Abstract
Social network analysis is increasingly used by behavioral ecologists and primatologists to describe the patterns and quality of interactions among individuals. We provide an overview of this methodology, with examples illustrating how it can be used to study social behavior in applied contexts. Like most kinds of social interaction analyses, social network analysis provides information about direct relationships (e.g. dominant–subordinate relationships). However, it also generates a more global model of social organization that determines how individual patterns of social interaction relate to individual and group characteristics. A particular strength of this approach is that it provides standardized mathematical methods for calculating metrics of sociality across levels of social organization, from the population and group levels to the individual level. At the group level these metrics can be used to track changes in social network structures over time, evaluate the effect of the environment on social network structure, or compare social structures across groups, populations or species. At the individual level, the metrics allow quantification of the heterogeneity of social experience within groups and identification of individuals who may play especially important roles in maintaining social stability or information flow throughout the network.
Keywords: Social networks, Social structure, Animal groups, Social behavior
1. An introduction to the social networks approach
The behavior of individuals both affects and is affected by the presence and behavior of others within their social networks. Social interactions can, for instance, influence how new information or behaviors are transmitted throughout groups. Understanding how such behaviors arise and spread has many potential applications. For example, Nicol (1995) suggested that social learning could be used to increase the efficiency of animal training, and that understanding the social mechanisms underlying the spread of behavior patterns such as feather pecking by poultry could allow researchers to target remediation strategies. Here we describe a powerful methodology, social network analysis, that can be used to explore these types of questions.
Social network analysis is an approach for examining and quantifying the patterns of relationships that arise among interacting social entities, typically individuals. An explicit assumption of this approach is that indirect relationships (i.e. “friends of friends”) in social groups matter. A particular strength of social network analysis is that it provides standardized mathematical methods for calculating measures of sociality across levels of social organization, from the population and group levels to the individual level (Freeman, 1984; Sih et al., 2009; McCowan et al., 2011). These quantitative measures can be used to describe and compare components of social network structures and to statistically test hypotheses about factors affecting social structures of groups or the effects of social structure on behavior. For example, at the group level these metrics can be used to track changes in social network structures over time, evaluate the effect of the environment on social network structure, or compare social structures across groups, populations or species. At the individual level, these measures allow quantification of the heterogeneity of social experience within groups. Additionally, they can be used to test hypotheses about how an individual's social role affects social stability or information flow throughout the network (e.g. Flack et al., 2006; Lusseau, 2007; Beisner et al., 2011a,b; McCowan et al., 2011).
The use of network analysis to understand emergent structure is not novel (Freeman, 1984). Rooted in mathematical graph theory, this approach has been employed across disciplines, including in studies of transportation systems (e.g. Sen et al., 2003), business and economics (e.g. Levine, 1972), and the spread of information (e.g. Albert et al., 1999; Liben-Nowell and Kleinberg, 2008) and computer viruses (e.g. Newman et al., 2002) through the internet. In the biological sciences, network approaches have been used to explore diverse phenomena ranging from cellular organization (reviewed by Barabási and Oltvai, 2004; Bullmore and Sporns, 2009) to food webs (reviewed by Ings et al., 2009) and disease spread (reviewed by Martínez-López et al., 2009). The use of social network analysis for studying sociality is also not new. The social network approach has a long history in social psychology and sociology (reviewed by Wasserman and Faust, 1994; Scott, 2000). While its value for studying social interactions of animals was recognized over three decades ago (Wilson, 1975), it was not until the mid-1990s that the methodology gained popularity (e.g. Sade and Dow, 1994). Over the past 15 years, social network analysis has become an increasingly common tool for studying animal behavior, particularly in the fields of primatology (e.g. Flack et al., 2005; McCowan et al., 2008; Beisner et al., 2011a,b; McCowan et al., 2011), behavioral ecology (e.g. Lusseau, 2003; Croft et al., 2004; Edenbrow et al., 2011) and epidemiology (e.g. Corner et al., 2003; Otterstatter and Thomson, 2007).
The development and widespread availability of comprehensive social network analysis software (discussed below), and the publication of a series of review articles highlighting the utility of social network analysis approaches in animal behavior research (e.g. Krause et al., 2007; Wey et al., 2008; Krause et al., 2009; Sih et al., 2009; Sueur et al., 2011), have contributed to the observed increase in the tool's popularity. These reviews have especially highlighted the value of social network analysis for assessing the effects of social network structure on disease spread, information flow, group dynamics, and future reproductive fitness. The utility of social network analysis for assessing the heterogeneity of social environments experienced by individual group members and for identifying individuals who may play especially important social roles, for example in maintaining group stability (Flack et al., 2005; McCowan et al., 2008; Beisner et al., 2011a,b; McCowan et al., 2011), has also been emphasized. Interestingly, despite suggestions that the social network approach could be used to provide new insights with respect to animal management and welfare issues (Krause et al., 2007), few such studies have been conducted (but see Durell et al., 2004; Webb, 2005; McCowan et al., 2008; Böhm et al., 2009; Cañon Jones et al., 2010; Beisner et al., 2011a,b; McCowan et al., 2011).
In this paper we provide an overview of the potential importance of social network analysis for applied animal behavior science. The sections that follow present an introduction to social network analysis visualization and quantification tools, and discuss the utility of these tools for applied animal behavior research.
2. Social network analysis tools
Multiple software packages that are capable of analyzing relationships among tens of thousands of individuals are currently available, many offering free trial versions (for reviews see Huisman and van Duijn, 2005; Martínez-López et al., 2009). Most social network analysis software packages include programs for visually exploring social network structure, calculating a variety of network metrics and conducting some statistical network analyses. Further analyses, including hypothesis testing (see Croft et al., 2011, for a review of constraints and limitations), can be conducted by exporting network measures into standard statistical software.
