Abstract
Fibromyalgia syndrome (FMS) is a widespread musculoskeletal pain condition with unclear physiologic mechanisms. The purpose of this investigation was to compare the responsiveness of nociceptive flexion reflex (NFR) pathways between women with and without FMS. A secondary purpose was to examine the influence of depression, fibromyalgia symptom severity, and cardiovascular health on NFR responses among women with FMS. Fifteen women with FMS and 14 healthy controls participated in an experimental session to assess NFR responses to sural nerve stimulation, resting mean arterial pressure (MAP) and heart rate (HR), and scores on the Beck Depression Inventory (BDI) and Fibromyalgia Impact Questionnaire (FIQ). NFR responses were successfully elicited from all healthy individuals, but only eight (53 %) of the women with FMS. These women did not differ in the minimum stimulus intensity required to elicit an NFR response compared to healthy controls (p ≥ 0.35). Further, these women had lower BDI (p = 0.04) and FIQ (p = 0.02) scores compared to women with FMS from whom NFR responses could not be elicited. Resting HR was higher in both groups of women with FMS compared to healthy individuals (p <0.05), and MAP was strongly associated with NFR thresholds only among women with FMS (r = 0.88, p <0.01). Findings from this preliminary investigation suggest that NFR pathways are impaired in women who are more severely impacted by symptoms of depression and fibromyalgia, potentially due to desensitization of NFR pathways with chronic autonomic arousal.
Keywords: Central sensitization, Electromyography, Pain, Muscle, Depression, Autonomic nervous system
Introduction
Fibromyalgia syndrome (FMS) is a chronic, widespread musculoskeletal pain condition that is often accompanied by comorbid depression, anxiety, fatigue, disturbed sleep, and cognitive dysfunction [1–3]. Patients with FMS exhibit increased sensitivity to a variety of noxious stimuli, including heat, cold, pressure, and electrical stimulation [4, 5]. It has been suggested that sensitization of the pathways involved in central pain processing may contribute to the development and maintenance of chronic musculoskeletal pain conditions such as FMS [6, 7].
One experimental approach used to assess central sensitization is the nociceptive flexion reflex (NFR), a poly-synaptic reflex responsible for withdrawal of a limb from noxious stimuli that present a potential source of injury [8, 9]. Previous research demonstrates that the NFR is primarily spinal in origin and includes contributions from A-delta and C nociceptive afferent fibers, interneurons, and alpha motoneurons [8]. Further evidence indicates that the minimum intensity of electrical stimulation required to elicit the NFR, termed the NFR threshold (NFRT), corresponds to subjective pain thresholds [10–12]. Previous comparisons of NFRT among individuals with and without FMS have reported conflicting results [13]. Two studies found no difference in the NFRT between patients with FMS and healthy controls [14, 15], whereas two other studies reported that patients with FMS had a lower NFRT compared to healthy controls suggesting greater sensitization of nociceptive pathways in FMS [16, 17]. More recent research suggests that depressive symptoms can modulate NFR responses [18], as well as other measures of central pain processing [19], among patients with FMS. The presence and severity of depressive symptoms were not considered in previous comparisons of NFR responses between individuals with and without FMS [14–17], which may explain the discrepant findings across studies.
The purpose of this investigation was to compare NFRT between individuals with and without FMS and to examine the influence of depression and fibromyalgia symptom severity on NFR responses. We expected to observe greater reflex responsiveness to noxious stimuli (lower NFRT) in patients with FMS compared to healthy controls, with greater impairment of the NFRT among patients with higher levels of depression and fibromyalgia symptom severity.
Materials and methods
Participants
Fifteen women with FMS and 14 women without FMS participated in this study. The two groups of women were matched based on age and body mass index (BMI). Participants were recruited through referrals from the University of Colorado Hospital, and study advertisements distributed through newspapers, electronic mailings, and local FMS support groups. Patients who had been diagnosed with FMS by their physician based on the diagnostic criteria of the American College of Rheumatology [20] were eligible for participation. Those who were taking selected medications known to affect pain perception (e.g., low dose narcotics) were asked to temporarily discontinue the use of these medications 48 h before participation in the experimental session, with the consent of their physician. Pain medications associated with known adverse withdrawal effects (e.g., higher dose narcotics, anti-depressants) were documented but were not discontinued prior to testing. Use of pain medications was found not to differ among women with FMS (χ2 = 0.05–2.02, p >0.05; Table 1).
