl-lapatite microspheres suspended in a carrier gel | Moderate to severe facial folds and wrinkles. | At least 1 year; average 12–15 months |
| Nonbiodegradable products | ||||
| Artecoll | Rofil medical | Contains PMMA enclosed in a solution of 3.5% collagen and 0.3% lidocaine | Deeper wrinkles: collagen is absorbed, leaving PMMA to stimulate new collagen. | 1 year, but may be permanent |
| Aquamid | Contura | 97.5% sterile water and 2.5% polyacrylamide | Facial volume restoration and contouring. | At least 5 years |
Abbreviations: HA, hyaluronic acid; PMMA, polymethylmethacrylate microspheres; vs, versus.
Fillers with biodegradable particles that stimulate the body to produce its own collagen have a longer duration of effect; such products include calcium hydroxylapatite (CaHA; Radiesse®; Merz Pharmaceuticals GmbH) and poly-L-lactic acid (PLLA; Sculptra®; Valeant, West Laval, QC, Canada). CaHA consists of synthetic CaHA microspheres suspended in a carrier gel. Injection provides immediate visual improvement with long-term deposition of new collagen surrounding the microspheres, which contributes to an average duration of effect of around 15 months.7 PLLA is a synthetic polymer that provides soft tissue augmentation through stimulation of an inflammatory tissue response with subsequent collagen deposition.8 Each injection session with PLLA produces a gradual treatment effect and limited correction. Three injection sessions are generally required, but once the final correction is achieved, results last up to 2 years.
The nonbiodegradable fillers provoke a foreign body reaction that stimulates a fibroblastic deposition of collagen around the nonabsorbable microspheres.9 Products in this category include polymethylmethacrylate (PMMA; Artecoll®; Rofil Medical International B.V., Breda, the Netherlands), the polyacrylamide hydrogel Aquamid® (Contura International, Soeborg, Denmark), and Silikon® 1000 (Alcon Laboratories, Inc., Fort Worth, TX, USA), a medical-grade pure form of silicone. PMMA consists of 80% bovine dermal collagen plus 20% PMMA microspheres. The collagen vehicle is degraded within 1–3 months, leaving the microspheres encapsulated by a fine fibrous capsule. Aquamid® is a hydrophilic polyacrylamide gel composed of 97.5% sterile water bound to 2.5% cross-linked acrylamide polymer. A continuous exchange of fluid occurs between the hydrogel and surrounding tissue, which becomes integrated in the soft tissue.10 Silikon® 1000 is injected in very small quantities using a microdroplet technique. Similar to the other permanent fillers, the body forms collagen around the silicone particles. The permanent nature of the nonbiodegradable fillers can make their complications more long-lasting and difficult to treat.
Dermal filler complications: avoidance and treatment
All fillers are associated with a risk of both short-duration and long-duration complications. Expanded indications and number of treatments performed will increase the number and spectrum of adverse events. While most adverse reactions are mild and transient, more serious adverse events can occur, leaving patients with long-lasting or permanent functional and aesthetic deficits. Some reactions occur immediately after treatment, whereas some have a delayed onset. The types of adverse event by time of onset are illustrated in Table 2.
Table 2.
Types of dermal filler complication by onset of adverse event
| Early events (occurring up to several days post-treatment) | Delayed events (occurring from weeks to years post-treatment) |
|---|---|
| Injection site reactions | Infection (atypical; eg, mycobacterial) |
| Erythema | Erythema |
| Edema | Edema |
| Pain/tenderness | Pain/tenderness |
| Bruising | Nodule/abscess |
| Itching | Systemic responses to infection Biofilm |
| Infection | Foreign body granuloma |
| Erythema | Varying from subclinical histologic changes to disfiguring nodules |
| Edema | |
| Pain/tenderness | |
| Acne papule formation | |
| Nodule/abscess | |
| Hypersensitivity | Migration of implant material |
| Erythema | |
| Edema | |
| Pain/tenderness | |
| Nonfluctuant nodules | |
| Lumps, asymmetries, contour irregularities caused by technique and placement errors | Immune reactions |
| Local and site of injection and generalized | |
| Skin discoloration | Persistent discoloration |
| Redness | Persistent scarring |
| Whiteness | |
| Hyperpigmentation | |
| Local tissue necrosis caused by vascular occlusion | Malar edema |
Notes: Reproduced with permission. Copyright © 2006, John Wiley and Sons. Lowe NJ, Maxwell CA, Patnaik R. Adverse reactions to dermal fillers: review. Dermatol Surg. 2005;31(11 Pt 2):1616–1625.70
Bruising
All dermal fillers have the potential to cause bruising (Figure 1). Bruising is observed more frequently after injection into the dermal and immediate subdermal planes using fanning and threading techniques.11 Less bruising is seen when materials are injected using the depot technique at the preperiosteal level. Bruising is treated with cold compresses after the procedure and vitamin K cream.12 For persistent staining, treatment with pulsed dye light (Vbeam®; Syneron Candela, Irvine, CA, USA) or potassium titanyl phosphate (KTP) lasers may be effective; the light emitted from these is more precisely absorbed by hemoglobin than other colors.
Figure 1.
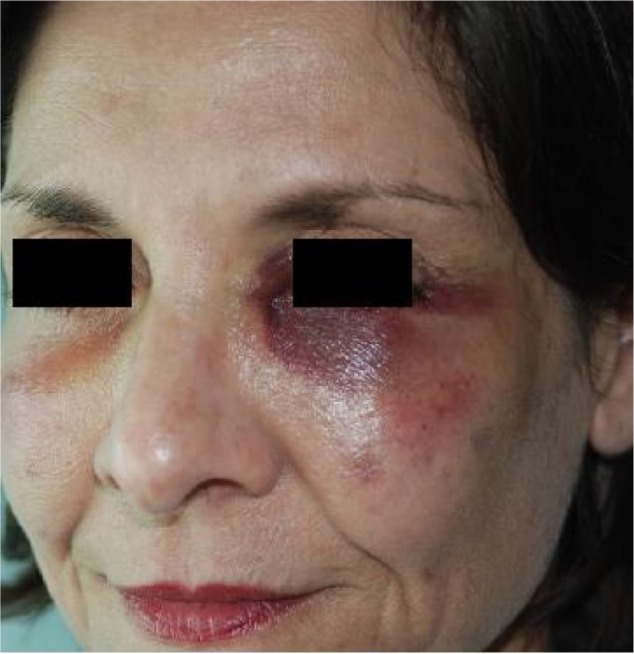
Bruising may be immediate or worsen over 3 days.
A number of steps can be taken to minimize bruising, including avoiding all blood-thinning medications starting 1 week prior to the procedure (aspirin, warfarin, dipyridamole, clopidogrel, nonsteroidal anti-inflammatory drugs [NSAIDs], fish oil, vitamin E supplements, St John’s Wort, garlic tablets, gingko biloba, and ginseng).13 Patients should be advised to stay out of the sun as long as bruising persists, and vigorous exercise should be avoided for the first 24 hours to avoid raising blood pressure. Fillers that incorporate lidocaine and epinephrine (adrenaline) may reduce the amount of postinjection bruising; the former improves the comfort of injection while the latter inhibits activation of eosinophils that play a role in bruising and cause vasoconstriction as the procedure proceeds. The patient’s head should be elevated throughout the procedure and remain so for 24 hours. Bruising can be further limited by use of the smallest gauge needle that can deliver the filler, a slow injection technique with small aliquots of product, use of blunt cannulas,14 and limiting the number of transcutaneous puncture sites.
Edema
Short-term post-traumatic edema
Some transient swelling in the immediate postprocedural period is normal and occurs with all dermal fillers. This type of edema occurs very shortly after injection and is related to injection volume and technique. Treatment and avoidance is as for bruising. The majority of cases of postinjection, trauma-related edema dissipate within 1 week.
Antibody-mediated edema (angioedema)
Dermal fillers are essentially foreign bodies, and some patients may develop hypersensitivity to injected products due to an immunoglobulin E (IgE)-mediated immune response (Type I hypersensitivity reaction). This may occur after initial or repeated exposure. IgE stimulates mast cells to degranulate, releasing proteases, heparin, histamine, cytokines, prostaglandins, leukotrienes, and platelet-activating factor, which result in the edema, erythema, pain, and itching characteristic of an allergic response. Angioedema occurs within hours of exposure. Reactions can be severe and can last for several weeks.15 Edema may be confined to the injection sites, but may also be more generalized. An acute idiopathic allergic response can also occur in which no allergen can be identified; the reaction may be localized, or there may be acute generalized facial edema (Figure 2).
Figure 2.
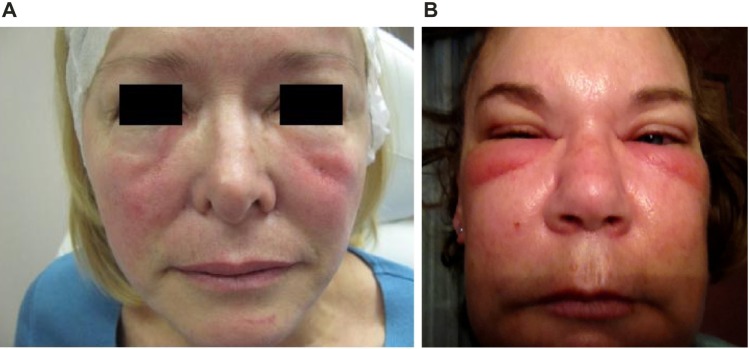
Acute generalized facial edema.