2.1. Visualizing social networks
The notion of graphically representing social relationships is not new (e.g. Foster, 1963). However, the relatively recent development and widespread accessibility of network visualization software has improved the ease with which such complex social structures can be visualized and explored. In addition to instantly graphing the relationships of thousands of individuals and highlighting relationship characteristics and individuals’ attributes, most software packages provide graph manipulation features which facilitate the exploration of emergent social patterns.
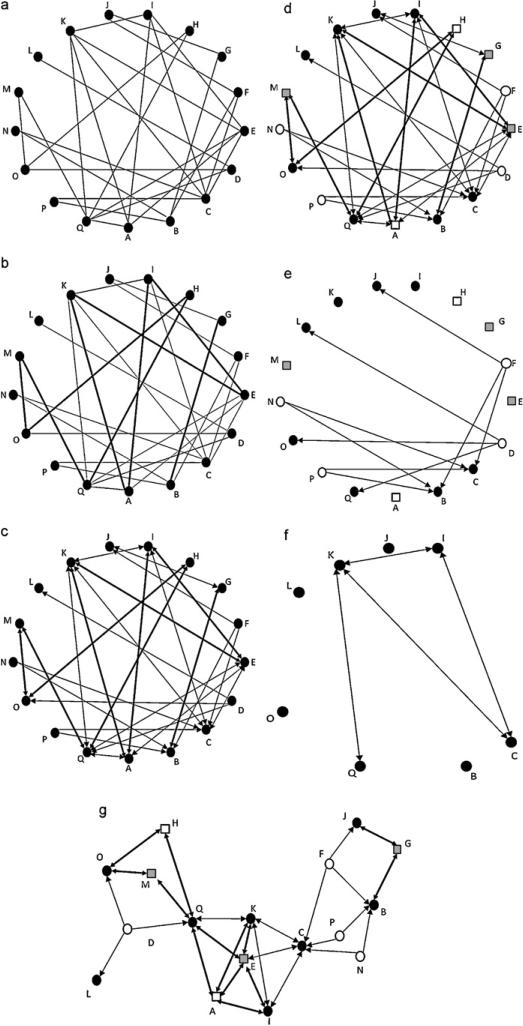
Social networks are typically represented as sociograms. Interacting entities are drawn as nodes. In behavioral studies nodes typically represent individuals. However, they can represent any interacting entities, such as groups of individuals or populations. Relationships between the nodes are represented by edges. The most basic sociograms therefore simply show the presence or absence of a relationship between each pair of nodes in the network (Fig. 1a). Complexity can be added to the graph by mapping on relationship characteristics and node attributes. For example, the thickness of each edge in a weighted graph (Fig. 1b) shows relative relationship strength, such as interaction frequency, rate or duration. Arrows can be used to indicate the directionality of the relationships (Fig. 1c). Such directed graphs are especially useful for exploring interaction data, such as grooming events or instances of aggression. When the individuals’ attributes (e.g. sex, age, relatedness, dominance status, etc.) are of interest, these can be represented by node size, shape or color (Fig. 1d).
Fig. 1.
(a) A sample binary sociogram. Nodes (○) represent interacting individuals. Edges (-) represent relationships between the nodes. (b) A weighted network. In weighted networks interaction strengths are represented by the thickness of the edge. (c) A weighted and directed network. Direction of the interactions is mapped on using arrows, which point away from the actor and toward the receiver. (d) A weighted and directed network highlighting node attributes. Characteristics of the nodes are drawn on by manipulating node size, shape or color. In this example shading has been use to denote gender: females (•) versus males (○). Shape indicates life stage: adults (○) versus juveniles (□). (e) The weighted and directed network shown in Fig. 1d filtered for unidirectional relationships. All reciprocal relationships have been removed. (f) The weighted and directed network shown in Fig. 1d filtered for adult female members. All other nodes have been removed. (g) The weighted and directed network shown in Fig. 1d shown in a layout that clumps highly connected individuals.
Most social network visualization software offers a wide variety of tools to aid in data exploration and hypothesis formation. Of these, perhaps the most broadly useful ones are the data filtering and layout options. Data filtering facilitates the exploration of just one component of the network. For example, for research questions about unidirectional relationships one may opt to filter all reciprocal interactions from the graph. Fig. 1e shows a spectrogram of the sample data filtered in this manner. A quick investigation of the spectrogram makes it clear that within this group only males interact in a unidirectional manner, always directing their behavior toward the adult females. The filtering tool can also be used on nodes. Fig. 1f shows the result of using this option to highlight the relationships among adult females in the sample group. Once again clear patterns of interaction immediately emerge, namely that the adult females are involved in reciprocal relationships.
Layout tools can be used to reformat the graph to highlight properties of relationships. In the previously discussed spectrograms (Fig. 1a–f) individuals have been represented as nodes positioned in random order around the circumference of a circle. The position of the node can be manipulated to reference relative geographic location or node attributes, such as relatedness. An especially useful layout clumps highly connected individuals (Fig. 1g). This layout emphasizes the variation in roles of individuals within the social network by, for example, highlighting the position of so-called cutpoints. Cutpoints are individuals who connect otherwise separate subgroups (Croft et al., 2008). Due to their position in the network they likely play a key role in social interactions, for example in the dissemination of information throughout the network. Identification of cutpoints may therefore have implications for animal management, as the removal of cutpoint individuals from the group is likely to disrupt the flow of information, which may in turn negatively impact the structure of the group.