Table 1.
Pain medications
| Healthy (n = 14) | NFR-R (n = 8) | NFR-NR (n = 7) | |
|---|---|---|---|
| No pain medication | 100.0 | 12.5 | 28.6 |
| Antidepressant | 0.0 | 62.5 | 57.1 |
| Opioid | 0.0 | 25.0 | 0.0 |
| NSAID | 0.0 | 25.0 | 14.3 |
NFR-R Nociceptive flexion reflex responders, NFR-NR NFR nonresponders, NSAID nonsteroidal anti-inflammatory drug
Numbers indicate percentage of women in each group who were taking various classes of medication for pain relief prior to enrollment. Note that, percentages do not always sum to 100 % within each group because some women reported taking more than one pain medication. Chi-square analyses revealed no difference in the distribution of medication usage between NFR-R and NFR-NR groups (p >0.05)
Healthy control subjects reported no history of chronic widespread or regional musculoskeletal pain. Individuals with and without FMS were excluded from participation if they were younger than 18 years of age, pregnant or breast-feeding, or reported a history of cardiovascular or neurological conditions that could impair sensory or motor function. The study procedures were approved by the Colorado Multiple Institutional Review Board, and all participants provided written informed consent prior to enrollment.
Experimental procedures
Participants completed one familiarization session and one experimental session on two separate days. During the familiarization session, all participants filled out a general health survey and questionnaire to assess the severity of depressive symptoms (Beck Depression Inventory; BDI) [21]. Patients with FMS completed an additional questionnaire to assess the overall impact of FMS on daily functioning (Fibromyalgia Impact Questionnaire; FIQ) [22, 23]. Participants were then exposed to electrical stimuli across a range of intensities to become familiar with the experimental procedures.
Neurophysiological testing
During the experimental session, resting blood pressure and heart rate were assessed using a commercially available monitor (Omron HEM-711DLX, Bannockburn, IL). Neurophysiological testing was then conducted to assess perceptual threshold (PT) and NFRT. Two 8-mm Ag–AgCl recording electrodes with an interelectrode distance of 2 cm [24] were placed over the right biceps femoris at half the distance between the ischial tuberosity and the lateral tibial condyle, with a reference electrode on the ipsilateral patella. Electromyographic (EMG) signals were amplified (×1,000) and filtered (13–1,000 Hz; Coulbourn Instruments, Allentown, PA) prior to A/D conversion at 2,000 Hz (Power 1401, 16-bit resolution; Cambridge Electronic Design, Cambridge, United Kingdom).
Participants sat upright in an experimental chair with the hip joint flexed approximately 90° and the right ankle positioned on a foot rest with the right knee flexed 60° from the horizontal. A series of 1 mA test stimuli were delivered with a constant current stimulator (Digitimer DS7AH, Hertfordshire, England) while manually adjusting the placement of a stimulating electrode along the retromalleolar pathway of the sural nerve until a localized, pricking sensation was reported which radiated along the distribution of the sural nerve at higher stimulus intensities. For all subsequent tests, trains of five 1-ms rectangular pulses with a 3-ms interpulse interval were delivered to the sural nerve at this location.
Perceptual threshold was first assessed to control for inter-individual differences in the efficacy of sural nerve stimulation [25]. During the PT assessment, participants first received a train of stimuli at an intensity of 2 mA, for which all participants reported feeling a localized pricking sensation under the stimulating electrode. The stimulus intensity was then decreased in 0.5 mA increments until the pricking sensation was no longer detectable. From this subthreshold intensity, the stimulus intensity was increased in 0.1 mA increments until the participant reported feeling a weak, pricking sensation in response to the stimulation. The lowest stimulus intensity required to elicit this sensation was defined as the PT [25].