Treatment of angioedema, whether of known cause or idiopathic, depends on the severity of the condition. In many cases, the swelling resolves spontaneously after a few hours or days. If mast-cell mediated, the swelling is short lived and responsive to antihistamines. For persistent edema or edema not responsive to antihistamines, oral prednisone is the mainstay of treatment (Table S1). The patient should be closely monitored to make sure the edema is not a result of an infectious etiology. Rapidly progressing angioedema is treated as a medical emergency because of the risk of airway obstruction.
Chronic angioedema refers to episodes that last more than 6 weeks. These cases are often difficult to treat and have a variable response to medication. Table S1 highlights the treatment steps recommended. Each step is added to the previous one if an inadequate response is obtained. Edema should be controlled with the smallest dose of oral steroids that is effective. One of the authors (DF) has had success in reducing the steroid dose with concurrent use of the leukotriene receptor antagonist montelukast (Singulair® 10 mg per day [Merck & Co., Inc., Whitehouse Station, NJ, USA]). This agent binds with high affinity and specificity to the cysteinyl leukotriene binding sites.
Nonantibody-mediated (delayed) edema
Delayed hypersensitivity reactions are characterized by induration, erythema, and edema, and are mediated by T lymphocytes rather than antibodies. They typically occur 1 day after injection, but may be seen as late as several weeks after injection and may persist for many months.16,17 Delayed hypersensitivity reactions are nonresponsive to antihistamines. The allergen should be removed (Table S1). In the case of HA, this will involve treatment with hyaluronidase. Other fillers may require treatment with steroids until the filler resorbs, laser treatment, and/or extrusion.18 Excision is a last resort.
Symptoms should be controlled with the lowest possible dose of oral steroids (prednisone).
Malar edema
Malar edema is a serious complication that has been reported with all fillers when injected into the infraorbital hollow and tear troughs (Figure 3).19 In a retrospective study of 51 patients who received placement of an HA gel to the infraorbital hollows, 12 patients had prolonged periorbital edema, which lasted an average of 5.4 months.20 Edema was defined as a clinically significant degree of swelling that lasted ≥1 month following injection.
Figure 3.
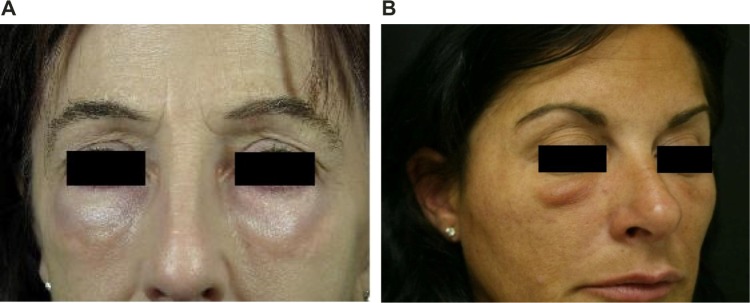
Malar edema.
The infraorbital hollow is the area bounded by the medial canthus medially, the lateral canthus laterally, and the inferior border of the orbicularis oculi. This region is particularly susceptible to adverse events. Malar edema after dermal filler injection arises because the malar septum, a band of connective tissue, divides the superficial suborbicularis oculi fat into a superficial and deep compartment. The superficial compartment has compromised lymphatic drainage, while the lymphatic drainage of the deep compartment is contiguous with the cheek drainage (Figure 4).21 Injection of fillers superficial to the malar septum may augment the impermeable barrier of the malar septum, further impeding lymphatic drainage and resulting in fluid accumulation and malar edema.19 Fillers injected superficial or deep to the malar septum may also cause edema by direct pressure on the lymphatics when injection volumes are too large. In addition, as the viscosity or G’ of the filler increases, the lifting force also increases, and the lymphatics may be more compressed. Some patients demonstrate malar edema without filler treatment, demonstrating existing lymphatic compromise. Malar edema is likely related to the volume of injectate, its depth of injection, the physical qualities of the injectate, and the patient’s degree of pre-existing lymphatic compromise.
Figure 4.
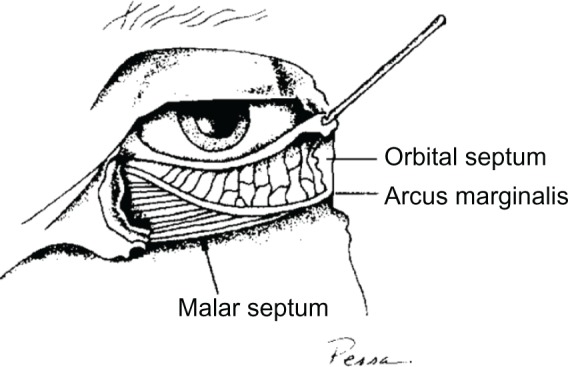
The anatomic basis of malar edema.
Notes: Pessa JE, Garza JR., Aesthet Surg J. 17(1):11–17. Copyright © 1997 by MOSBY, INC. Reprinted by Permission of SAGE Publications.21
Malar edema is long lasting and responds poorly to treatment. Initial measures include head elevation, cold compresses, manual compression multiple times daily, and a methylprednisolone (Medrol® Dosepak; Pfizer Inc., New York, NY, USA) dose pack (Table S2). Intralesional hyaluronidase treatment should be given to patients injected with HAs. The incidence of malar edema can be reduced by proper patient and filler selection, limiting filler volume, and by placing filler material deep to the malar septum at the immediate preperiosteal level.19 The authors strongly recommend the use of an HA when treating the infraorbital hollow so that hyaluronidase may be used to dissolve the material if adverse events occur. A filler of low elasticity and viscosity is preferred, as this limits lymphatic compression. In addition, a filler may be placed at the immediate subcutaneous level superficial to the area prone to lymphatic obstruction. A material such as Belotero® Balance or Soft that does not cause a Tyndall effect when injected superficially should be used in small quantities. Superficial injection, if properly placed and limited in volume, should not result in malar edema. Persistent malar edema should be distinguished from overcorrection.
Skin discoloration
Erythema
Immediately after injection, some skin redness is normal (Figure 5). If erythema persists for more than a few days, a hypersensitivity reaction is likely. Treatments for rosacea may be effective, including oral tetracycline or isotretinoin. A medium-strength topical steroid is advocated for persistent erythema. Long-term use of high-potency steroids should be avoided, as they may cause atrophy and telangiectasias. Lasers can be effective for the treatment of telangiectasias and erythema (for details of laser treatment, see Bruising section). Vitamin K cream is useful in accelerating resolution of erythema in addition to facial bruising.22 Patients with rosacea have a higher risk of developing postinjection erythema and should be warned of this prior to beginning the procedure.
Figure 5.
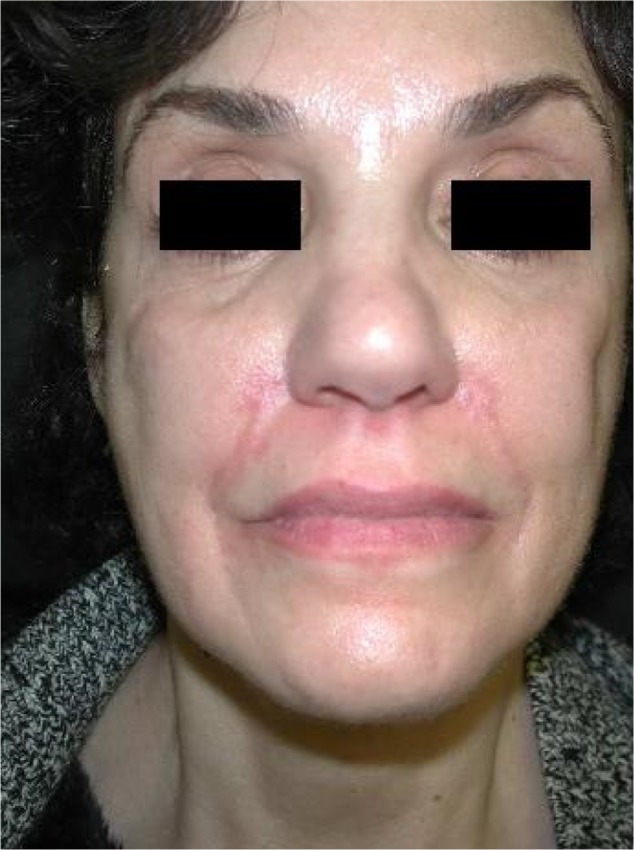
Erythema at site of injection.
Neovascularization
New capillaries, arterioles, and venules may occur at the site of dermal filler injection. These tiny vessels can appear days or weeks after the procedure, but should fade within 3–12 months without further treatment. They are caused by tissue trauma as a result of tissue expansion by the product and/or by excessive molding and massage of the product. Lasers shown to be effective in the treatment of telangiectasias include the 532-nm KTP, the 532-nm diode copper vapor, the 585-nm pulsed dye lasers, and intense pulsed light (IPL). The laser of choice will depend on how large the vessel is (for details of laser treatment, see Bruising section).