Although for simplicity we have been referring to nodes as individuals, an important point to reiterate is that a node can be any number of entities (individual, subgroup, group, behavior, location). Edges can likewise represent a variety of relationships (social behaviors, affiliations, vectors). For example, the network of information flow during pollen foraging by honeybees can be depicted as the behaviors associated with pollen foraging (nodes) connected by vectors (edges) that represent the individual bees that regulate the performance of these behaviors (see Fig. 1 in Fewell, 2003). The resulting sociogram highlights how the behavior of particular classes of worker bees modulates the behavior of other classes of worker bees. Similarly, the potential for disease spread among groups of cattle can be modeled by mapping the movement of animals (edges) between farms, auction yards and slaughterhouses (nodes; e.g. Bigras-Poulin et al., 2006).
2.2. Social network metrics
Social network analysis is comprised of five major principles and a battery of quantitative techniques that measure variables derived from each of these principles at multiple levels of analysis (Wasserman and Faust, 1994; summarized in Table 1). These principles include:
Prominence, an indicator of who is “in charge”.
Range, an indicator of the extent of the actor's or node's network.
Cohesion, which is the grouping of actors or nodes according to strong common relationships with each other.
Structural equivalence, which is the grouping of actors or nodes according to similarity in their overall social environments.
Brokerage, which indicates the bridging of otherwise unconnected components within or between networks.
Table 1.
Summary of key terms and network measures.
| Terminology | Description | Level of analysis |
|---|---|---|
| Node | Social entity (i.e. individual, group) | |
| Edge | Relationship between two nodes | |
| Measures of prominence | ||
| Degree centrality | Number of direct connections a focal node has to other nodes in the network | Individual |
| Degree centralization | Variation in degree centralities of nodes within the network | Network |
| Eigenvalue centrality | A weighted version of degree centrality | Individual |
| Closeness centrality | Sum of shortest paths that connect a focal node to all other nodes in the network | Individual |
| Closeness centralization | Variation in closeness centrality measures of nodes within the network | Network |
| Betweenness centrality | Proportion of shortest paths between any two nodes in the network that pass through the focal node | Individual |
| Betweenness centralization | Variation in betweenness centrality measures of nodes within the network | Network |
| Information centrality | A weighted version of betweenness centrality | Individual |
| Measures of range | ||
| Reach | Number of edges separating the focal node from other nodes of interest | Individual |
| Diameter | Longest distance between any two nodes in the network | Network |
| Measures of cohesion | ||
| Density | Proportion of all possible connections that are present in the network | Network |
| Reciprocity | Extent to which pairs of nodes make reciprocal connections to each other | Network |
| Clustering coefficient | Tendency of groups of nodes to be interconnected with each other | Network |
| Fragmentation | An inverse measure of the amount of connection redundancy in a network | Network |
| Assortativity | Tendency of nodes with certain attributes (sex, age, node degree centrality) to interact with each other | Network |
| Affiliation networks | A special type of social network that explores relationships between entities and events |
Using these principles, network analysis can explore relational properties of social networks, such as how cohesive a group is or what subgroups of interconnected nodes exist, and positional properties, such as who occupies different roles and positions in a network. As such, roles and positions in networks are identified by examining similarities in connections among the nodes in a network.
For applied animal behaviorists, the most useful types of measures are found under the principles of prominence, range and cohesion. In addition, structural equivalence seems an interesting method to evaluate dominance or other hierarchical relationships in some animal groups (e.g. how similarities in social connections of different hens in a flock relate to their individual positions within the pecking order) and brokerage might be useful for looking at the bridges of connections between animal subgroups with different attributes (e.g. the role specific matrilines play in connecting other matrilines in a large rhesus macaque group). These measures can be quantified at multiple levels of analysis, but most commonly are used at the individual and group (or subgroup) levels. Below we define and describe the utility of some of the more common measures under each principle; brief definitions are also provided in Table 1. The mathematical derivations and detailed descriptions of these measures can be found in many excellent previous publications (e.g. Wasserman and Faust, 1994; Carrington et al., 2005; Whitehead, 2008; Croft et al., 2008).
2.2.1. Prominence: centrality and centralization
A primary use of graph theory in social network analysis is to identify “important” or “key” actors. Centrality concepts quantify theoretical ideas about an individual actor's or node's prominence within a network. Group-level measures of centrality, known as centralization, assess the inequality among the prominences of all individuals across a network. These measures quantify the extent to which a set of actors are organized around a central node. Especially useful metrics of prominence include degree, closeness, betweenness, information centrality and eigenvalue centrality.
2.2.1.1. Degree
Degree refers to the number of direct connections an actor or node has with other network nodes. For example, in Fig. 1b individual B is connected to four other individuals, and therefore has a node degree of 4. In directed networks, a node's in-degree, the number of connections incoming from others to the node, can be distinguished from out-degree, the number of connections that are outgoing from the node to others. For example, in Fig. 1c individual N has an in-degree of 0 and an out-degree of 2, while individual O has an in-degree of 3 and an out-degree of 2. At the individual level, degree centrality could be used to look at the differential roles that individuals play in behavioral networks (e.g. if certain chickens are overall more involved in aggressive pecking behavior than others). Analyses of interaction asymmetries (in-degree versus out-degree) may uncover important differences in individual's roles within the network (e.g. how incoming and outgoing grooming behavior in nonhuman primate groups relates to dominance interactions). At the group level, degree centralization can be used to compare the extent to which groups are centered around one or two highly connected individuals (that is, have a high level of centralization) or in which dispersion of degree centrality is more uniformly distributed across individuals or nodes (that is, have a low level of centralization). The extent of centralization in a network can have a profound effect on its underlying cohesiveness and thus could be used to compare stability across different networks or groups.