Nociceptive flexion reflex threshold was assessed using a 4-2-1 staircase method previously described by others [24]. The stimulus intensity was increased in 4 mA increments until an NFR response was detected using an online script written with Signal 4.05 software (Cambridge Electronic Design, Cambridge, United Kingdom). A positive NFR response was automatically identified when the mean value of the rectified EMG signal from the biceps femoris muscle during a 90–150 ms post-stimulus time window exceeded the mean value of the pre-stimulus rectified EMG signal (−60–0 ms time window) by at least one standard deviation. This assessment method allowed for online verification that stimuli were delivered with the muscle at rest. Once an initial NFR was detected, the stimulus intensity was then decreased in 2 mA increments until the NFR could no longer be detected. From this subthreshold intensity, the stimulus intensity was adjusted in 1 mA increments and decrements until the NFR appeared and subsided two more times. The NFRT was then defined as the average of the stimulus intensities during the two ascending sequences obtained from the second and third series of the staircase assessment. To minimize prediction of the stimulus times, trains were delivered to the sural nerve at random intervals 5–10 s after participants were verbally instructed to relax. The NFR assessment was discontinued if a maximum stimulus intensity of 40 mA was reached without a detectable NFR response, or if the participant requested the assessment be stopped at any time during the experiment.
Data processing and statistical analyses
The FIQ and BDI questionnaires were scored according to standard procedures [21, 22]. Mean arterial pressure (MAP) was calculated using the following formula: MAP = diastolic blood pressure + (systolic blood pressure−diastolic blood pressure)/3. NFRT data were expressed in terms of both absolute (NFRT (mA)) and relative (NFRT (mA)/PT (mA)) stimulation intensities. Expressing the NFRT as a ratio of the minimum stimulus intensity that can be perceived by each individual accounts for any differences in the efficacy of sural nerve stimulation across participants [26]. In addition, the average of the maximum stimulus intensity delivered during the NFR assessment was calculated for each group to identify any differences in tolerance to electrical stimulation.
Nociceptive flexion reflex responses were successfully elicited from all healthy women, but the NFRT could only be measured in a subgroup of 8 women with FMS (NFR-Responders; see “Results”). Therefore, independent t-tests were used to compare NFR responses in this subgroup of women with FMS to healthy control subjects. Independent t-tests were also used to compare demographic, cardiovascular, and self-report measures between the full sample of FMS patients (n = 15) and healthy controls (n = 14), as well as between NFR-Responders (n = 8) and the subgroup of women with FMS from whom the NFRT could not be measured (NFR-Nonresponders; n = 7). Finally, an exploratory Pearson’s correlation analysis was performed separately for NFR-Responders and healthy controls to identify associations between the NFRT and demographic, cardiovascular, and self-report measures. The significance level was set at α = 0.05 for all analyses.
Results
Comparison of women with FMS and healthy controls
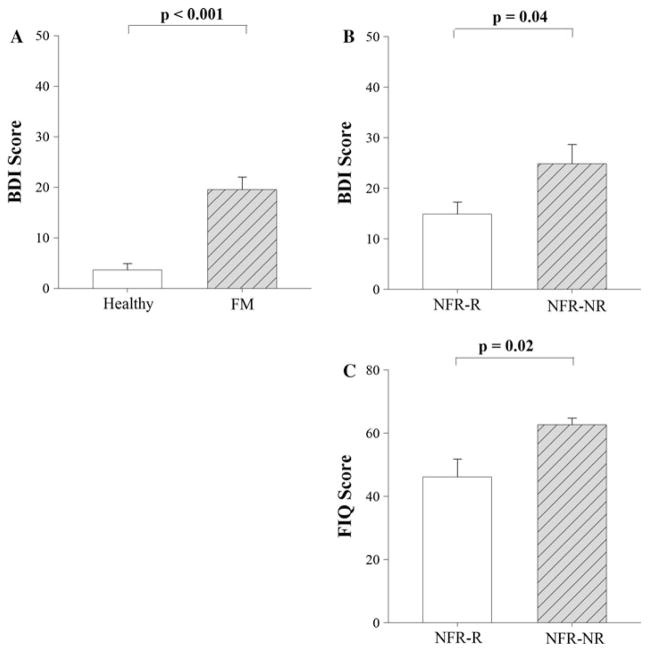
Comparisons between women with FMS and healthy controls (Table 2) revealed no significant differences in age, BMI, MAP, PT, or the maximum intensity of stimulation used in the NFR assessment between groups (t = 0.05–1.96, p >0.05). Women with FMS had a significantly higher resting HR (t = 2.49, p = 0.02) compared to healthy controls. Women with FMS also reported a significantly higher score on the BDI questionnaire compared to healthy controls (t = 5.56, p <0.01; Fig. 1a).
