Abstract
The incidence and economic burden of cancer in sub-Saharan Africa is increasing, and innovative strategies are needed to improve prevention and care in this population. This article utilizes a case of cutaneous T-cell lymphoma (CTCL) in Uganda to propose guidelines for diagnosis and treatment of this disease in resource-limited settings. These guidelines were developed from the consensus opinion of specialists at the Uganda Cancer Institute and Fred Hutchinson Cancer Research Center as part of an established collaboration. Areas for future investigation that can improve the care of patients in this region are identified.
INTRODUCTION
Cutaneous T-cell lymphomas (CTCL) comprise 2% of all non-Hodgkin lymphoma diagnoses in the United States (US). The most common subtypes are Mycosis Fungoides (MF) and its leukemic presentation, Sézary Syndrome (SS) [1]. CTCLs were noted to occur with a higher incidence rate among African-Americans in a recent analysis of US SEER data [2], and while there are no studies that estimate the prevalence of CTCL or MF in sub-Saharan Africa (SSA) many case series have suggested that the diagnosis is more frequent than currently recognized [3]. There are insufficient data to clearly recommend one treatment regimen over another, and while a number of treatment guidelines for CTCL exist in resource-abundant regions [4-6], this is not true for resource-limited settings.
In 2008, a formal collaboration between the Uganda Cancer Institute (UCI) and the Fred Hutchinson Cancer Research Center (FHCRC) was initiated with a tripartite mission: 1) conduct research to reduce the incidence, morbidity and mortality due to cancers seen commonly in both the US and SSA; 2) build the capacity for global cancer research and care; 3) develop novel strategies for the treatment and prevention of infection-related cancers and cancers of high incidence in resource-limited settings. As part of this ongoing collaboration, two clinical cases are selected each month from the more than 800 seen monthly at the UCI that represent opportunities for improving cancer care. Multidisciplinary experts from both institutions discuss cases by web-conference between the Seattle and Kampala clinical sites, and come to consensus regarding knowledge gaps and opportunities to develop standards of care in resource-limited settings. This article describes a recent case of CTCL in Uganda from the conference and summarizes the subsequent discussion to outline an approach to care which may be appropriate for other resource-limited settings in SSA.
CASE PRESENTATION
A 75 year-old man from Kampala, Uganda presented to the Mulago hospital reporting a 7-year history of gradually increasing pruritis and diffuse, scaly skin lesions. More recently he reported mild weight loss and anorexia though denied drenching night-sweats or fevers. He denied any palpable adenopathy, but described the development of a nodular lesion on his face over the past year. Upon presentation to the Dermatology Department at Mulago hospital he was given a presumptive diagnosis of dermatitis thought to be secondary to exposures in his capacity as a building inspector, and was started on empiric anti-histamines and topical corticosteroid therapy. Due to an inadequate response to these agents, he returned for further evaluation, which included a skin biopsy of a lesion on his left shoulder in March 2012.
Histopathologic evaluation suggested an atypical dermal lymphoid infiltrate, though a more specific diagnosis was not possible with the available samples. Therefore he underwent a repeat skin biopsy of a patch lesion on his anterior chest, which revealed a diffuse dermal infiltrate of lymphoid cells, some with cerebriform nuclei within a collagenous background. There was no evidence of large cell transformation noted. To confirm the diagnosis the local pathologist used his personal supply of antibodies, as routine immunohistochemistry is not available in Uganda, which confirmed that the lymphoid cells stained positive for CD3 and CD45 without CD20 expression.