Hyperpigmentation
ASAPS 2011 statistics indicate that racial and ethnic minorities constituted 21% of all cosmetic procedures.1 The skin of patients of color has a tendency to hyperpigment following trauma, and dermal filler procedures are a common cause of postinflammatory hyperpigmentation in individuals with Fitzpatrick Skin Types IV–VI,23,24 although postinjection hyperpigmentation can also be seen in other skin types (Figure 6). If persistent hyperpigmentation develops following dermal filler injection, first-line treatment should be with a bleaching agent such as topical hydroquinone (2%–8%) and Retin-A (tretinoin) combined with daily full-spectrum sunscreen application. Chemical peels may also be used to treat resistant postinflammatory hyperpigmentation. If unsuccessful, the next step is treatment with IPL, a pulsed dye laser or fractional laser; the low fluence Q-switched Nd:YAG 1,064 nm laser is also effective. Current lasers have limited use in the treatment of Fitzpatrick skin types IV–VI, but the long wavelength Nd:YAG laser allows darker skin to be treated without disrupting natural skin color, and IPL systems can treat Fitzpatrick skin types I–IV. Limiting the number of skin punctures during the injection process by using the linear threading or fanning technique or injecting at the preperiosteal level may reduce postinjection erythema and therefore postinflammatory hyperpigmentation.
Figure 6.
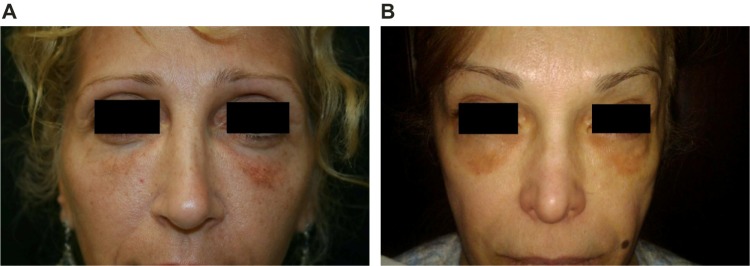
Dyschromia and visible material.
Dyspigmentation
When particulate HA fillers are inappropriately implanted into the superficial dermis or epidermis, a bluish hue may occur as a result of the Tyndall effect (scattering of light by particles in suspension). Blue light waves have a higher frequency than red and are more easily scattered, so that when a ray of light hits the skin’s surface, it is reflected in many different directions, with blue becoming the prominent color that emerges. This effect can occur with all particulate HA fillers, but does not appear to be caused by Belotero®, a monophasic polydensified nonparticulate gel.25 The more superficial the placement of material, the longer the duration of the discoloration. Hyaluronidase should be the initial approach to treatment. For HAs that are less susceptible to hyaluronidase because of a high degree of cross-linking or large particle size, multiple treatments may be necessary. As a last resort, dyspigmentation can be treated by nicking the skin with a small-gauge needle (30 gauge) or surgical scalpel (#11 blade) and expressing the superficial, unwanted dermal filler.26,27 This procedure can be performed immediately, or as long as 12 months or more after placement.27
Infection
As with any procedure that breaks the surface of the skin, dermal filler injections are associated with a risk of infection. To minimize this risk, sterilize the injection site with an effective topical disinfectant, carefully remove the needle and syringe from sterilized individual packaging, wear gloves throughout the procedure, and ensure that the needle is not contaminated during the procedure.13 Do not wipe excessive filler material from the needle tip with nonsterile gauze; residual amounts of material should be flicked off the needle. Although rare, resistant postfiller infections can occur, and may be bacterial, fungal, or viral. Virulent late infections (biofilms) can also occur.
Erysipelas and phlegmon
Erysipelas and phlegmon are a diffuse inflammation of the skin or connective tissue due to infection. The responsible organisms are usually Staphylococcus aureus or Streptococcus pyogenes, but the presentation of a new lesion more than 2 weeks postprocedure may be suggestive of an atypical infection. Differential diagnosis includes delayed hypersensitivity reactions, which also cause erythema, but with the latter there is usually pruritus and an absence of fever. If untreated, the conditions may lead to sepsis, particularly in elderly people and those with diabetes or other illnesses that alter the immune system. Mild forms may be treated with oral antibiotics, while more serious cases require intravenous antibiotics and hospitalization. Antibiotics with activity against S. aureus are necessary, such as cephalexin, dicloxacillin, or nafcillin. To avoid spreading infection, the area should not be massaged.
Abscess
Abscess formation is a rare complication occurring any time from 1 week to several years after treatment; it may persist for weeks, and periodically recur for months. Abscesses should be treated with incision, drainage, and antibiotics. Cultures should be obtained. Treatment should then be tailored to the obtained sensitivity reports.28 Midfacial and periorbital infection can in rare cases result in intracerebral complications.
Herpetic outbreak
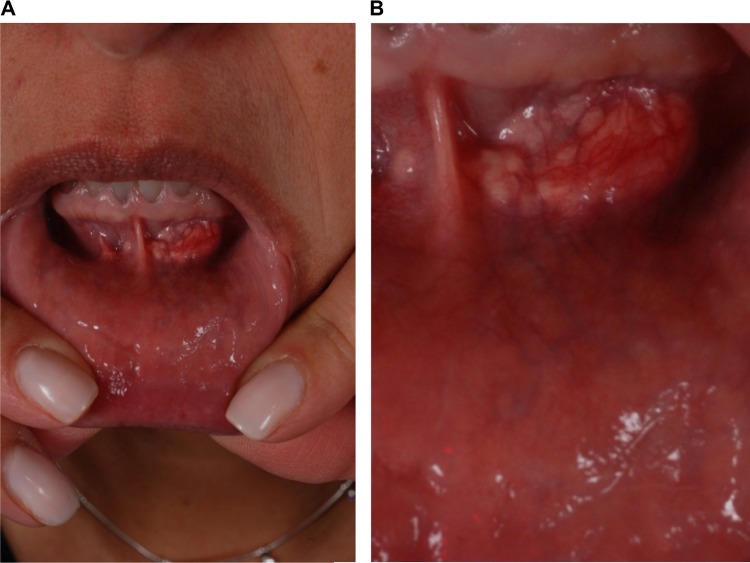
Dermal filler injections can lead to reactivation of herpes virus infections (Figure 7). If the treatment is targeting the lips or mouth area and the individual has a history of cold sores, prophylactic treatment with valaciclovir (500 mg twice daily [bid] for 3–5 days) can be started prior to injection to reduce the likelihood of this occurring. If the patient has not received prophylactic treatment, but infection is recognized early, valaciclovir at a dose of 2 g bid for 1 day should be given. If suprainfection occurs, the patient should be treated with appropriate antibiotics. The majority of herpetic recurrences occur in the perioral area, nasal mucosa, and mucosa of the hard palate. Shingles after injection is very rare. When a blistering reaction occurs outside of the areas of recurrent herpes simplex virus infection (lip skin and vermillion, nasal mucosa, and mucosa of hard palate), vascular compromise should be seriously considered.
Figure 7.
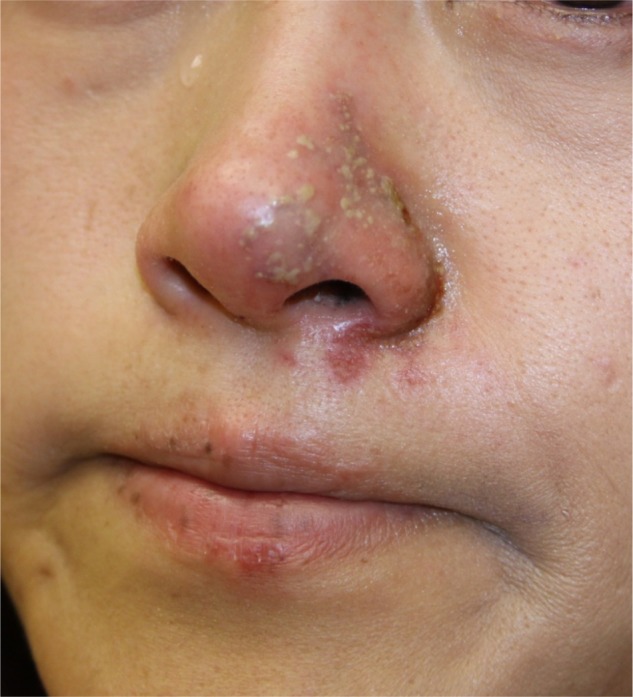
Dermal filler injection leading to herpes virus reactivation.
Nodular masses
Nodules are frequently observed after soft tissue augmentation. As they can arise from a number of causes, investigation may be required to establish a diagnosis. Visible material is more common in areas of thinner skin. Nodules must be categorized as inflammatory or noninflammatory.
Noninflammatory nodules
When too much material accumulates in an area as a result of poor technique (overcorrection, too superficial placement of a filler, or use of a filler for an incorrect indication such as intramuscular placement in a sphincteric muscle; Figure 8), a noninflammatory nodule may result that is palpable and may be visible. Such implant nodules form isolated lumps in the area of injection that do not grow, and which are well defined from the surrounding tissue. They appear early after the procedure and should be differentiated from foreign body granulomas, or biofilms, which are a result of an inflammatory reaction around the product or site of infection and occur later. Poor filler placement and the use of particulate fillers (eg, PMMA, CaHA) in highly mobile areas such as the lips can cause delayed-onset noninflammatory nodules.29
Figure 8.
Noninflammatory nodule.