2.2.1.2. Closeness
A node that is close to many others can quickly interact and communicate without going through many intermediaries. Its interactions are more efficient and accurate, resulting in less information loss due to disruption of transmission. Closeness is a measure of the minimum cumulative distance at which a node is connected to others in the network. It differs from degree in that, while degree takes into account only direct relationships, closeness takes into account both direct and indirect connections. Closeness centrality measures might be used to model disease transmission or social transmission of abnormal behaviors in captive social groups or to identify high risk individuals (those with relatively high closeness centrality). Closeness centralization measures might be used to evaluate how different groups compare in behaviors that affect health outcomes in captive groups. For example, transmission of lice or mites may be expected to occur faster in groups with high closeness centralization of grooming behavior than those with lower closeness centralization measures.
2.2.1.3. Betweenness
A node with high betweenness occupies a “between” position on the paths connecting many pairs of other nodes in the network. This measure differs from closeness in that it is the number of other paths through which others in the network can travel that is the important measure, not the directness or distance of edges. If the number of alternate paths (i.e. paths that do not include the focal node) is low, the individual has a high betweenness. Cutpoint individuals that connect subgroups within the network (e.g. individuals C and Q in Fig. 1g) represent a case of extremely high betweenness centrality, as they are necessarily on all paths between members of the different subgroups. Clearly, cutpoints and other high betweenness individuals are important as they may control the flow of information or the exchange of resources between group members. Betweenness centrality may therefore be especially useful for looking at the roles individuals play in maintaining cohesiveness of a network (Lusseau, 2007; but see Flack et al., 2006). At the group level, betweenness centralization measures the extent to which a few individuals are fundamental to maintaining group cohesiveness relative to other groups. Betweenness centralization may therefore be useful in testing hypotheses about the effects of age or group membership on group cohesiveness.
2.2.1.4. Information centrality and eigenvalue centrality
Two other measures of centrality that are worth mentioning in the applied animal behavior context are information centrality and eigenvalue centrality. Information centrality is similar to betweenness centrality except that it takes into account node degree. Individuals with high degree (many direct connections) may be more likely to be hubs of information flow (have high betweenness) simply because they have more connections than others in the network, a phenomenon which is certainly seen in animal social networks. Therefore, information centrality re-calculates betweenness centrality based upon node degree measures.
Eigenvalue centrality (as well as Boncich's Power) takes into account not only how well an individual or node is connected (degree centrality) but also how well connected each of its “friends” are. This measure of centrality highlights that, all else being equal, an individual is more likely to play a key role in disease or information flow when his/her immediate “friends” are themselves well-connected. One can imagine multiple scenarios in captive animal groups in which such indirect degree information would be crucial to the understanding of patterns of relationships. For example, individuals may be expected to preferentially direct their affiliative interactions at group members that have influence over many others in the network.
2.2.2. Range
At the individual level range, or the extent of the network, can be evaluated using a variety of measures. One of these is reach, which calculates the number of network nodes that a focal individual can reach within a specified distance (i.e. within a specified number of edges). Special cases of these measures are used to calculate closeness, degree, and other measures as described above. The most common metric of range at the network level is diameter. The diameter of a network is an indicator of the network's size: that is, how many steps along edges are necessary to get from one side of a network to the other. Reach can be used to test hypotheses about the influence of indirect relationships on behavior or dominance rank. For example, it can be used to evaluate whether only direct or secondary connections matter, or if more distantly connected nodes also have an impact on the focal individual's behavior or rank. Reach and diameter are also useful quantities in that they can be used to set an upper bound on the lengths of connections under study. Many researchers limit their explorations of the connections among actors to involve connections that are no longer than the diameter of their networks.
2.2.3. Cohesion
As discussed above, many measures can be used to examine the role(s) that specific individuals play in maintaining cohesion in a network, such as betweenness and cutpoints. At the group-level, centralization (degree to which a set of nodes are organized around a central node; see above) can be used as a measure of network cohesion. Other measures that can provide insight into animal behavior processes include density, reciprocity, transitivity (represented by the clustering coefficient), fragmentation, assortativity and community structure.
2.2.3.1. Density and reciprocity
Density measures the degree to which all members of a population interact with all other members. Dense networks contain redundant connections, and may therefore be less likely to be affected by the removal of a randomly selected node. Reciprocity represents the degree to which individuals in a group reciprocate the connections among one another. It is useful for measuring cohesion in groups, because when the connections are mutual there are more paths and directions through which information can flow in the network.
2.2.3.2. Clustering coefficient
The clustering coefficient is a measure of the degree to which nodes in a graph tend to cluster together. As a measure of transitivity (A connected to B who is connected to C who is connected to A) it can be used to measure an individual's neighborhood cliquishness using the local clustering coefficient. The network's overall cliquishness can be determined using an averaged measure of the local clustering coefficient across individuals. The global clustering coefficient measures the degree to which the entire network is composed of transitive relationships and thus measures the degree to which the network is connected. These measures of clustering could be used to evaluate robustness or cohesiveness in grooming or other affiliative networks and thus both individuals’ positions in such networks as well as overall group stability.
2.2.3.3. Fragmentation
Using the concept of cutpoint, fragmentation is defined as the proportion of mutually reachable nodes as each node is removed from the network (Borgatti, 2003). Fragmentation is an inverse measure of the amount of connectedness or connection redundancy in a network. McCowan et al. (2008) measured the fragmentation of captive rhesus macaque dominance interaction networks. They hypothesized that lower fragmentation (thus a higher degree of redundancy in dominance interactions among individuals) should result in less ambiguity about dominance relationships. Less ambiguity about dominance relationships should result in lower levels of aggression during displacement activities. Fragmentation was positively associated with wounding rates in captive rhesus macaques, indicating its utility as an inverse measure of cohesiveness or connectedness in animal networks.
2.2.3.4. Assortativity
The assortativity coefficient is a measure of the amount of mixing between and across subgroups of animals with certain attributes (sex, age, node degree) as compared to that expected by chance. Flack et al. (2006) used the assortativity coefficient to assess whether changes in affiliative behaviors occurred when key individuals were temporarily removed from a pigtail macaque group and found that more assortative mixing occurred when key individuals were absent.