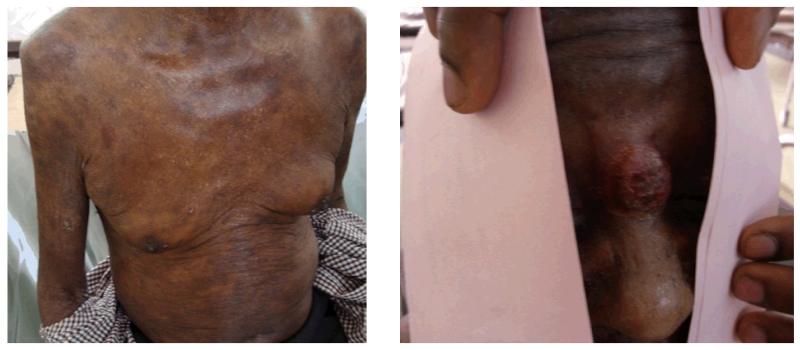
Based on these results, the patient was diagnosed with CTCL consistent with MF, and started on 25mg of oral methotrexate weekly. Because of an inadequate response to methotrexate, he was referred for evaluation at the UCI, where his ECOG performance status was 1 and he was without additional significant past medical history. He was noted to be thin but in no acute distress. The skin lesions were immediately evident on evaluation, and were found to be diffuse, scaly patch and plaque lesions involving approximately 30% of his body surface area. Most of the involved areas were on the scalp, trunk and arms. A tumor stage lesion measuring 3cm in maximum dimension was present at the bridge of his nose. There was no significant adenopathy or splenomegaly. Laboratory evaluation revealed eosinophilia with mild anemia and normal platelet count. He had renal insufficiency (Cr 2.35mg/dL [208.6 μmol/l]) and was HIV-negative. Peripheral blood smear examination did not reveal any circulating Sézary cells, though confirmed the eosinophilia.
Further radiological evaluation included a chest radiograph without evidence of an enlarged mediastinum, and abdominal ultrasound did not reveal any adenopathy or splenomegaly. Therefore, he was diagnosed with stage IIB (T3N0M0B0) MF upon completion of this evaluation.
DISCUSSION
Diagnosis
The diagnosis of CTCLs is difficult even in resource-abundant regions of the world. A long-duration of follow-up and evaluation of multiple biopsies is often necessary to confirm the diagnosis. If a tumor stage lesion is present, it is typically biopsied to evaluate for large-cell transformation. Otherwise, the most indurated lesion is usually selected. Prior to biopsy of an involved early-stage skin lesion, therapy is typically held for 2-4 weeks since the treatment effect can obscure the characteristic histologic features including epidermotropism and the ‘cerebriform’ nuclear appearance. To facilitate the diagnosis in resource-rich settings, clinicopathologic criteria were proposed by Pimpinelli in 2005 [7]. While these criteria can support a diagnosis of MF with clinical and histopathologic findings alone, most cases require adjunctive PCR and/or immunohistochemistry including CD2, CD3, CD4, CD5, CD7, and CD8 [7].
Since cost constraints preclude assessment of multiple immunophenotypic markers in SSA, a consensus was reached that in resource-limited settings the combination of characteristic clinical findings, morphologic appearance on hematoxylin and eosin staining, and a positive CD3 or CD4 immunohistochemical result is sufficient for a diagnosis of MF (Table 1). Clearly, new approaches are needed to develop cost-effective methods to increase diagnostic capacity in SSA.
Table 1. Clinicopathologic Criteria for the Diagnosis of Mycosis Fungoides in resource-limited settings.