Table 2.
Characteristics of study participants
| Healthy (n = 14) | Total (n = 15) | FMS
|
||
|---|---|---|---|---|
| NFR-R (n = 8) | NFR-NR (n = 7) | |||
| Age (years) | 41.93 (11.46) | 47.07 (12.10) | 47.63 (16.46) | 46.43 (4.96) |
| BMI (kg/m2) | 25.84 (3.94) | 26.99 (5.39) | 24.53 (5.56) | 29.80 (3.79) |
| MAP (mmHg) | 83.29 (11.05) | 90.73 (9.37) | 88.58 (10.07) | 93.19 (8.58) |
| HR (bpm) | 69.79 (7.47)* | 79.07 (11.91) | 77.88 (15.09) | 80.43 (7.83) |
| PT (mA) | 0.78 (0.29) | 0.79 (0.51) | 0.75 (0.43) | 0.83 (0.62) |
| NFRT (mA) | 14.82 (7.90) | – | 11.88 (4.67) | – |
| NFRT/PT | 20.90 (10.51) | – | 18.84 (9.45) | – |
| Highest stimulus intensity (mA) | 17.21 (7.34) | 23.13 (11.27) | 14.25 (4.46)†† | 33.29 (6.90) |
Numbers are group mean (SD)
NFR-R Nociceptive flexion reflex responders, NFR-NR NFR nonresponders, BMI body mass index, MAP mean arterial pressure, HR heart rate, PT perceptual threshold, NFRT NFR threshold
Significant difference between FMS Total and Healthy groups (p <0.05)
Significant difference between NFR-R and NFR-NR groups (p <0.01)
Fig. 1.
a Beck Depression Inventory (BDI) scores were significantly lower for healthy women (n = 14) compared to women with fibromyalgia syndrome (FMS; n = 15). b BDI scores were significantly lower for women with fibromyalgia in the nociceptive flexion reflex responder (NFR-R) group compared to the NFR nonresponder (NFR-NR) group. c Fibromyalgia Impact Questionnaire (FIQ) scores were also significantly lower for the NFR-R group compared to the NFR-NR group. Bars indicate group mean (SEM)
Unexpectedly, NFRT could not be measured from a subgroup of seven women with FMS (NFR nonresponders). For two of these women, an NFR response was initially detected at 28 mA (40 × PT) and 37 mA (37 × PT), respectively. The full NFRT assessment could not be completed, however, due to the patients’ inability to tolerate additional stimuli at these higher intensities. Full NFRT assessments were completed for the remaining five women at maximum tolerated stimulus intensities of up to 40 mA without a detectable NFR response.