2.2.3.5. Communities
Communities are structural relationships in groups that are above the individual level but below the population level. Girvan and Newman (2002) developed an algorithm to quantify the number of communities in a network using the notion of edge betweenness, which is the number of shortest paths between nodes that make use of an edge or connection. They determined the number of communities by successively removing the edge with the highest betweenness until each individual in a network was its own community. To determine whether the communities detected using this method were meaningful, they calculated a stopping parameter, known as modularity or Q. Q measures the extent to which edges between individuals are intra- rather than inter-community, and is defined as the number of edges within the community minus the expected number of edges within the community if connections were random (Newman and Girvan, 2004). Numerous studies have used this measure of community (e.g. Lusseau and Newman, 2004; Kasper and Voelkl, 2009; Clark, 2011). One recent study looked at the effect of degree of relatedness in captive rhesus macaque matrilines, called matriline fragmentation, on grooming community structure (Beisner et al., 2011a). It found that higher matriline fragmentation resulted in a greater number of grooming communities, which in turn was associated with higher rates of aggression and wounding.
2.2.4. Affiliation networks
A special type of social network is the affiliation network. Unlike previously described social networks that focus on relationships between interacting entities (e.g. individuals, groups), affiliation networks explore relationships that arise from common involvement in activities or other social events. To that end, affiliation network analysis simultaneously concentrates on the interacting entities (individuals, groups, etc.) and the events in which those actors are involved. In affiliation networks both the actors and the events are represented as nodes; the edges connect the actors to the social events in which they partake. Affiliation networks can therefore be viewed from the perspective of the actors (since co-participation in events links actors together) or the perspective of the events (since participation of the same actors in multiple events links the events together; Faust, 2005). Such approaches have previously been used to examine a variety of social affiliations, including patterns of connections between boards of major banks and major industries (Levine, 1972), membership in voluntary associations (McPherson, 1982), and co-authorship of scientific publications (Newman, 2001). These types of networks can also be used to look at how animals interact with components of their environment.
3. Examples of the utility of social network analysis for applied animal behavior and welfare research
The ability to gain a new understanding of complex social structures by quantifying the social networks of animal groups and identifying the social roles of individual group members has important implications for applied animal behavior and welfare research. Here we provide a few examples of how the social network approach has been or could be used to generate and test hypotheses, including related to the development and spread of abnormal or undesirable behaviors in stable social groups, the occurrence of aggression during mixing of animals, and the effects of social structure on health outcomes or resource use.
3.1. Development and spread of abnormal or undesirable behaviors
Social network tools that are used to evaluate the spread of information (e.g. Fewell, 2003; Lusseau, 2003) and disease (e.g. Corner et al., 2003; Otterstatter and Thomson, 2007) across groups can also be used to answer questions about the development and spread of abnormal or undesirable behaviors. For example, if affiliative interactions mitigate the spread of abnormal behavior, one may expect high redundancy in the affiliation networks (indicated by high network density or low network fragmentation) to result in low frequencies of abnormal behavior. It is possible for the speed of spread to be, in turn, correlated with the density or closeness centralization of the interaction network, with the spread being slower when interaction network density or closeness centralization is low. The degree to which the behavior spreads across the group will also depend on the social environments of the individuals who display the behavior. Individual-level measures, such as closeness centrality and betweenness centrality may therefore prove useful for identifying individuals who, because of their social position, play key roles in promoting the spread of the behavior to the entire group.
Social network metrics have recently been used to evaluate the factors affecting outbreaks of aggressive behavior in groups of Atlantic salmon and rhesus macaques. Cañon Jones et al. (2010) examined how feed restriction of Atlantic salmon affected social structure and the occurrence of fin biting. Feed restricted groups of fish were found to form denser affiliative and aggressive networks, indicating that the individuals in these groups interacted with more of their group members than in non-restricted groups. In- and out-degree centrality measures of the aggression network indicated that individuals in the feed restricted group played different roles in the aggressive interactions in terms of whether they were the instigator or receiver of attacks. The authors concluded that the ability to identify these key individuals has important implications in the control of fin damage in situations when feed is restricted (e.g. during transport). McCowan et al. (2008, 2011) explored components of social structure that promote social stability and reduce aggression among captive macaques. They concluded that monitoring quantitative changes in measures of centrality and cohesion, including degree within the network of subordination behaviors and network fragmentation, could be used to predict the onset of aggressive outbreaks. These kinds of metrics could be useful for testing hypotheses about the development and spread of many behaviors of interest in applied ethology, for example hypotheses about the relationships between flock size, social structure, and rates of aggression or cannibalism in poultry (Mench and Keeling, 2001).
3.2. Regrouping of animals
The regrouping of animals is a common component of research, farm and zoo animal husbandry protocols. While some species habituate to even repeated changes in group composition (e.g. calves: Veissier et al., 2007), for others this practice can lead to the destabilization of the social structure resulting in injurious aggression (e.g. pigs: Coutellier et al., 2007; rhesus macaques: McCowan et al., 2008, 2011). Gaining a more complete understanding of the interaction between social structure and group stability has clear applications for managing aggression related to the practice of remixing. For instance, the removal of animals at random from highly interconnected networks (networks that are characterized by high global clustering coefficients and low network fragmentation measures), might be expected to have less of an effect on group stability than removing animals from less cohesive groups. Conversely, the addition of animals to a group may have the least effect when the individuals within that group are loosely connected (as indicated by low density, high diameter or a low global clustering coefficient). The extent to which a few group members share the responsibility for maintaining group stability (betweenness centralization), is also important for assessing the relative resilience of groups to remixing events. The ability to identify individuals who play key roles in maintaining group cohesiveness is undoubtedly relevant to management decisions regarding which animals to move. Such information can be obtained by calculating individual-level centrality measures, including betweenness or eigenvalue centrality.