| Diagnostic Guidelines |
Clinical | Histopathologic | Immunohistochemistry |
|---|---|---|---|
| Required | Persistent and/or progressive patches/thin plaques |
Superficial lymphoid infiltrate |
Infiltrating cells are CD3 or CD4 positive |
| Supportive | Non-sun exposed skin |
Epidermotropism without spongiosis |
|
| Size/shape variation | Lymphoid atypia (enlarged hyperchromatic nuclei and irregular or cerebriform nuclear contours) |
CD2, CD3, CD5 positive CD7, CD8, CD25 negative |
|
| Poikiloderma | Circulating Sézary Cells Absence of Flower Cells |
Epidermal/dermal discordance of CD2, CD3, and CD5 (aberrant loss in epidermis)[7] |
|
|
Large-Cell
Transformation |
Systemic symptoms Tumor stage lesions |
>25% large lymphoid cells within dermal infiltrates |
CD3 positive May be CD30+/− |
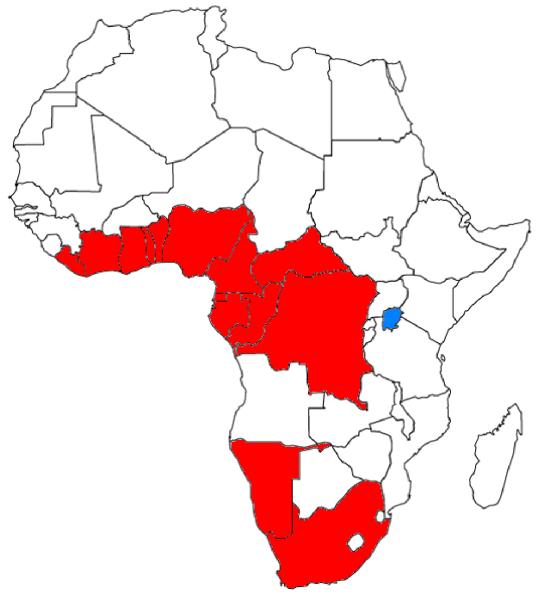
In addition to overcoming these challenges associated with diagnosing CTCL, an additional potential neoplasm, adult T-cell leukemia/lymphoma (ATLL), should be considered when making the diagnosis of MF in Africa. Human T-cell lymphotropic virus 1 (HTLV-1) is endemic in parts of Africa, Japan, and the Caribbean (Figure 2) and was first identified in cells from a patient diagnosed with CTCL [8]. Since this discovery, a smoldering clinical subtype of ATLL was described that can mimic CTCL clinically and pathologically [9-11]. According to the Shimoyama classification, these patients may have progressive skin lesions that develop over many years, a normal serum calcium concentration and lactate dehydrogenase (LDH), no visceral organ involvement, and a skin biopsy with small T-lymphocytes and even Pautrier’s microabscesses, just as in CTCL. A diagnosis of smoldering ATLL requires either the presence of >5% abnormal circulating T-lymphocytes in the peripheral blood or histological confirmation of typical ‘flower-cells’ in a T-cell infiltrate (not present in this case) [12]. While there are rare patients who carry HTLV-1 that have a negative serum antibody test to the virus, a serologic test for HTLV-1 can help differentiate smoldering ATLL of the skin from CTCL. Immunohistochemistry for CD25 may also be helpful, as it is expressed strongly in almost all cases of ATLL. Since HTLV-1 is not endemic in Uganda, these tests were not necessary in the described case.
Figure 2. African Countries with 1-5% prevalence of Human T-Cell Leukemia Virus-1 (adapted from Harrison’s Internal Medicine, 18th Ed.[27]).
Treatment
Patients with CTCL are typically divided into early-stage (IA, IB, IIA) and advanced-stage (IIB, III, IV) because the first group should be treated with skin-directed therapy (SDT) while the latter is treated predominately with systemic therapy (Table 2).
Table 2. Treatment Strategies for Mycosis Fungoides/Sézary Syndrome in Resource-Limited Settings.
| Stage | Treatment Options in Resource-Limited Settings |
|---|---|
| Early-Stage (IA, IB, IIA) |
First Line Topical corticosteroids Heliotherapy Radiation Therapy Second Line Topical chemotherapy (mechlorethamine or carmustine) Low-dose oral methotrexate |
| Advanced-Stage (IIB, III, IV) |
First Line Doxorubicin Gemcitabine Second Line Chlorambucil Cyclophosphamide Etoposide High-dose methotrexate (consideration can be given to combining with topical therapies, above) |
| Large Cell Transformation (if aggressive growth rate) |
First Line CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) Second Line DHAP (dexamethasone, cytarabine, cisplatin), GDP (gemcitabine, dexamethasone, cisplatin) ICE (ifosfamide, carboplatin, etoposide) Monotherapy: ifosfamide, gemcitabine |
Early-Stage Disease
Early-stage (IA, IB, IIA) CTCL (65-85% of all patients) is not associated with a change in overall survival when compared to an age-matched population and early therapy has not been shown to impact the rate of progression. Therefore the goal of treatment in this setting is improving symptoms with minimal associated toxicity [13]. In the absence of significant symptoms, patients with early-stage CTCL can be expectantly managed without additional therapy.