If nodules occur after treatment with an HA filler, they will resolve with hyaluronidase treatment. Early nodules occurring after treatment with a non-HA filler may respond to vigorous massage; the material can also be extruded (Table S3). Nodules may also be disrupted with lidocaine or saline followed by vigorous massage.30 Nodules that do not resolve may respond to intralesional steroids (only small amounts should be injected to avoid skin atrophy). Additional treatment options are a series of three or more injections of 5-fuorouracil (5-FU), triamcinolone, and lidocaine, or 5-FU and lidocaine. The exact dosage is 0.5 mL of 50 mg/mL 5-FU, 0.3 mL of 10 mg/mL triamcinolone (or 40 mg/mL triamcinolone, depending on localization and degree of inflammation), and 0.2 mL 2% lidocaine with adrenalin. The injection amount is between 0.1 mL (tear trough or lips) and a maximum of 0.5 mL (in the cheeks) per nodule. In all cases, the patient should maintain disruption with home massage. Fractional lasers, which are used after the carrier gel has been absorbed, have been reported to improve visible material in the lower eyelids and the lips.31,32 Excision of implanted material is only used as a last resort. Chronic, intractable nodules that persist for months despite disruption and become increasingly fibrotic are in all likelihood foreign body granulomas and require excision.
Implant nodules are one of the most common adverse events following dermal filler procedures,33 but their incidence can be reduced by taking care to avoid too superficial placement of filler, selecting the appropriate filler for the tissue site, massaging after injection to ensure even distribution and smoothness, and avoiding intramuscular injection.
Inflammatory nodules
Biofilms
Biofilms are widespread in nature and consist of densely packed communities of bacteria that surround themselves with secreted polymers. When a material is injected into the skin or subcutaneous tissue, it can become coated with bacteria and form a biofilm. These complex collections of bacteria secrete a protective and adhesive matrix that allows them to irreversibly adhere to a living structure or inert surface, where they give rise to a low-grade chronic infection that is resistant to antibiotics.34,35 A mature biofilm will release individual free-swimming bacteria in the tissues. Many bacterial species form biofilms, and as biofilms progress they become more antibiotic and culture resistant. When activated, for example by trauma from a further dermal filler procedure, the biofilm can cause a local infection, a systemic infection, or a granulomatous or inflammatory response.
Distinguishing inflammation due to a bacterial biofilm from a low-grade hypersensitivity reaction is difficult. Furthermore, these infections are difficult to treat, and so the focus should be on prevention. If a red, indurated area appears at any time after treatment, regardless of duration, a biofilm should be suspected.36,37 Persistent inflammatory conditions not showing improvement with other therapy and inflammatory nodules that recur after resolution may also indicate a biofilm. As they are usually culture negative, these nodules were previously thought to be due to an allergic or a foreign body reaction to the filler substance. However, the technique of fluorescence in situ hybridization confirms an infective etiology in the majority of cases that are culture negative.38 The use of scintigraphy with radiolabeled autologous white blood cells is also an accurate method for diagnosing infection in patients with long-term dermal filler complications.39 Most lesions when resected and sent for pathology return as foreign body granulomas, so the importance of biofilm as a result of inflammatory nodules is still in question. A course of antibiotic therapy should be considered prior to excision of the nodule. If there is a nodule that is resistant to antibiotic therapy and is becoming increasingly fibrotic, it is most likely a foreign body granuloma.
Antibiotic treatment is the first step in treating a patient with a suspected biofilm, even if the culture is negative, and should be initiated with a broad-spectrum agent (quinolone) such as ciprofoxacin 500 mg bid and a macrolide such as clarithromycin XL 500 mg bid for 4–6 weeks (Table S3). If there is a strong suspicion of biofilm, intralesional steroids should not be used before antibiotics; doing so can prolong the problem. With any biofilm, whether a result of a biodegradable or nonbiodegradable product, removal of the filler will reduce the postinflammatory potential of the biofilm. Hyaluronidase should be used if the patient was injected with an HA. If a long-term indurated area persists, or if steroids have already been used, treatment with 5-FU injection, up to 50 mg/mL (0.5 cc), alone or in combination with steroids (for exact dosage, see Table S3) should be initiated and repeated every 2–4 weeks. If induration persists despite 5-FU treatment, a further option includes laser lysis. Surgical excision should be used as a last resort, and is dictated by nonresponse to antibiotics. The differential diagnosis is foreign body granuloma. However, exposing the inflammatory nodule to the body’s immune system is always beneficial.
Laser technology has been reported to have beneficial effects in the treatment of infectious filler complications. The intralesional subcutaneous introduction of an optic laser microfiber and the subsequent heat production result in a theoretical decrease of bacterial counts on the biofilm, and in liquefaction of the filler microparticles. Radiofrequency heating has been used in the same fashion. These minimally invasive techniques could be an intermediate step before attempting surgical excision of an inflamed area.
The incidence of biofilms as a result of filler injections is not known, and diagnosis is difficult. Fortunately, biofilms are very rare with most filler products, but it is important to take precautions to prevent infection when injecting fillers. Strategies to reduce risk of biofilm development include thorough cleansing of the face before injection, avoiding injecting through oral or nasal mucosa, avoiding hydrophilic permanent materials, not injecting over previous filler or into traumatized tissue, and aggressive treatment of any postfiller infection.
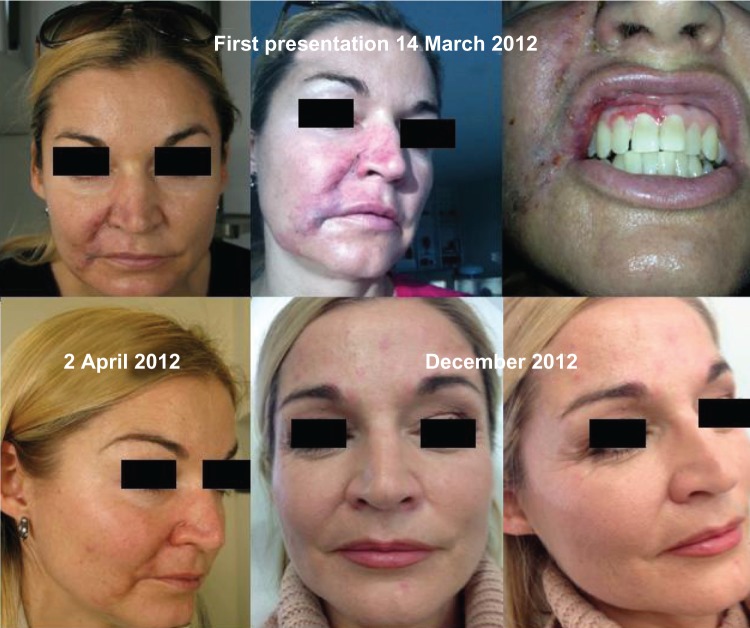
Foreign body granulomas
Foreign body, longstanding inflammatory nodules are most frequently foreign body granulomas (Figures 9 and 10). The function of such reactions is to isolate and prevent the migration of foreign bodies that cannot immediately be removed by enzymatic breakdown or phagocytosis by enclosing them in a capsule of monocytes and macrophages.40 The engulfed material may resist degradation and remain sequestered in the macrophages. These activated macrophages secrete a variety of cytokines and other inflammatory products that attract additional macrophages and blood monocytes. Individual macrophages may become larger (epithelioid histiocytes) or fuse to form multinucleated foreign body giant cells. These cells are characteristic of granulomas, and if a biopsy can be obtained, a true granulomatous reaction can be confirmed histologically. Clinically, foreign body granulomas appear as red papules, nodules, or plaques (with or without ulceration); any material expressed is culture negative. The lesions become firmer over time due to fibrosis.
Figure 9.
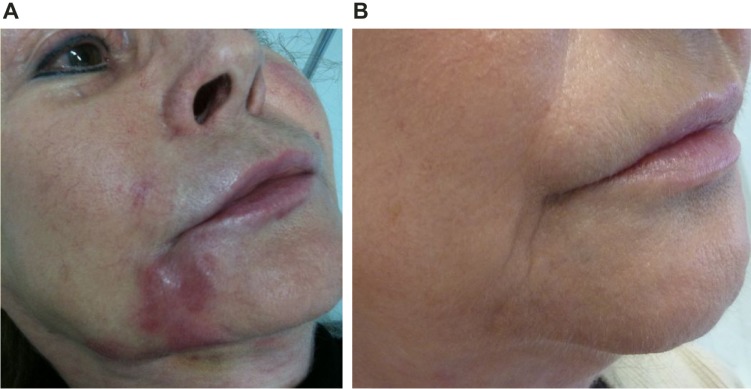
Inflammatory foreign body granuloma before and after treatment with an antibiotic, 5-FU, triamcinolone, and local anesthetic.
Abbreviation: 5-FU, 5-fluorouracil.
Figure 10.
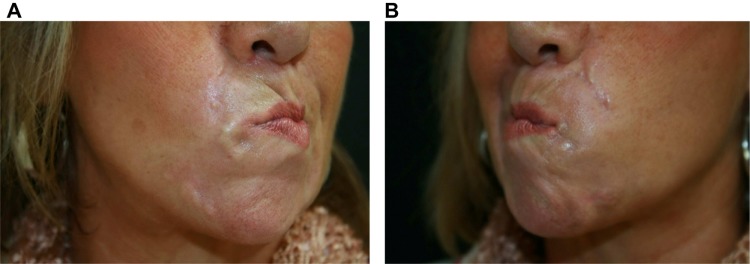
Nodularity that proved to be foreign body granuloma on surgical biopsy.