Several studies have used social network analysis to examine how changes in social group composition affect social stability. Lusseau (2003) quantified the diameter and clustering coefficient of a network of dolphins. He developed a computer model of the responses of the network to the removal of individuals from the group. He found that the diameter of the network increased only minimally even when up to 30% of the group members were removed, indicating that the dolphin group was highly resilient to changes in membership. Flack et al. (2006) examined changes in the social structure of pigtail macaque groups following the physical removal of individuals previously identified as important for managing conflict resolution (individuals with high weighted degree metrics). Removal of such key individuals resulted in the destabilization of the social network structure, characterized by higher assortativity measures (indicating decreased diversity of social partners), lower reach among the majority of individuals (indicating fewer social partners), and higher local clustering coefficients (indicating a preference for interacting with socially proximate individuals and hence a less open social environment).
3.3. Impact of social environment on health
Social status has been shown to affect the ability of individuals to cope with stress, and thus their subsequent health outcomes (e.g. pigs: Sutherland et al., 2006; primates: Sapolsky, 2005). Social network analysis can be used to explore these relationships further by using other measures of social position, such as local clustering coefficients or betweenness, and health. Additionally, social network analysis can explore the relationship between social structure and disease spread, since the likelihood of disease spread throughout a group of animals may be dependent on the structure of the interaction network of the group. For example, pathogens can be more quickly transmitted when the social network is highly dense, its diameter is small and its global clustering coefficient is high. Determining local clustering coefficient, betweenness centrality and reach may thus be useful for assessing the role of individuals in propagating a disease. Such approaches have already been employed in preventative veterinary medicine. Corner et al. (2003) showed that centrality measures, including closeness and betweenness, were accurate predictors of risk for Mycobacterium bovis transmission via den sharing among captive brushtail possums. Böhm et al. (2009) studied networks of social contact within and between wild badger and cattle groups. Using social network metrics they identified individuals who were central for disease transmission within and between the species. The authors suggested that the efficiency of disease management could be increased by targeting these high-risk individuals for testing and preventative measures.
3.4. Evaluating resource use
Another area in which social network analysis might prove useful is in evaluating resource use by populations of animals. Bode et al. (2011) have recently used conceptual models to explore the effects of social network structure on aspects of collective movement, including group cohesion, individual spatial position and hierarchical group dynamics. A similar approach can be used to explore the relationships between social structure, behavioral synchrony, leadership and resource use. Interesting questions include how measures of network centralization and fragmentation affect synchrony of resource use, and whether measures of reach, information centrality or betweenness centrality can be used to identify individuals that play leadership roles in initiating synchronous behaviors.
Another approach to addressing resource use questions is to use affiliation networks. Typically such networks graph co-membership of individuals or groups of individuals in social events. One might, however, use affiliation networks to analyze how cattle utilize water troughs to ensure that the troughs are appropriately placed for most efficient use. In this case, the troughs and individual cows would be mapped as nodes, with edges representing the use of specific troughs by the individuals. The network analysis could then focus on connection among water troughs, giving information about the importance of each water trough to cows in relationship to their location. Conversely, one can also focus the analysis on the individuals to see how cows are connected through use of water troughs, which could be related to probability or spread of disease. Affiliation networks could therefore provide a unique way to look at networks such as resource networks both from the top down (e.g., troughs as nodes, shared cow use as edges) or the bottom up (e.g. cows as nodes, shared trough use as edges).
4. Conclusions
Like other methods for evaluating social behavior and social structures, social network analysis has limitations. For very small groups more traditional methods provide the same information while using a simpler approach. For very large groups, or short-term groups where individual animals are used as nodes, it may not be possible to collect enough data to construct meaningful networks. These limitations may have contributed to the under-utilization of this tool by applied ethologists. However, when collection of representative data is possible, social network analysis provides a very powerful approach for generating and testing hypotheses about social behavior relevant to a variety of applied questions, allowing one to evaluate the importance of indirect social connections, gain a detailed understanding of complex social interrelationships in groups, assess whether or how information or behavior flows through groups, and evaluate the effects of social structure on resource use and welfare outcomes.
Acknowledgement
We thank the organizers of the 44th ISAE Congress (Uppsala, Sweden) for supporting this plenary lecture.
Footnotes
Conflict of interest
None.