The main therapeutic options for early-stage CTCL in resource-limited settings include topical corticosteroids, radiation therapy, and heliotherapy, the latter to take the place of controlled phototherapy which is not usually available. Topical corticosteroids lead to a complete response (CR) in T1 patients in about 60% of cases with an additional 30% experiencing a partial response (PR). T2 patients also fare well with a 25% CR and 50% PR, respectively [14].
In the setting of diffuse lesions or inadequate response to corticosteroids, heliotherapy should be considered. The optimal dose of heliotherapy, though never specifically studied in CTCL, can be estimated with data from a South African study that measured the natural UVB exposure at meteorological centers in Cape Town, Durban, and Pretoria throughout an entire year. In patients with Fitzpatrick skin type VI, 0.08 J/cm2 is the initial recommended daily treatment dose (0.03 J/cm2 in type III skin) for CTCL. Previous studies show that 70% of the minimal erythema dose (MED) is effective in treating MF, with the daily dose increased by 15% every three days until a maximal tolerated dose (MTD) is reached [15]. This MTD is then maintained throughout the treatment course. To reach these UVB targets with heliotherapy a patient in Pretoria at 10am with type VI skin, for example, requires 77 minutes of sun exposure to all affected areas in December, 113 minutes in March, and 228 minutes in June [16] (1/3 of this time is required for type III skin in each instance). Since an equatorial country such as Uganda has less seasonal variation and more intense UVB exposure these durations are likely over-estimates there, though a significant amount of time is still required to achieve therapeutic UVB doses using natural sunlight, especially in dark-skinned individuals.
While the availability of radiation therapy is limited in sub-Saharan Africa, some centers such as the UCI do have access to this modality, usually with a cobalt-60 source. Prior studies support the efficacy of cobalt-60 radiation for the treatment of CTCL, with the most durable responses reported for lesions treated with at least 3000 cGy at 200 cGy per fraction [17]. Since more recent reports suggest acceptable disease control (94% CR) is possible with lower doses (400-900 cGy) of radiation delivered in a single fraction [18], consideration should be given to this approach in SSA to optimize the utilization of radiation therapy and associated costs.
Second-line options for early-stage patients in resource-limited settings include topical chemotherapeutics such as mechlorethamine or carmustine, as well as low-dose oral methotrexate (MTX). While there are no prospective studies investigating weekly oral MTX, Zackheim et al. described 69 previously-treated patients (60 with T2 disease) who received weekly oral MTX and reported a CR rate of 12% in T2 patients and a 22% PR rate with a median time to treatment failure of 15 months. Notably, almost half of these patients received another concurrent therapy at the same time as MTX. The median weekly dose was 25mg with a maximum dose of 75mg. In patients that did not initially respond, the dose was increased until response or dose-limiting toxicity was noted. 7 patients with tumor stage disease were described in this retrospective analysis, with only 1 PR described, suggesting that this strategy is of limited value in advanced-stage disease [19].
Advanced-stage disease
In contrast to patients with early-stage disease, advanced-stage disease impacts overall survival. Optimal skin care is crucial in these patients since a common cause of death in patients with CTCL is infection originating from the skin. Since therapy is not expected to be curative, optimal control of symptoms while minimizing the risk of infection is the goal of treatment. Due to cost constraints, many agents are not yet available in resource-limited settings (e.g. vorinostat, bexarotene, romidepsin, praletrexate), and therefore this discussion of advanced-stage disease will focus on the use of traditional cytotoxic chemotherapy such as gemcitabine and doxorubicin.
Duvic and colleagues reported an overall response rate to gemcitabine 1000mg/m2 days 1, 8, and 15 q28 days of 68% (8%CR, n=25) in previously treated patients with a median of 5 prior regimens [20]. In the front-line setting, Marchi et al reported a 75% overall response rate (22% CR, n=32) at a dose of 1200mg/m2 days 1, 8, and 15 q28days for 6 cycles. Toxicities included mild cytopenias and otherwise was well tolerated. The median duration of response in those that achieved a CR was 10 months [21].