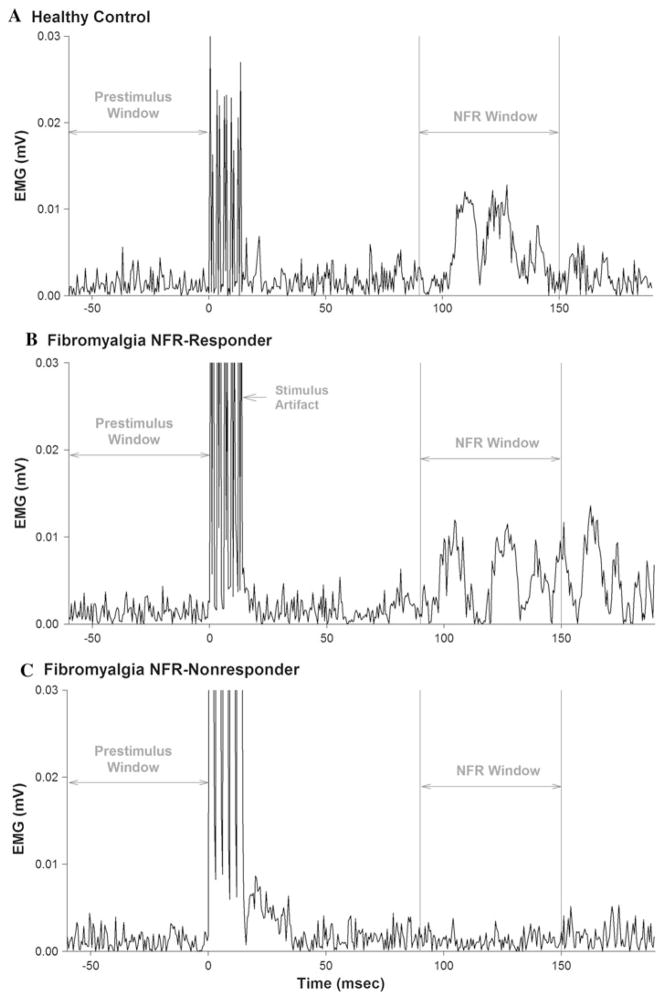
Results from the NFRT assessment revealed that the NFR responders did not differ significantly from healthy controls in the absolute (NFRT: t = −0.96, p = 0.35) or relative (NFRT/PT: t = −0.46, p = 0.65) threshold of stimulation required to elicit an NFR response (Table 2). NFR responses to sural nerve stimulation from representative women in the healthy control group, the NFR responder group, and the NFR nonresponder group are illustrated in Fig. 2.
Fig. 2.
Nociceptive flexion reflexes (NFR) in the biceps femoris muscle are shown for representative women from the healthy control group (a), the NFR responder group (b), and the NFR nonresponder group (c). A positive NFR response was defined as an increase in the rectified electromyographic (EMG) signal within a time window corresponding to the NFR latency (90–150 ms after delivery of a train of electrical stimuli to the sural nerve) that exceeded the mean pre-stimulus EMG by ≥1 SD. Note that no NFR response was detected for the individual shown in (c) at the maximum stimulus intensity of 40 mA (relative stimulus intensity = 19 × perceptual threshold)
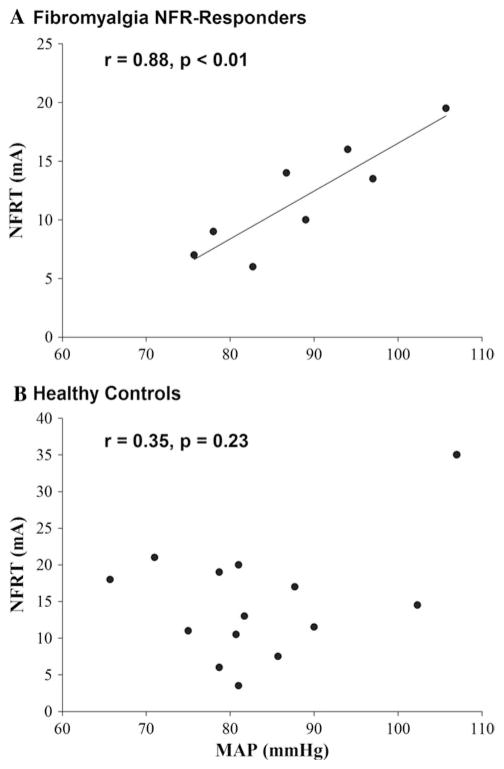
Exploratory correlation analyses indicated that the NFRT was significantly associated with resting MAP in NFR-Responders (r = 0.88, p <0.01; Fig. 3a), but not in healthy controls (r = 0.35, p = 0.23; Fig. 3b). No significant correlations were found between the NFRT and any other cardiovascular, demographic, or self-report measures for either group.
Fig. 3.