References
- Albert R, Jeong H, Barabási A-L. Internet: diameter of the worldwide web. Nature. 1999;401:130–131. [Google Scholar]
- Barabási A-L, Oltvai ZN. Network biology: understanding the cell's functional organization. Nat. Rev. Genet. 2004;2:101–113. doi: 10.1038/nrg1272. [DOI] [PubMed] [Google Scholar]
- Beisner BA, Jackson ME, Cameron AN, McCowan B. Detecting instability in animal social networks: genetic fragmentation is associated with social instability in rhesus macaques. PLoS ONE. 2011a;6:e16365. doi: 10.1371/journal.pone.0016365. doi:10.1371/journal.pone.0016365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisner BA, Jackson ME, Cameron A, McCowan B. Effects of natal male alliances on aggression and power dynamics in rhesus macaques. Am. J. Primatol. 2011b;73:790–801. doi: 10.1002/ajp.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigras-Poulin M, Thompson RA, Chriel M, Mortsen S, Greiner M. Network analysis of Danish cattle industry trade patterns as an evaluation of risk potential for disease spread. Prev. Vet. Med. 2006;76:11–39. doi: 10.1016/j.prevetmed.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Bode NW, Wood AJ, Franks DW. The impact of social networks on collective motion. Anim. Behav. 2011;82:29–38. [Google Scholar]
- Böhm M, Hutchings MR, White PCL. Contact networks in a wildlife-livestock host community: identifying high-risk individuals in the transmission of bovine TB among badgers and cattle. PLoS ONE. 2009;4:e5016. doi: 10.1371/journal.pone.0005016. doi:10.1371/journal.pone.0005016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgatti S. The key player problem. In: Breiger RCK, Pattison P, editors. Dynamic Social Network Modeling and Analysis: Workshop Summary and Papers. The National Academies Press; Washington, DC: 2003. pp. 241–252. [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Cañon Jones HA, Hansen LA, Noble C, Damsgård B, Broom DM, Pearce GP. Social network analysis of behavioural interactions influencing fin damage development in Atlantic salmon (Salmo salar) during feed-restriction. Appl. Anim. Behav. Sci. 2010;127:139–151. [Google Scholar]
- Carrington P, Wasserman S, Scott J. Models and Methods in Social Network Analysis. Cambridge University Press; Cambridge: 2005. [Google Scholar]
- Clark FE. Space to choose: network analysis of social preference in a captive chimpanzee community, and implications for management. Am. J. Primatol. 2011;73:748–755. doi: 10.1002/ajp.20903. [DOI] [PubMed] [Google Scholar]
- Corner LA, Pfeiffer D, Morris RS. Social network analysis of Mycobacterium bovis transmission among captive brushtail possums (Trichosurus vulpecula). Prev. Vet. Med. 2003;59:147–167. doi: 10.1016/s0167-5877(03)00075-8. [DOI] [PubMed] [Google Scholar]
- Coutellier L, Arnould C, Boissy A, Orgeur P, Prunier A, Veissier I, Meunier-Salaün MC. Pig's responses to repeated social regrouping and relocation during the growing-finishing period. Appl. Anim. Behav. Sci. 2007;105:102–114. [Google Scholar]
- Croft DP, Krause J, James R. Social networks in the guppy (Peocilia reticulata). Proc. R. Soc. Lond. B. 2004;271:S516–S519. doi: 10.1098/rsbl.2004.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft DP, James R, Krause J. Exploring Animal Social Networks. Princeton University Press; Princeton, NJ: 2008. [Google Scholar]
- Croft DP, Madden JR, Franks DW, James R. Hypothesis testing in animal social networks. TREE. 2011;26:503–507. doi: 10.1016/j.tree.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Durell JL, Sneddon IA, O'Connell NE, Whitehead H. Do pigs form preferential associations? Appl. Anim. Behav. Sci. 2004;89:41–52. [Google Scholar]
- Edenbrow M, Darden SK, Ramnarine IW, Evans JP, James R, Croft DP. Environmental effects on social interaction networks and male reproductive behaviour in guppies Poecilia reticulate. Anim. Behav. 2011;81:551–558. [Google Scholar]
- Faust K. Using correspondence analysis for joint displays of affiliation networks. In: Carrington PJ, Scott J, Wasserman S, editors. Models and Methods in Social Network Analysis. Cambridge University Press; Cambridge, MA: 2005. pp. 117–147. [Google Scholar]
- Fewell JH. Social insect networks. Science. 2003;301:1867–1870. doi: 10.1126/science.1088945. [DOI] [PubMed] [Google Scholar]
- Flack JC, Krakauer DC, de Waal FBM. Robustness mechanisms in primate societies: a perturbation study. Proc. R. Soc. Lond. B. 2005;272:1091–1099. doi: 10.1098/rspb.2004.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flack JC, Girvan M, de Waal FBM, Krakauer DC. Policing stabilizes construction of social niches in primates. Nature. 2006;439:426–429. doi: 10.1038/nature04326. [DOI] [PubMed] [Google Scholar]
- Foster CC. A study of a large sociogram II. Elimination of free parameters. Behav. Sci. 1963;8:56–65. [Google Scholar]
- Freeman LC. Turning a profit from mathematics: the case of social networks. J. Math. Sociol. 1984;10:343–360. [Google Scholar]
- Girvan M, Newman MEJ. Community structure in social and biological networks. PNAS. 2002;99:7821–7826. doi: 10.1073/pnas.122653799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman M, van Duijn MAJ. Software for statistical analysis of social networks. In: Carrington PJ, Scott J, Wasserman S, editors. Models and Methods in Social Network Analysis. Cambridge University Press; Cambridge, MA: 2005. pp. 270–316. [Google Scholar]
- Ings TC, Monotya JM, Bascompte J, Blüthgen N, Brown L, Dormann CR, Edwards F, Figueroa D, Jacob U, Jones JI, Lauridsen RB, Ledger ME, Lewis HM, Olesen JM, van Veen FJF, Warren PH, Woodward G. Ecological networks-beyond food webs. J. Anim. Ecol. 2009;78:253–269. doi: 10.1111/j.1365-2656.2008.01460.x. [DOI] [PubMed] [Google Scholar]