Most of the literature on anthracycline monotherapy in CTCL reports on the use of liposomal doxorubicin with an overall response rate of 56% (20% CR) reported in a prospective study of advanced-stage CTCL [22]. Of note, there are no published reports of the response rate to doxorubicin monotherapy in CTCL as prior studies used this agent in combination regimens [23]. Evaluating the activity of doxorubicin monotherapy could contribute to more cost-effective treatment of CTCL and help lessen the impact of any future shortages of the liposomal variant.
In the setting of refractory or transformed disease with an aggressive growth rate, combination chemotherapy can be considered as detailed in Table 2. However, extrapolation of the experience with aggressive regimens developed in resource-abundant settings can lead to significant toxicity in SSA. For example, treatment of endemic Burkitt lymphoma in Malawi with a dose-reduced version of the LMB 89 Group B protocol led to a 33% treatment-related mortality compared with <1% reported in the original European cohort [24, 25]. Therefore combination regimens should be delivered with caution, and only in centers able to provide adequate supportive care.
Based on the consensus reached at the conference, this patient was treated with gemcitabine at 1000mg/m2 days 1, 8, and 15 q28 days with a significant response after 2 cycles with minimal toxicities to date.
CONCLUSIONS
The incidence and economic burden of cancer in sub-Saharan Africa is increasing, and innovative strategies are needed to improve care and control in this population [26]. This report proposes guidelines for the diagnosis and treatment of CTCL in resource-limited settings, and identifies areas for potential investigation to improve cancer care in this region.
Figure 1. Skin lesions present on exam at the UCI, a) patch lesions and b) tumor stage lesion on the nose.
REFERENCES
- 1.Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sezary syndrome. Blood. 2009;114(20):4337–53. doi: 10.1182/blood-2009-07-202895. [DOI] [PubMed] [Google Scholar]
- 2.Bradford PT, et al. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113(21):5064–73. doi: 10.1182/blood-2008-10-184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fouchard N, et al. Cutaneous T cell lymphomas: mycosis fungoides, Sezary syndrome and HTLV-I-associated adult T cell leukemia (ATL) in Mali, West Africa: a clinical, pathological and immunovirological study of 14 cases and a review of the African ATL cases. Leukemia. 1998;12(4):578–85. doi: 10.1038/sj.leu.2400956. [DOI] [PubMed] [Google Scholar]
- 4.Horwitz SM, et al. Review of the treatment of mycosis fungoides and sezary syndrome: a stage-based approach. J Natl Compr Canc Netw. 2008;6(4):436–42. doi: 10.6004/jnccn.2008.0033. [DOI] [PubMed] [Google Scholar]
- 5.Dummer R, Dreyling M. Primary cutaneous lymphoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19(Suppl 2):ii72–6. doi: 10.1093/annonc/mdn095. [DOI] [PubMed] [Google Scholar]
- 6.Trautinger F, et al. EORTC consensus recommendations for the treatment of mycosis fungoides/Sezary syndrome. Eur J Cancer. 2006;42(8):1014–30. doi: 10.1016/j.ejca.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Pimpinelli N, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53(6):1053–63. doi: 10.1016/j.jaad.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 8.Poiesz BJ, et al. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77(12):7415–9. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi K, et al. A proposal for smoldering adult T-cell leukemia: a clinicopathologic study of five cases. Blood. 1983;62(4):758–66. [PubMed] [Google Scholar]