The nociceptive flexion reflex threshold (NFRT) was positively correlated with resting mean arterial pressure (MAP) in women with FMS (a), but not in healthy women (b)
Comparison of NFR responders and nonresponders
Comparisons between NFR responders and nonresponders revealed no significant differences in age, BMI, MAP, HR, or PT between groups (t = −2.11–0.20, p >0.05). However, the NFR nonresponders reported significantly higher scores on the FIQ (t = −2.74, p = 0.02; Fig. 1c) and BDI (t = −2.28, p = 0.04; Fig. 1b) questionnaires. Additionally, the NFR nonresponder group was exposed to significantly higher intensities of electrical stimulation (t = −6.43, p <0.01); yet, attempts to elicit the NFR remained unsuccessful. The results for group comparisons between NFR responders and nonresponders did not change when repeated for only the subgroup of five women who completed the full NFR protocol without any detectable reflex response.
Discussion
The purpose of this investigation was to compare NFRT between women with FMS and healthy controls, and to examine the influence of depression and fibromyalgia symptom severity on NFR responses. Contrary to expectation, our results indicated that NFRT could only be measured in a subgroup of women with FMS and that neither the absolute nor relative threshold of stimulation required to elicit an NFR response differed for NFR responders compared to healthy controls. Further, this subgroup of women reported fewer symptoms of depression and a lesser impact of FMS on daily function compared to women with FMS from whom the NFRT could not be measured. Finally, we observed a strong association between NFRT and resting blood pressure among the NFR responders that was not present in healthy controls. These findings suggest an impairment of nociceptive reflex pathways in women with FMS who are more severely impacted by symptoms of depression and fibromyalgia.
Previous comparisons of NFRT between individuals with and without FMS have produced conflicting results, with some studies reporting a lower NFRT among patients with FMS [16, 17] and other studies reporting no difference in NFRT compared to healthy controls [14, 15]. These discrepant findings may be explained by methodological differences across studies, including characteristics of the study population and NFR assessment protocol. We attempted to minimize methodological variability by controlling for individual differences in background muscle activity and stimulation efficacy because it is well-established that these two variables directly affect reflex responses [26, 27]. We found no significant difference in the absolute or relative threshold of stimulation required to elicit an NFR response in the subgroup of women with FMS for whom NFRT could be measured. Therefore, previous findings of reduced NFRT among patients with FMS [16, 17] may have been caused by higher levels of background EMG due to increased resting muscle tension, or a greater efficacy of electrical stimulation in the patient groups.
It is also possible that the failure to identify differences in NFRT between women with and without FMS in this and previous studies [14, 15] was due to an insufficient sample size. Although the NFRT was assessed in 15 women with FMS in the present study, we were only able to analyze responses from a smaller subset of 8 women for whom the NFRT could be reliably measured in a resting muscle. Previous investigations have not reported whether subjects with FMS were excluded from study due to an inability to elicit NFR responses. Our results indicate that nearly half of women with FMS may not exhibit a reproducible NFR response, particularly those who are more severely impacted by symptoms of pain and depression. This may suggest a selection bias in previous studies of NFR for this clinical population. However, the absence of NFR responses among more symptomatic women with FMS in the present investigation is supported by previous reports that patients with more severe depressive symptoms exhibit greater impairments of both NFRT [18] and diffuse noxious inhibitory control [19] compared to patients with less depressive symptoms. Longitudinal studies are needed to determine the time course of changes in central pain processing, depression, and fibromyalgia symptom severity to identify causal relations among these variables.
Our results indicate that the NFR nonresponder group did not differ from the NFR responder group in the use of pain medications, and also received higher intensities of electrical stimulation in attempts to elicit an NFR response. Therefore, failure of the NFRT assessment among the NFR nonresponder group cannot be explained by medications or reduced tolerance to electrical stimulation. Alternatively, failure of the NFRT assessment may suggest an impairment of central pathways underlying the NFR among this subgroup of women with FMS. Some investigators have speculated that central pain modulatory systems may become exhausted by the sustained activation of nociceptive pathways in patients with FMS [4], whereas others have suggested that FMS symptoms may be maintained by chronic hyperactivity of the sympathetic nervous system [28]. Exploratory correlation analyses in the present study indicated that reflex thresholds were strongly associated with resting blood pressure in patients with FMS. This preliminary observation suggests that impairment of the protective withdrawal reflex among more symptomatic patients with FMS is potentially mediated by desensitization of central pain pathways with chronic activation of the autonomic fight-or-flight response. A positive relation between the NFRT and mean arterial blood pressure among women with FMS is consistent with the well-known analgesic effects of the cardiac baroreflex [29, 30], and the absence of a reproducible NFR response in some women with FMS may reflect a failure of this protective mechanism against noxious stimuli. Given the few number of women with FMS who exhibited NFR responses that could be included in the correlation analysis, this hypothesis requires validation in a larger patient sample.
