Abstract
The bacterial chloramphenicol acetyltransferase (CAT) gene was expressed in protoplasts of three important graminaceous plant species after introduction of the gene by electroporation. Gene transfer occurred when high-voltage electric pulses were applied either directly or indirectly (without anode contact) to a solution containing plasmid DNA and protoplasts of rice, wheat, or sorghum. The indirect method was more rapid, resulted in higher protoplast viability, and was less subject to contamination than the direct-contact method. Gene expression of approximately equal magnitude resulted when the CAT gene was fused to either the 35S promoter of cauliflower mosaic virus or the copia long terminal repeat promoter of Drosophila. Together with recent advances in regeneration of callus and whole plants from protoplasts, this system makes it possible to study inheritance and expression of genes introduced into graminaceous monocotyledonous plants.
Keywords: chloramphenicol acetyltransferase, transformation, chimeric gene
Full text
PDF
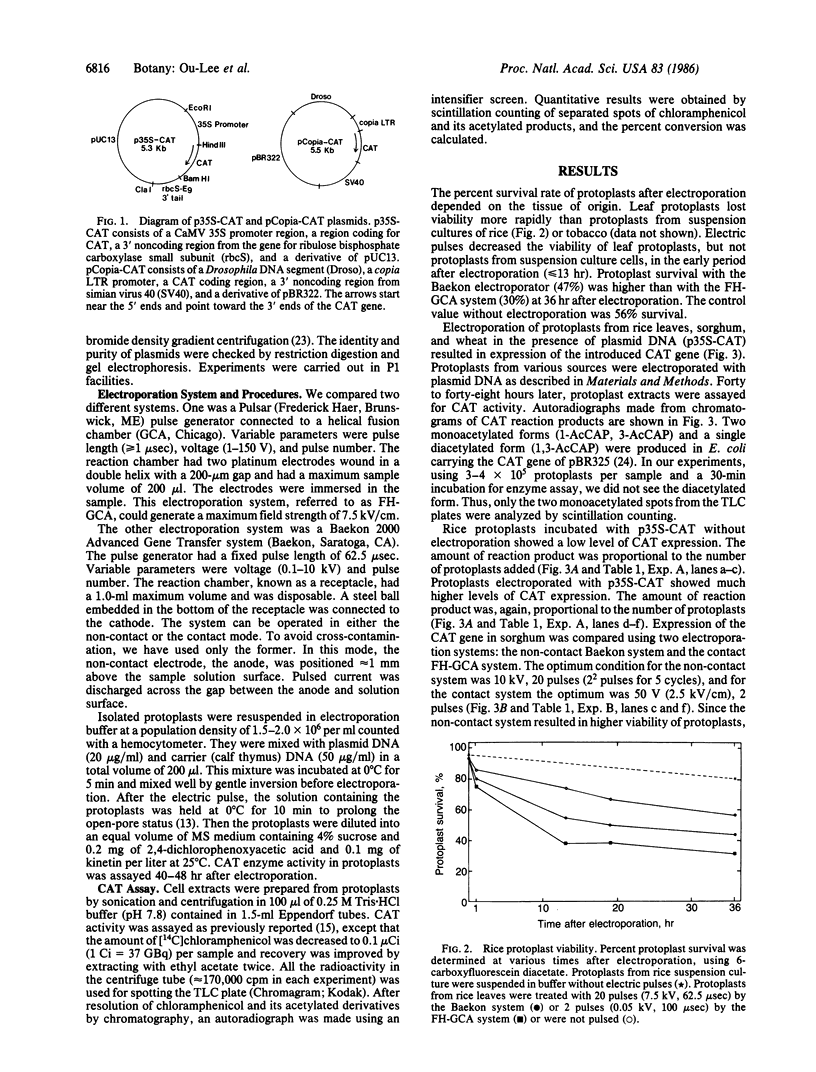



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Baba M. L., Darga L. L., Goodman M., Czelusniak J. Evolution of cytochrome C investigated by the maximum parsimony method. J Mol Evol. 1981;17(4):197–213. doi: 10.1007/BF01732758. [DOI] [PubMed] [Google Scholar]
- Bischoff R., Eisert R. M., Schedel I., Vienken J., Zimmerman U. Human hybridoma cells produced by electro-fusion. FEBS Lett. 1982 Oct 4;147(1):64–68. doi: 10.1016/0014-5793(82)81012-0. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Di Nocera P. P., Dawid I. B. Transient expression of genes introduced into cultured cells of Drosophila. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7095–7098. doi: 10.1073/pnas.80.23.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell A. J., Levis R., Simon M. A., Rubin G. M. The 5' termini of RNAs encoded by the transposable element copia. Nucleic Acids Res. 1981 Dec 11;9(23):6279–6291. doi: 10.1093/nar/9.23.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R., Janda M., Ahlquist P. Bacterial gene inserted in an engineered RNA virus: efficient expression in monocotyledonous plant cells. Science. 1986 Mar 14;231(4743):1294–1297. doi: 10.1126/science.231.4743.1294. [DOI] [PubMed] [Google Scholar]
- Fromm M. E., Taylor L. P., Walbot V. Stable transformation of maize after gene transfer by electroporation. 1986 Feb 27-Mar 5Nature. 319(6056):791–793. doi: 10.1038/319791a0. [DOI] [PubMed] [Google Scholar]
- Fromm M., Taylor L. P., Walbot V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5824–5828. doi: 10.1073/pnas.82.17.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall H., Johnson M. H. Use of carboxyfluorescein diacetate to study formation of permeable channels between mouse blastomeres. Nature. 1982 Feb 11;295(5849):524–526. doi: 10.1038/295524a0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hohn T., Richards K., Geneviève-Lebeurier Cauliflower mosaic virus on its way to becoming a useful plant vector. Curr Top Microbiol Immunol. 1982;96:194–236. [PubMed] [Google Scholar]
- Neumann E., Schaefer-Ridder M., Wang Y., Hofschneider P. H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W. Direct transfer of cloned genes from bacteria to mammalian cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2163–2167. doi: 10.1073/pnas.77.4.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spena A., Hain R., Ziervogel U., Saedler H., Schell J. Construction of a heat-inducible gene for plants. Demonstration of heat-inducible activity of the Drosophila hsp70 promoter in plants. EMBO J. 1985 Nov;4(11):2739–2743. doi: 10.1002/j.1460-2075.1985.tb03997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. K., Neumann E. Electric field mediated gene transfer. Biochem Biophys Res Commun. 1982 Jul 30;107(2):584–587. doi: 10.1016/0006-291x(82)91531-5. [DOI] [PubMed] [Google Scholar]