- 10.Sakamoto FH, et al. Cutaneous T-cell lymphoma with HTLV-I infection: clinical overlap with adult T-cell leukemia/lymphoma. Int J Dermatol. 2006;45(4):447–9. doi: 10.1111/j.1365-4632.2006.02687.x. [DOI] [PubMed] [Google Scholar]
- 11.Takasaki Y, et al. Long-term study of indolent adult T-cell leukemia-lymphoma. Blood. 2010;115(22):4337–43. doi: 10.1182/blood-2009-09-242347. [DOI] [PubMed] [Google Scholar]
- 12.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87) Br J Haematol. 1991;79(3):428–37. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 13.Zackheim HS, et al. Prognosis in cutaneous T-cell lymphoma by skin stage: long-term survival in 489 patients. J Am Acad Dermatol. 1999;40(3):418–25. doi: 10.1016/s0190-9622(99)70491-3. [DOI] [PubMed] [Google Scholar]
- 14.Zackheim HS, Kashani-Sabet M, Amin S. Topical corticosteroids for mycosis fungoides. Experience in 79 patients. Arch Dermatol. 1998;134(8):949–54. doi: 10.1001/archderm.134.8.949. [DOI] [PubMed] [Google Scholar]
- 15.Gathers RC, et al. Narrowband UVB phototherapy for early-stage mycosis fungoides. J Am Acad Dermatol. 2002;47(2):191–7. doi: 10.1067/mjd.2002.120911. [DOI] [PubMed] [Google Scholar]
- 16.Moosa Y, Esterhuyse DJ. Heliotherapy: A South African perspective. S Afr Med J. 2010;100(11):728–33. doi: 10.7196/samj.4008. [DOI] [PubMed] [Google Scholar]
- 17.Cotter GW, et al. Palliative radiation treatment of cutaneous mycosis fungoides--a dose response. Int J Radiat Oncol Biol Phys. 1983;9(10):1477–80. doi: 10.1016/0360-3016(83)90321-8. [DOI] [PubMed] [Google Scholar]
- 18.Thomas TO, et al. Outcome of Patients Treated With a Single-Fraction Dose of Palliative Radiation for Cutaneous T-Cell Lymphoma. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 19.Zackheim HS, Kashani-Sabet M, McMillan A. Low-dose methotrexate to treat mycosis fungoides: a retrospective study in 69 patients. J Am Acad Dermatol. 2003;49(5):873–8. doi: 10.1016/s0190-9622(03)01591-3. [DOI] [PubMed] [Google Scholar]
- 20.Duvic M, et al. Phase II evaluation of gemcitabine monotherapy for cutaneous T-cell lymphoma. Clin Lymphoma Myeloma. 2006;7(1):51–8. doi: 10.3816/CLM.2006.n.039. [DOI] [PubMed] [Google Scholar]
- 21.Marchi E, et al. Gemcitabine as frontline treatment for cutaneous T-cell lymphoma: phase II study of 32 patients. Cancer. 2005;104(11):2437–41. doi: 10.1002/cncr.21449. [DOI] [PubMed] [Google Scholar]
- 22.Quereux G, et al. Prospective multicenter study of pegylated liposomal doxorubicin treatment in patients with advanced or refractory mycosis fungoides or Sezary syndrome. Arch Dermatol. 2008;144(6):727–33. doi: 10.1001/archderm.144.6.727. [DOI] [PubMed] [Google Scholar]
- 23.Winkler CF, et al. Combined modality treatment of cutaneous T cell lymphoma: results of a 6-year follow-up. J Clin Oncol. 1986;4(7):1094–100. doi: 10.1200/JCO.1986.4.7.1094. [DOI] [PubMed] [Google Scholar]
- 24.Hesseling P, et al. The 2000 Burkitt lymphoma trial in Malawi. Pediatr Blood Cancer. 2005;44(3):245–50. doi: 10.1002/pbc.20254. [DOI] [PubMed] [Google Scholar]
- 25.Patte C, et al. The Societe Francaise d’Oncologie Pediatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97(11):3370–9. doi: 10.1182/blood.v97.11.3370. [DOI] [PubMed] [Google Scholar]
- 26.Knaul FM, et al. Global Task Force on Expanded Access to Cancer Care and Control in Developing Countries . Closing the Cancer Divide: A Blueprint to Expand Access in Low and Middle Income Countries. Harvard Global Equity Initiative; Boston, MA: 2011. [Google Scholar]
- 27.Longo DL FA. The Human Retroviruses. In: F.A. Longo DL, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison’s Principles of Internal Medicine. 18th ed McGraw-Hill; New York: 2012. [Google Scholar]