Acknowledgments
We thank Jackie Balter, Carly Nagel, Michaela Nofsinger, and Kelly Santo for their assistance refining the experimental procedures, and with data collection and processing. Funding provided by a John J. Bonica Fellowship from the International Association for the Study of Pain to MU, and a Career Development award from the Colorado Clinical & Translational Institute (NIH KL2RR025779) to KSM.
Footnotes
Conflict of interest Authors declare no conflicts of interest.
Contributor Information
Masataka Umeda, Department of Health, Exercise, and Sport Sciences, Texas Tech University, Lubbock, TX, USA.
Lisa W. Corbin, Center for Integrative Medicine, University of Colorado Hospital, Aurora, CO, USA
Katrina S. Maluf, Email: katrina.maluf@ucdenver.edu, Physical Therapy Program, Department of Physical Medicine and Rehabilitation, University of Colorado, Anschutz Medical Campus, MS C244 Education 2 South, Bldg #L28 13121 E. 17th Ave, Room 3108, Aurora, CO 80045, USA
References
- 1.Buskila D, Cohen H. Comorbidity of fibromyalgia and psychiatric disorders. Curr Pain Headache Rep. 2007;11:333–338. doi: 10.1007/s11916-007-0214-4. [DOI] [PubMed] [Google Scholar]
- 2.White KP, Speechley M, Harth M, et al. The London fibromyalgia epidemiology study: comparing the demographic and clinical characteristics in 100 random community cases of fibromyalgia versus controls. J Rheumatol. 1999;26:1577–1585. [PubMed] [Google Scholar]
- 3.Wolfe F, Ross K, Anderson J, et al. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 4.Gracely RH. Augmented central pain processing in fibromyalgia patients. In: Thomas Graven-Nielsen LA-N, Siegfried Mense, editors. Foundations of musculoskeletal pain. International Association for the Study of Pain; Seattle: 2008. pp. 311–326. [Google Scholar]
- 5.Williams DA, Clauw DJ. Understanding fibromyalgia: lessons from the broader pain research community. J Pain. 2009;10:777–791. doi: 10.1016/j.jpain.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeSantana JM, Sluka KA. Central mechanisms in the maintenance of chronic widespread noninflammatory muscle pain. Curr Pain Headache Rep. 2008;12:338–343. doi: 10.1007/s11916-008-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen LA, Henriksson KG. Pathophysiological mechanisms in chronic musculoskeletal pain (fibromyalgia): the role of central and peripheral sensitization and pain disinhibition. Best Pract Res Clin Rheumatol. 2007;21:465–480. doi: 10.1016/j.berh.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Sandrini G, Serrao M, Rossi P, et al. The lower limb flexion reflex in humans. Prog Neurobiol. 2005;77:353–395. doi: 10.1016/j.pneurobio.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Skljarevski V, Ramadan NM. The nociceptive flexion reflex in humans—review article. Pain. 2002;96:3–8. doi: 10.1016/s0304-3959(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 10.Chan CW, Dallaire M. Subjective pain sensation is linearly correlated with the flexion reflex in man. Brain Res. 1989;479:145–150. doi: 10.1016/0006-8993(89)91344-9. [DOI] [PubMed] [Google Scholar]
- 11.Willer JC. Comparative study of perceived pain and nociceptive flexion reflex in man. Pain. 1977;3:69–80. doi: 10.1016/0304-3959(77)90036-7. [DOI] [PubMed] [Google Scholar]
- 12.Willer JC, Boureau F, Berny J. Nociceptive flexion reflexes elicited by noxious laser radiant heat in man. Pain. 1979;7:15–20. doi: 10.1016/0304-3959(79)90103-9. [DOI] [PubMed] [Google Scholar]