True foreign body granulomas are rare with an estimated incidence between 0.01% and 1%. They can occur with all injectable dermal fillers and usually appear after a latent period, which can be several months to years after injection, as compared with 2–4 weeks for early implant nodules.41–44 Factors influencing increased foreign body granuloma development include the properties of the filler, larger volumes injected, intramuscular injection, and previous infections or trauma. The shape of the microspheres appears to be an important factor, with granulomatous reactions occurring less frequently after implantation of microspheres with smooth surfaces. Irregular and sharp-edged particles may also induce more severe granulomatous reactions.45 Foreign-body granulomatous reactions have also been described after dermal filler injections in two patients treated with interferon for chronic hepatitis C46 and in a patient treated with omalizumab, a monoclonal antibody that targets circulating IgE, for severe persistent allergic asthma.47
Nonbiodegradable fillers are not characterized by a higher rate of foreign body granulomas per se than temporary fillers, but their clinical appearance is more pronounced and their persistence longer, if not treated appropriately. The structural changes that some nonbiodegradable fillers undergo during years in situ in human tissue may be one reason why adverse reactions to these permanent fillers occur clinically with a delay of several years.48 However, the mechanism of late inflammation or granuloma formation is still unknown.
Initial treatment is with intralesional corticosteroids (triamcinolone, betamethasone, or prednisone; Table S3). Granulomatous reactions to HA fillers can be treated with hyaluronidase.49 Many patients with lesions unresponsive to steroid alone will respond to the addition of 5-FU to the corticosteroids to discourage additional fibroblast activity and fibrosis in light of its antimetabolite function. Furthermore, this combination has the added advantage of reducing the amount of steroid injected, and thereby the risk of steroid-related adverse events such as tissue atrophy and telangiectasia.48 In case of the repeated failure of other therapies, surgical excision is the treatment of choice.42
Paresthesia
Inadvertent nerve damage is a rare complication of dermal filler procedures and can occur as result of direct trauma where the nerve is pierced or partially lacerated by the needle, injection of filler into the nerve, tissue compression by product, and by excessive molding and massage of product into a nerve foramina. Nerve injury may be transient and reversible, or permanent. Neurapraxia refers to injury to a nerve without axonal disruption. This type of injury may result in sensory or motor deficits, but improvement should occur within 2–3 weeks. Transection of small cutaneous sensory nerves (axonolytic injury) may result in a patch of anesthesia in that nerve’s zone of innervation. This type of injury is also reversible; sensation typically returns within several months. In cases of complete nerve transection, damage may be permanent. The most common site of dysesthesias, paresthesias, and anesthesia is the infraorbital nerve. It is the authors’ opinion that this occurs more often through the intraoral approach. Treatment is with small doses of triamcinolone at the infraorbital foramen, and disruption of palpable material with lidocaine or saline. One of the authors (TP) also reports good results with Keltican forte® capsules (Trommsdorff GmbH & Co, KG Medicines, Alsdorf, Germany), which contain vitamin B12, folic acid, and uridine monophosphate, and have neurotrophic properties.
A thorough knowledge of facial anatomy, with special attention to known danger zones, is required to anticipate vital structures as they are approached. If a deep injection is placed with the needle tip in contact with bone, an intraneural or intraforminal injection is not possible. However, material can be compacted into the foramen through overly vigorous massage.
Vascular compromise
Vascular compromise following filler injection is a major, immediate complication that is almost always the result of intravascular injection into an artery, causing an embolism that impedes blood fow. All injectors should have a thorough knowledge of facial anatomy. Recognition of a vascular event and swift and aggressive treatment is necessary to avoid serious, potentially irreversible complications.50–55 Injection of filler into an artery can cause necrosis of tissues in both an anterograde and retrograde fashion. There have been a few reports of acute vision loss and hemiplegia after autologous facial fat injection as a result of ocular and cerebral embolism, respectively.56–59 Acute fatal stroke has also been reported after autologous fat injection into the glabella.60 In all these cases, it is thought that fragments of the fatty tissue reached the ocular and cerebral arteries via reverse fow immediately after the fat injection and caused an embolism.
Retinal artery occlusion
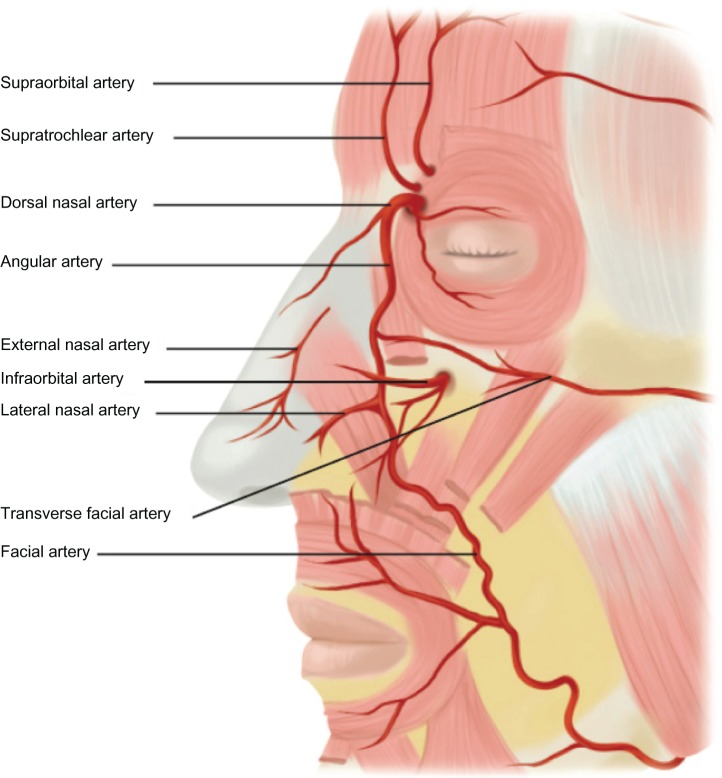
Retinal artery occlusion is a rare event that occurs when dermal filler enters the ocular circulation through retrograde arterial fow after inadvertent intra-arterial injection into one of the distal branches of the ophthalmic artery.51 These include the angular artery and zygomatico temporal, zygomatico facial, and dorsal nasal arteries (Figure 11). The supratrochlear and supraorbital arteries are also terminal branches of the ophthalmic artery. When there is an intravascular injection into one of these arteries that exceeds intra-arterial pressure, the injectate can move proximal to the origin of the central retinal artery; when the pressure is released, the material moves distally into the retinal artery, blocking blood supply to the retina and potentially causing visual impairment or blindness.50–52,61 Immediate blurring or loss of vision may occur (Figure 12). If there is any evidence of a visual problem after facial injection of a dermal filler, prompt consultation with an ophthalmologist is essential. When injecting in the area of the mentioned vessels, injections should be directly in contact with bone; if obstruction of the needle occurs, it should be removed, cleared, and then replaced at the preperiosteal level. At the level of the periostea, there are no vessels and the injector cannot be within a vessel or a foramina.
Figure 11.
Facial artery anatomy illustrating the most common sites of vascular occlusion.
Notes: Reproduced with permission. Copyright © 2009, John Wiley and Sons. Grunebaum LD, Bogdan Allemann I, Dayan S, Mandy S, Baumann L. The risk of alar necrosis associated with dermal filler injection. Dermatol Surg. 2009;35 Suppl 2:1635–1640. 53
Figure 12.
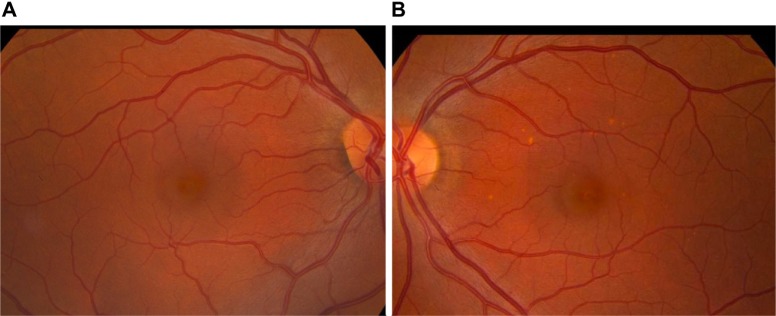
Retinal artery occlusion as a result of calcium hydroxylapatite in the central retinal artery.
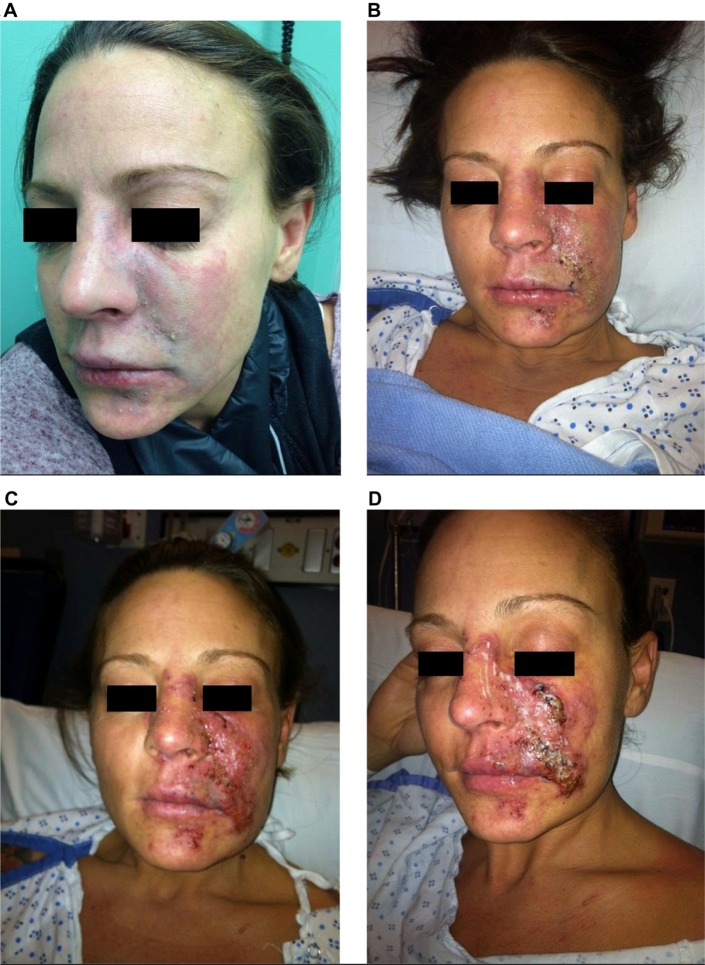
Tissue necrosis
Impending tissue necrosis may occur as a result of inadvertent injection of filler into vessels supplying the mucosa or the skin, resulting in vessel occlusion (Figures 13 and 14). During injection, the filler may fow antegrade, retrograde, or both in the vessel. Once the pressure from the injection is stopped, the product is carried through the vasculature and may result in local or distal necrosis with disastrous consequences (Figure 15). Necrosis may also occur secondary to local edema or to occlusion of adjacent vasculature secondary to the hydrophilic properties of the product.62 Necrosis can occur following injection with any dermal filler, although it is more likely with particulate fillers. Areas most vulnerable are those in which blood supply depends strongly on a single arterial branch (axial pattern blood supply), such as the glabellar and nasolabial folds.63,64 There are case reports documenting necrosis of the alar base, lip, and nose.53,65–68
Figure 13.