- Kasper C, Voelkl B. A social network analysis of primate groups. Primates. 2009;50:343–356. doi: 10.1007/s10329-009-0153-2. [DOI] [PubMed] [Google Scholar]
- Krause J, Croft DP, James R. Social network theory in the behavioural sciences: potential applications. Behav. Ecol. Sociobiol. 2007;62:15–27. doi: 10.1007/s00265-007-0445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J, Lusseau D, James R. Animal social networks: an introduction. Behav. Ecol. Sociobiol. 2009;63:967–973. [Google Scholar]
- Levine JH. The sphere of influence. Am. Sociol. Rev. 1972;37:14–27. [Google Scholar]
- Liben-Nowell D, Kleinberg J. Tracing information flow on a global scale using internet chain-letter data. PNAS. 2008;105:4633–4638. doi: 10.1073/pnas.0708471105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusseau D. The emergent properties of a dolphin social network. Proc. R. Soc. Lond. B. 2003;270:S186–S188. doi: 10.1098/rsbl.2003.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusseau D, Newman MEJ. Identifying the role that animals play in their social networks. Proc. R. Soc. Lond. B. 2004;271:S477–S481. doi: 10.1098/rsbl.2004.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusseau D. Evidence for social role in a dolphin social network. Evol. Ecol. 2007;21:357–366. [Google Scholar]
- Martínez-López B, Perez AM, Sánczez-Vizcaíno J. Social network analysis: review of general concepts and use in preventative veterinary medicine. Transbound. Emerg. Dis. 2009;56:109–120. doi: 10.1111/j.1865-1682.2009.01073.x. [DOI] [PubMed] [Google Scholar]
- McCowan B, Anderson A, Heagarty A, Cameron A. Utility of social network analysis for primate behavioral management and well-being. Appl. Anim. Behav. Sci. 2008;109:396–405. [Google Scholar]
- McCowan B, Beisner BA, Capitanio JP, Jackson ME, Cameron AN, Seil S, Atwill ER, Fushing H. Network stability is a balancing act of personality, power, and conflict dynamics in rhesus macaque societies. PLoS ONE. 2011;6:e22350. doi: 10.1371/journal.pone.0022350. doi:10.1371/journal.pone.0022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson JM. Hypernetwork sampling: duality and differentiation among voluntary organizations. Soc. Netw. 1982;3:225–249. [Google Scholar]
- Mench JA, Keeling LJ. The social behaviour of domestic birds. In: Keeling LJ, Gonyou H, editors. Social Behaviour in Farm Animals. CAB International; Wallingford, Oxon, UK: 2001. pp. 177–210. [Google Scholar]
- Newman MEJ. Scientific collaboration networks. 1. Network construction and fundamental results. Phys. Rev. E. 2001;64:016131. doi: 10.1103/PhysRevE.64.016131. doi:10.1103/PhysRevE.66.016131. [DOI] [PubMed] [Google Scholar]
- Newman MEJ, Forrest S, Balthrop J. Email networks and the spread of computer viruses. Phys. Rev. E. 2002;66:035101(R). doi: 10.1103/PhysRevE.66.035101. doi:10.1103/PhysRevE.66.035101. [DOI] [PubMed] [Google Scholar]
- Newman MEJ, Girvan M. Finding and evaluating community structure in networks. Phys. Rev. E. 2004;69:026113. doi: 10.1103/PhysRevE.69.026113. doi:10.1103/PhysRevE.69.026113. [DOI] [PubMed] [Google Scholar]
- Nicol CJ. The social transmission of information and behavior. Appl. Anim. Behav. Sci. 1995;44:79–98. [Google Scholar]
- Otterstatter MC, Thomson JD. Contact networks and transmission of an intestinal pathogen in bumble bee (Bombus impatiens) colonies. Oecologia. 2007;154:411–421. doi: 10.1007/s00442-007-0834-8. [DOI] [PubMed] [Google Scholar]
- Sade DS, Dow M. Primate social networks. In: Wasserman S, Galaskiewicz J, editors. Advances in Social Network Analysis: Research in the Social and Behavioral Sciences. Sage; California: 1994. pp. 152–166. [Google Scholar]
- Sapolsky RM. The influence of social hierarchy primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Scott J. Social Network Analysis. Sage; London: 2000. [Google Scholar]
- Sih A, Hanser SF, McHugh KA. Social network theory: new insights and issues for behavioral ecologists. Behav. Ecol. Sociobiol. 2009;63:975–988. [Google Scholar]
- Sueur C, Jacobs A, Amblard F, Petit O, King AJ. How can social network analysis improve the study of primate behavior? Am. J. Primatol. 2011;71:1–17. doi: 10.1002/ajp.20915. [DOI] [PubMed] [Google Scholar]
- Sen P, Dasgupta S, Chatterjee A, Sreeram PA, Mukherjee G, Manna SS. Small-world properties of the Indian railway network. Phys. Rev. E. 2003;67:036106. doi: 10.1103/PhysRevE.67.036106. doi:10.1103/PhysRevE.67.036106. [DOI] [PubMed] [Google Scholar]
- Sutherland MA, Niekamp SR, Rodriguez-Zas SL, Salak-Johnson JL. Impacts of chronic stress and social status on various physiological and performance measures in pigs of different breeds. J. Anim. Sci. 2006;84:588–596. doi: 10.2527/2006.843588x. [DOI] [PubMed] [Google Scholar]
- Veissier I, Boissy A, dePassille AM, van Reenen CG, Roussel S, Andanson S, Pradel P. Calves’ responses to repeated social regrouping and relocation. J. Anim. Sci. 2007;79:2580–2593. doi: 10.2527/2001.79102580x. [DOI] [PubMed] [Google Scholar]
- Wasserman S, Faust K. Social Network Analysis: Methods and Applications. Cambridge University Press; Cambridge: 1994. [Google Scholar]
- Webb CR. Farm animal networks: unravelling the contact structure of the British sheep population. Prev. Vet. Med. 2005;68:3–17. doi: 10.1016/j.prevetmed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Wey T, Blumstein DT, Shen W, Jordan F. Social network analysis of animal behaviour: a promising tool for the study of sociality. Anim. Behav. 2008;75:333–344. [Google Scholar]
- Whitehead H. Analyzing Animal Societies: Quantitative Methods for Vertebrate Social Analysis. University of Chicago Press; Chicago, IL: 2008. [Google Scholar]
- Wilson EO. Sociobiology: The New Synthesis. Harvard University Press; Cambridge, MA: 1975. [Google Scholar]