- 13.Lim EC, Sterling M, Stone A, et al. Central hyperexcitability as measured with nociceptive flexor reflex threshold in chronic musculoskeletal pain: a systematic review. Pain. 2011;152:1811–1820. doi: 10.1016/j.pain.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 14.Boureau F, Luu M, Doubrere JF. Study of experimental pain measures and nociceptive reflex in chronic pain patients and normal subjects. Pain. 1991;44:131–138. doi: 10.1016/0304-3959(91)90126-I. [DOI] [PubMed] [Google Scholar]
- 15.Guieu R, Serratrice G, Pouget J. Counter irritation test in primary fibromyalgia. Clin Rheumatol. 1994;13:605–610. doi: 10.1007/BF02243002. [DOI] [PubMed] [Google Scholar]
- 16.Banic B, Petersen-Felix S, Andersen OK, et al. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain. 2004;107:7–15. doi: 10.1016/j.pain.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Desmeules JA, Cedraschi C, Rapiti E, et al. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48:1420–1429. doi: 10.1002/art.10893. [DOI] [PubMed] [Google Scholar]
- 18.Ang DC, Chakr R, France CR, et al. Association of nociceptive responsivity with clinical pain and the moderating effect of depression. J Pain. 2011;12:384–389. doi: 10.1016/j.jpain.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 19.De Souza JB, Potvin S, Goffaux P, et al. The deficit of pain inhibition in fibromyalgia is more pronounced in patients with comorbid depressive symptoms. Clin J Pain. 2009;25:123–127. doi: 10.1097/AJP.0b013e318183cfa4. [DOI] [PubMed] [Google Scholar]
- 20.Wolfe F, Smythe HA, Yunus MB, et al. The American college of rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA, Ball R, et al. Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 22.Bennett R. The fibromyalgia impact questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol. 2005;23:S154–S162. [PubMed] [Google Scholar]
- 23.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18:728–733. [PubMed] [Google Scholar]
- 24.France CR, Suchowiecki S. Assessing supraspinal modulation of pain perception in individuals at risk for hypertension. Psychophysiology. 2001;38:107–113. [PubMed] [Google Scholar]
- 25.Zehr EP, Komiyama T, Stein RB. Cutaneous reflexes during human gait: electromyographic and kinematic responses to electrical stimulation. J Neurophysiol. 1997;77:3311–3325. doi: 10.1152/jn.1997.77.6.3311. [DOI] [PubMed] [Google Scholar]
- 26.Floeter MK, Gerloff C, Kouri J, et al. Cutaneous withdrawal reflexes of the upper extremity. Muscle Nerve. 1998;21:591–598. doi: 10.1002/(sici)1097-4598(199805)21:5<591::aid-mus5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Pearson K, Gordon J. Spinal reflexes. In: Kandel Eric R, Schwartz JH, Jessell Thomas M, editors. Principles of neural science. McGraw-Hill; New York: 2000. pp. 713–736. [Google Scholar]
- 28.Sarzi-Puttini P, Atzeni F, Diana A, et al. Increased neural sympathetic activation in fibromyalgia syndrome. Ann N Y Acad Sci. 2006;1069:109–117. doi: 10.1196/annals.1351.009. [DOI] [PubMed] [Google Scholar]
- 29.Bruehl S, Chung OY. Interactions between the cardiovascular and pain regulatory systems: an updated review of mechanisms and possible alterations in chronic pain. Neurosci Biobehav Rev. 2004;28:395–414. doi: 10.1016/j.neubiorev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Ghione S. Hypertension-associated hypalgesia. Evidence in experimental animals and humans, pathophysiological mechanisms, and potential clinical consequences. Hypertension. 1996;28:494–504. doi: 10.1161/01.hyp.28.3.494. [DOI] [PubMed] [Google Scholar]