Skin necrosis after dermal filler injection.
Figure 14.
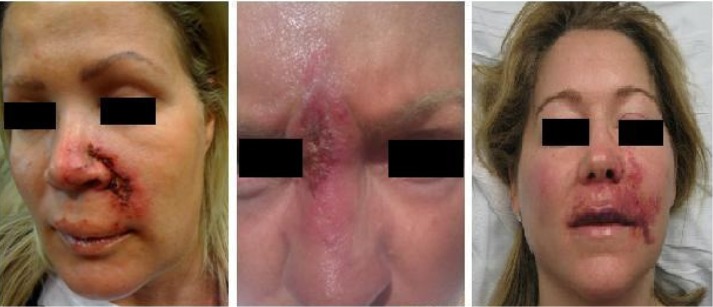
Examples of tissue necrosis after vascular compromise.
Figure 15.
Progression of vascular compromise after an embolic event.
The first signs of impending necrosis are pain inconsistent with that of the injection and an area of blanching. If this is observed, immediate action is required. Injection should be stopped immediately. A crash cart for impending necrosis allows for an immediate response (Table S4). The patient should receive hyaluronidase into the injection site regardless of the filler used, and have a 2% nitroglycerin paste massaged into the affected area.69 The area should be massaged and warm compresses applied to increase vasodilation. Patients should also receive a methylprednisolone (Medrol) dose pack. Lovenox injections bid and aspirin (unless contraindicated for the patient) are started to prevent further clot formation due to vascular compromise, with an antacid to prevent aspirin-associated gastritis. Nitroglycerin paste massages should be continued until improvement is seen. Sildenafil 100 mg may also be used to dilate the compromised vasculature. In severe cases, hyperbaric oxygen should be administered to help the survival of compromised tissue. Prophylactic antibiotic and antiviral therapy should be initiated.
Factors increasing the likelihood of vessel occlusion include large volume bolus injections, small sharp needles, a stationary rather than moving needle (bolus injections should only be performed when truly on the bone or in the dermis), high-pressure injections (as these are more likely to cause anterograde fow), and a deep plane of injection (larger vessels are found beneath the dermis in the subcutaneous fat). Areas of tissue necrosis are subject to secondary bacterial or viral infections.
Conclusion
The past decade has seen the arrival of a host of new soft tissue fillers for facial rejuvenation, which not only remove wrinkles but also have the ability to restore facial volume to create a balanced, more natural rejuvenated look. To achieve cosmetically pleasing results, it is essential that those practicing facial rejuvenation have a thorough understanding of the individual characteristics of available fillers, their indications, contraindications, benefits and drawbacks, and ways to prevent and avoid potential complications.
Side effects can occur with any dermal filler, but our knowledge of the frequency and potential risk factors is limited. A report from the Injectable Filler Safety Study, which obtained population-based data on the type and frequency of adverse reactions to injectable filler substances from dermatologists and plastic surgeons practicing in Berlin, Germany, confirmed that while adverse reactions are documented with all injectable fillers, time until reaction and the type of adverse reaction varied between the different fillers.5 For example, adverse reactions to biodegradable fillers occurred after a mean (standard deviation) of 4.9 ± 5.8 months, and reactions to nonbiodegradable fillers after 18.3 ± 19.0 months (P=0.005). PMMA with collagen (Artecoll®) showed the longest interval between injection and development of a reaction (37.1 ± 25.4 months). In this small case series, adverse reactions could not be classified by biodegradable or nonbiodegradable filler, but characteristic adverse event profiles could be defined for each substance. For example, adverse events in patients treated with HA-based fillers (mostly Restylane®) were mainly swelling, erythema, and nodules; PLLA (Sculptra®) patients mainly developed granulomas, as did patients treated with PMMA plus collagen (Artecoll®). Consensus is urgently required on the best course of treatment if complications occur.
Different injectable products have widely varying properties, associated risks, and injection requirements. The practicing physician should be suitably experienced to select and use these products accordingly, which will necessitate a detailed understanding of facial anatomy, proper patient selection, proper product selection for the anatomical site, correct placement of product, and proper preparation and injection techniques. Most adverse events are avoidable with proper planning and technique.
Supplementary tables
Table S1.
Algorithm for the management of antibody-mediated or nonantibody-mediated edema
|
ccid-6-295s1.tif (239.1KB, tif)
|
Abbreviation: HA, hyaluronic acid.
Table S2.
Algorithm for the management of malar edema
|
ccid-6-295s2.tif (223KB, tif)
|
Abbreviation: HA, hyaluronic acid.
Table S3.
Algorithm for the management of nodular masses
|
ccid-6-295s3.tif (303.4KB, tif)
|
Notes: *The exact dosage is 0.5 mL of 50 mg/mL 5-FU, 0.3 mL of 10 mg/mL triamcinolone (or 40 mg/mL triamcinolone depending on localization and degree of inflammation) and 0.2 mL 2% lidocaine with adrenalin. The injection amount is between 0.1 mL (tear trough or lips) and a maximum of 0.5 mL (in the cheeks) per nodule; †when in doubt as to whether dealing with foreign body or biofilm, initiate a course of antibiotics, especially if the nodule does not respond to injection therapy.
Abbreviations: 5-FU, 5-fluorouracil; HA, hyaluronic acid.
Table S4.
Algorithm for the management of vascular compromise
|
ccid-6-295s4.tif (168.8KB, tif)
|
Note: *For progressing necrosis resistant to above options. Crash cart contents should always be on hand for an immediate response.
Acknowledgment
Jenny Grice provided help with medical writing.
Footnotes
Disclosure
This work was supported in full by Merz Pharmaceuticals. The authors report no further conflicts of interest in this work.
References
- 1.American Society for Aesthetic Plastic Surgery Cosmetic surgery national data bank statistics 2012. [Accessed September 13, 2013]. Available from: http://www.surgery.org/sites/default/fles/ASAPS-2011-Stats.pdf.
- 2.Rzany B, Hilton S, Prager W, et al. Expert guideline on the use of porcine collagen in aesthetic medicine. J Dtsch Dermatol Ges. 2010;8(3):210–217. doi: 10.1111/j.1610-0387.2009.07321.x. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg DJ. Legal ramifications of off-label filler use. Dermatol Ther. 2006;19(3):189–193. doi: 10.1111/j.1529-8019.2006.00073.x. [DOI] [PubMed] [Google Scholar]
- 4.Glogau RG, Kane MA. Effect of injection techniques on the rate of local adverse events in patients implanted with nonanimal hyaluronic acid gel dermal fillers. Dermatol Surg. 2008;34(Suppl 1):S105–S109. doi: 10.1111/j.1524-4725.2008.34251.x. [DOI] [PubMed] [Google Scholar]
- 5.Zielke H, Wölber L, Wiest L, Rzany B. Risk profiles of different injectable fillers: results from the Injectable Filler Safety Study (IFS Study) Dermatol Surg. 2008;34(3):326–335. doi: 10.1111/j.1524-4725.2007.34066.x. [DOI] [PubMed] [Google Scholar]
- 6.Narins RS, Brandt FS, Lorenc ZP, Maas CS, Monheit GD, Smith SR. Twelve-month persistency of a novel ribose-cross-linked collagen dermal filler. Dermatol Surg. 2008;34(Suppl 1):S31–S39. doi: 10.1111/j.1524-4725.2008.34240.x. [DOI] [PubMed] [Google Scholar]
- 7.Bass LS, Smith S, Busso M, McClaren M. Calcium hydroxylapatite (Radiesse) for treatment of nasolabial folds: long-term safety and efficacy results. Aesthet Surg J. 2010;30(2):235–238. doi: 10.1177/1090820X10366549. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald R, Vleggaar D. Facial volume restoration of the aging face with poly-l-lactic acid. Dermatol Ther. 2011;24(1):2–27. doi: 10.1111/j.1529-8019.2010.01375.x. [DOI] [PubMed] [Google Scholar]
- 9.Hilinski JM, Cohen SR. Soft tissue augmentation with ArteFill. Facial Plast Surg. 2009;25(2):114–119. doi: 10.1055/s-0029-1220651. [DOI] [PubMed] [Google Scholar]
- 10.Pallua N, Wolter TP. A 5-year assessment of safety and aesthetic results after facial soft-tissue augmentation with polyacrylamide hydrogel (Aquamid): a prospective multicenter study of 251 patients. Plast Reconstr Surg. 2010;125(6):1797–1804. doi: 10.1097/PRS.0b013e3181d18158. [DOI] [PubMed] [Google Scholar]
- 11.Gladstone HB, Cohen JL. Adverse effects when injecting facial fillers. Semin Cutan Med Surg. 2007;26(1):34–39. doi: 10.1016/j.sder.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Shah NS, Lazarus MC, Bugdodel R, et al. The effects of topical vitamin K on bruising after laser treatment. J Am Acad Dermatol. 2002;47(2):241–244. doi: 10.1067/mjd.2002.120465. [DOI] [PubMed] [Google Scholar]
- 13.Bailey SH, Cohen JL, Kenkel JM. Etiology, prevention, and treatment of dermal filler complications. Aesthet Surg J. 2011;31(1):110–121. doi: 10.1177/1090820X10391083. [DOI] [PubMed] [Google Scholar]
- 14.Zeichner JA, Cohen JL. Use of blunt tipped cannulas for soft tissue fillers. J Drugs Dermatol. 2012;11(1):70–72. [PubMed] [Google Scholar]
- 15.Van Dyke S, Hays GP, Caglia AE, Caglia M. Severe Acute Local Reactions to a Hyaluronic Acid-derived Dermal Filler. J Clin Aesthet Dermatol. 2010;3(5):32–35. [PMC free article] [PubMed] [Google Scholar]
- 16.Geisler D, Shumer S, Elson ML. Delayed hypersensitivity reaction to Restylane®. Cosmetic Dermatol. 2007;20(12):784–786. [Google Scholar]
- 17.Arron ST, Neuhaus IM. Persistent delayed-type hypersensitivity reaction to injectable non-animal-stabilized hyaluronic acid. J Cosmet Dermatol. 2007;6(3):167–171. doi: 10.1111/j.1473-2165.2007.00331.x. [DOI] [PubMed] [Google Scholar]
- 18.Cassuto D, Marangoni O, De Santis G, Christensen L. Advanced laser techniques for filler-induced complications. Dermatol Surg. 2009;35(Suppl 2):1689–1695. doi: 10.1111/j.1524-4725.2009.01348.x. [DOI] [PubMed] [Google Scholar]
- 19.Funt DK. Avoiding malar edema during midface/cheek augmentation with dermal fillers. J Clin Aesthet Dermatol. 2011;4(12):32–36. [PMC free article] [PubMed] [Google Scholar]
- 20.Griepentrog GJ, Lucarelli MJ, Burkat CN, Lemke BN, Rose JG. Periorbital edema following hyaluronic acid gel injection: a retrospective review. Am J Cosmetic Surg. 2011;28(4):251–254. [Google Scholar]
- 21.Pessa JE, Garza JR. The malar septum: the anatomic basis of malar mounds and malar edema. Aesthet Surg J. 1997;17(1):11–17. doi: 10.1016/s1090-820x(97)70001-3. [DOI] [PubMed] [Google Scholar]
- 22.Cohen JL, Bhatia AC. The role of topical vitamin K oxide gel in the resolution of postprocedural purpura. J Drugs Dermatol. 2009;8(11):1020–1024. [PubMed] [Google Scholar]
- 23.Taylor SC, Burgess CM, Callender VD. Safety of nonanimal stabilized hyaluronic acid dermal fillers in patients with skin of color: a randomized, evaluator-blinded comparative trial. Dermatol Surg. 2009;35(Suppl 2):1653–1660. doi: 10.1111/j.1524-4725.2009.01344.x. [DOI] [PubMed] [Google Scholar]
- 24.Heath CR, Taylor SC. Fillers in the skin of color population. J Drugs Dermatol. 2011;10(5):494–498. [PubMed] [Google Scholar]
- 25.Pavicic T. Efficacy and tolerability of a new monophasic, double-crosslinked hyaluronic acid filler for correction of deep lines and wrinkles. J Drugs Dermatol. 2011;10(2):134–139. [PubMed] [Google Scholar]
- 26.Hirsch RJ, Narurkar V, Carruthers J. Management of injected hyaluronic acid induced Tyndall effects. Lasers Surg Med. 2006;38(3):202–204. doi: 10.1002/lsm.20283. [DOI] [PubMed] [Google Scholar]
- 27.Douse-Dean T, Jacob CI. Fast and easy treatment for reduction of the Tyndall effect secondary to cosmetic use of hyaluronic acid. J Drugs Dermatol. 2008;7(3):281–283. [PubMed] [Google Scholar]
- 28.Rousso JJ, Pitman MJ. Enterococcus faecalis complicating dermal filler injection: a case of virulent facial abscesses. Dermatol Surg. 2010;36(10):1638–1641. doi: 10.1111/j.1524-4725.2010.01699.x. [DOI] [PubMed] [Google Scholar]
- 29.Sclafani AP, Fagien S. Treatment of injectable soft tissue filler complications. Dermatol Surg. 2009;35(Suppl 2):1672–1680. doi: 10.1111/j.1524-4725.2009.01346.x. [DOI] [PubMed] [Google Scholar]
- 30.Voigts R, Devore DP, Grazer JM. Dispersion of calcium hydroxylapatite accumulations in the skin: animal studies and clinical practices. Dermatol Surg. 2010;36(Suppl s1):798–803. [Google Scholar]
- 31.Vrcek IM, Malouf P, Gilliland GD. A novel solution for superficially placed calcium hydroxylapatite (Radiesse) in the inferior eyelid. Orbit. 2012;31(6):431–432. doi: 10.3109/01676830.2012.694557. [DOI] [PubMed] [Google Scholar]
- 32.Reddy KK, Brauer JA, Anolik R, et al. Calcium hydroxylapatite nodule resolution after fractional carbon dioxide laser therapy. Arch Dermatol. 2012;148(5):634–636. doi: 10.1001/archdermatol.2011.3374. [DOI] [PubMed] [Google Scholar]
- 33.Heinz BC, Ladhoff U, Kahl Ch, Rzany B, von Mallek D. Survey of incidents associated with injectable dermal fillers reported to the German Medical Devices Vigilance System. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2008;51(7):787–792. doi: 10.1007/s00103-008-0587-5. German. [DOI] [PubMed] [Google Scholar]
- 34.Christensen LH. Host tissue interaction, fate, and risks of degradable and nondegradable gel fillers. Dermatol Surg. 2009;35(Suppl 2):1612–1619. doi: 10.1111/j.1524-4725.2009.01338.x. [DOI] [PubMed] [Google Scholar]
- 35.Monheit GD, Rohrich RJ. The nature of long-term fillers and the risk of complications. Dermatol Surg. 2009;35(Suppl 2):1598–1604. doi: 10.1111/j.1524-4725.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- 36.Narins RS, Coleman WP, Glogau RG. Recommendations and treatment options for nodules and other filler complications. Dermatol Surg. 2009;35(Suppl 2):1667–1671. doi: 10.1111/j.1524-4725.2009.01335.x. [DOI] [PubMed] [Google Scholar]
- 37.Dayan SH, Arkins JP, Brindise R. Soft tissue fillers and biofilms. Facial Plast Surg. 2011;27(1):23–28. doi: 10.1055/s-0030-1270415. [DOI] [PubMed] [Google Scholar]
- 38.Bjarnsholt T, Tolker-Nielsen T, Givskov M, Janssen M, Christensen LH. Detection of bacteria by fluorescence in situ hybridization in culture-negative soft tissue filler lesions. Dermatol Surg. 2009;35(Suppl 2):1620–1624. doi: 10.1111/j.1524-4725.2009.01313.x. [DOI] [PubMed] [Google Scholar]
- 39.Grippaudo FR, Pacilio M, Di Girolamo M, Dierckx RA, Signore A. Radiolabelled white blood cell scintigraphy in the work-up of dermal filler complications. Eur J Nucl Med Mol Imaging. 2013;40(3):418–425. doi: 10.1007/s00259-012-2305-7. [DOI] [PubMed] [Google Scholar]
- 40.Alijotas-Reig J, Fernández-Figueras MT, Puig L. Late-onset inflammatory adverse reactions related to soft tissue filler injections. Clin Rev Allergy Immunol. 2013;45(1):97–108. doi: 10.1007/s12016-012-8348-5. [DOI] [PubMed] [Google Scholar]
- 41.Lemperle G, Gauthier-Hazan N, Wolters M, Eisemann-Klein M, Zimmermann U, Duffy DM. Foreign body granulomas after all injectable dermal fillers: part 1. Possible causes. Plast Reconstr Surg. 2009;123(6):1842–1863. doi: 10.1097/PRS.0b013e31818236d7. [DOI] [PubMed] [Google Scholar]
- 42.Lemperle G, Gauthier-Hazan N. Foreign body granulomas after all injectable dermal fillers: part 2. Treatment options. Plast Reconstr Surg. 2009;123(6):1864–1873. doi: 10.1097/PRS.0b013e3181858f4f. [DOI] [PubMed] [Google Scholar]
- 43.Alijotas-Reig J, Garcia-Gimenez V, Miró-Mur F, Vilardell-Tarrés M. Delayed immune-mediated adverse effects of polyalkylimide dermal fillers: clinical findings and long-term follow-up. Arch Dermatol. 2008;144(5):637–642. doi: 10.1001/archderm.144.5.637. [DOI] [PubMed] [Google Scholar]
- 44.Chrastil-LaTowsky B, Wesley NO, MacGregor JL, Kaminer MS, Arndt KA. Delayed inflammatory reaction to bio-alcamid polyacrylamide gel used for soft-tissue augmentation. Arch Dermatol. 2009;145(11):1309–1312. doi: 10.1001/archdermatol.2009.266. [DOI] [PubMed] [Google Scholar]
- 45.Sachdev M, Anantheswar Y, Ashok B, Hameed S, Pai SA. Facial granulomas secondary to injection of semi-permanent cosmetic dermal filler containing acrylic hydrogel particles. J Cutan Aesthet Surg. 2010;3(3):162–166. doi: 10.4103/0974-2077.74493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Descamps V, Landry J, Francès C, Marinho E, Ratziu V, Chosidow O. Facial cosmetic filler injections as possible target for systemic sarcoidosis in patients treated with interferon for chronic hepatitis C: two cases. Dermatology (Basel) 2008;217(1):81–84. doi: 10.1159/000128281. [DOI] [PubMed] [Google Scholar]
- 47.Dammak A, Taillé C, Marinho E, Crestani B, Crickx B, Descamps V. Granulomatous foreign-body reaction with facial dermal fillers after omalizumab treatment for severe persistent allergic asthma: a case report. Br J Dermatol. 2012;166(6):1375–1376. doi: 10.1111/j.1365-2133.2012.10817.x. [DOI] [PubMed] [Google Scholar]
- 48.Wiest LG, Stolz W, Schroeder JA. Electron microscopic documentation of late changes in permanent fillers and clinical management of granulomas in affected patients. Dermatol Surg. 2009;35(Suppl 2):1681–1688. doi: 10.1111/j.1524-4725.2009.01347.x. [DOI] [PubMed] [Google Scholar]
- 49.Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31(8 Pt 1):893–897. doi: 10.1097/00042728-200508000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Kwon SG, Hong JW, Roh TS, Kim YS, Rah DK, Kim SS. Ischemic oculomotor nerve palsy and skin necrosis caused by vascular embolization after hyaluronic Acid filler injection: a case report. Ann Plast Surg. 2013;71(4):333–334. doi: 10.1097/SAP.0b013e31824f21da. [DOI] [PubMed] [Google Scholar]
- 51.Peter S, Mennel S. Retinal branch artery occlusion following injection of hyaluronic acid (Restylane) Clin Experiment Ophthalmol. 2006;34(4):363–364. doi: 10.1111/j.1442-9071.2006.01224.x. [DOI] [PubMed] [Google Scholar]
- 52.Kim YJ, Kim SS, Song WK, Lee SY, Yoon JS. Ocular ischemia with hypotony after injection of hyaluronic acid gel. Ophthal Plast Reconstr Surg. 2011;27(6):e152–e155. doi: 10.1097/IOP.0b013e3182082f37. [DOI] [PubMed] [Google Scholar]
- 53.Grunebaum LD, Bogdan Allemann I, Dayan S, Mandy S, Baumann L. The risk of alar necrosis associated with dermal filler injection. Dermatol Surg. 2009;35(Suppl 2):1635–1640. doi: 10.1111/j.1524-4725.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 54.Schanz S, Schippert W, Ulmer A, Rassner G, Fierlbeck G. Arterial embolization caused by injection of hyaluronic acid (Restylane) Br J Dermatol. 2002;146(5):928–929. doi: 10.1046/j.1365-2133.2002.04707.x. [DOI] [PubMed] [Google Scholar]
- 55.Coleman SR. Avoidance of arterial occlusion from injection of soft tissue fillers. Aesthet Surg J. 2002;22(6):555–557. doi: 10.1067/maj.2002.129625. [DOI] [PubMed] [Google Scholar]
- 56.Feinendegen DL, Baumgartner RW, Vuadens P, et al. Autologous fat injection for soft tissue augmentation in the face: a safe procedure? Aesthetic Plast Surg. 1998;22(3):163–167. doi: 10.1007/s002669900185. [DOI] [PubMed] [Google Scholar]
- 57.Egido JA, Arroyo R, Marcos A, Jiménez-Alfaro I. Middle cerebral artery embolism and unilateral visual loss after autologous fat injection into the glabellar area. Stroke. 1993;24(4):615–616. doi: 10.1161/01.str.24.4.615. [DOI] [PubMed] [Google Scholar]
- 58.Lee DH, Yang HN, Kim JC, Shyn KH. Sudden unilateral visual loss and brain infarction after autologous fat injection into nasolabial groove. Br J Ophthalmol. 1996;80(11):1026–1027. doi: 10.1136/bjo.80.11.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thaunat O, Thaler F, Loirat P, Decroix JP, Boulin A. Cerebral fat embolism induced by facial fat injection. Plast Reconstr Surg. 2004;113(7):2235–2236. doi: 10.1097/01.prs.0000123627.33690.9e. [DOI] [PubMed] [Google Scholar]
- 60.Yoon SS, Chang DI, Chung KC. Acute fatal stroke immediately following autologous fat injection into the face. Neurology. 2003;61(8):1151–1152. doi: 10.1212/wnl.61.8.1151. [DOI] [PubMed] [Google Scholar]
- 61.Silva MT, Curi AL. Blindness and total ophthalmoplegia after aesthetic polymethylmethacrylate injection: case report. Arq Neuropsiquiatr. 2004;62(3B):873–874. doi: 10.1590/s0004-282x2004000500025. [DOI] [PubMed] [Google Scholar]
- 62.Dayan SH, Arkins JP, Mathison CC. Management of impending necrosis associated with soft tissue filler injections. J Drugs Dermatol. 2011;10(9):1007–1012. [PubMed] [Google Scholar]
- 63.Hirsch RJ, Cohen JL, Carruthers JD. Successful management of an unusual presentation of impending necrosis following a hyaluronic acid injection embolus and a proposed algorithm for management with hyaluronidase. Dermatol Surg. 2007;33(3):357–360. doi: 10.1111/j.1524-4725.2007.33073.x. [DOI] [PubMed] [Google Scholar]
- 64.Bachmann F, Erdmann R, Hartmann V, Wiest L, Rzany B. The spectrum of adverse reactions after treatment with injectable fillers in the glabellar region: results from the Injectable Filler Safety Study. Dermatol Surg. 2009;35(Suppl 2):1629–1634. doi: 10.1111/j.1524-4725.2009.01341.x. [DOI] [PubMed] [Google Scholar]
- 65.Georgescu D, Jones Y, McCann JD, Anderson RL. Skin necrosis after calcium hydroxylapatite injection into the glabellar and nasolabial folds. Ophthal Plast Reconstr Surg. 2009;25(6):498–499. doi: 10.1097/IOP.0b013e3181b81082. [DOI] [PubMed] [Google Scholar]
- 66.Kassir R, Kolluru A, Kassir M. Extensive necrosis after injection of hyaluronic acid filler: case report and review of the literature. J Cosmet Dermatol. 2011;10(3):224–231. doi: 10.1111/j.1473-2165.2011.00562.x. [DOI] [PubMed] [Google Scholar]
- 67.Glaich AS, Cohen JL, Goldberg LH. Injection necrosis of the glabella: protocol for prevention and treatment after use of dermal fillers. Dermatol Surg. 2006;32(2):276–281. doi: 10.1111/j.1524-4725.2006.32052.x. [DOI] [PubMed] [Google Scholar]
- 68.Burt B, Nakra T, Isaacs DK, Goldberg RA. Alar necrosis after facial injection of hyaluronic Acid. Plast Reconstr Surg. 2010;125(5):199e–200e. doi: 10.1097/PRS.0b013e3181d5152e. [DOI] [PubMed] [Google Scholar]
- 69.Kleydman K, Cohen JL, Marmur E. Nitroglycerin: a review of its use in the treatment of vascular occlusion after soft tissue augmentation. Dermatol Surg. 2012;38(12):1889–1897. doi: 10.1111/dsu.12001. [DOI] [PubMed] [Google Scholar]
- 70.Lowe NJ, Maxwell CA, Patnaik R. Adverse reactions to dermal fillers: review. Dermatol Surg. 2005;31(11 Pt 2):1616–1625. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Algorithm for the management of antibody-mediated or nonantibody-mediated edema
|
ccid-6-295s1.tif (239.1KB, tif)
|
Abbreviation: HA, hyaluronic acid.
Table S2.
Algorithm for the management of malar edema
|
ccid-6-295s2.tif (223KB, tif)
|
Abbreviation: HA, hyaluronic acid.
Table S3.
Algorithm for the management of nodular masses
|
ccid-6-295s3.tif (303.4KB, tif)
|
Notes: *The exact dosage is 0.5 mL of 50 mg/mL 5-FU, 0.3 mL of 10 mg/mL triamcinolone (or 40 mg/mL triamcinolone depending on localization and degree of inflammation) and 0.2 mL 2% lidocaine with adrenalin. The injection amount is between 0.1 mL (tear trough or lips) and a maximum of 0.5 mL (in the cheeks) per nodule; †when in doubt as to whether dealing with foreign body or biofilm, initiate a course of antibiotics, especially if the nodule does not respond to injection therapy.
Abbreviations: 5-FU, 5-fluorouracil; HA, hyaluronic acid.
Table S4.
Algorithm for the management of vascular compromise
|
ccid-6-295s4.tif (168.8KB, tif)
|
Note: *For progressing necrosis resistant to above options. Crash cart contents should always be on hand for an immediate response.