Abstract
During an action potential, Ca2+ entering a presynaptic terminal triggers synaptic vesicle exocytosis and neurotransmitter release in less than a millisecond. How does Ca2+ stimulate release so rapidly and precisely? Work over the last decades revealed that Ca2+-binding to synaptotagmin triggers release by stimulating synaptotagmin-binding to a core machinery composed of SNARE and SM proteins that mediates membrane fusion during exocytosis. Complexin adaptor proteins assist synaptotagmin by activating and clamping this core fusion machinery. Synaptic vesicles containing synaptotagmin are positioned at the active zone, the site of vesicle fusion, by a protein complex containing RIM proteins. RIM proteins simultaneously activate docking and priming of synaptic vesicles and recruit Ca2+-channels to active zones, thereby connecting in a single complex primed synaptic vesicles to Ca2+-channels. This architecture allows direct flow of Ca2+-ions from Ca2+-channels to synaptotagmin, which then triggers fusion, thus mediating tight millisecond coupling of an action potential to neurotransmitter release.
INTRODUCTION
Arguably, Emil du Bois-Raymond (1818-1896) initiated modern neuroscience with the discovery of the action potential and of synaptic transmission at the neuromuscular junction. 100 years later, Bernard Katz (1911-2003), working also on the neuromuscular junction, established the fundamental pathway of synaptic transmission whereby an action potential arrives at a presynaptic nerve terminal and gates Ca2+-channels; the inflowing Ca2+ then triggers the exocytotic fusion of synaptic vesicles containing neurotransmitters, and the released neurotransmitters subsequently produce a postsynaptic signal (Katz, 1969). Katz’s brilliant work built on George Palade’s (1912-2008) studies on vesicular trafficking (Palay and Palade, 1955), and initiated a series of elegant electrophysiological experiments that characterized the process of synaptic transmission in exquisite detail. Among others, these studies revealed that Ca2+ triggers release in a highly cooperative manner (Dodge and Rahamimoff, 1967) within a few hundred microseconds (Sabatini and Regehr, 1996), which is not much slower than the opening of a voltage-gated ion channel. What molecular mechanisms enable fast vesicle fusion at a synapse, however, remained a mystery until molecular biology allowed mechanistic dissection of vesicle fusion and its control by Ca2+ (reviewed in Südhof and Rothman, 2009).
Katz’s work posed three basic questions:
How do vesicles fuse? This general question transcends neurobiology, and is important for all areas of vesicle traffic and cell biology since membrane fusion is a universal process in eukaryotic cells.
How does Ca2+ induce rapid membrane fusion? This question is crucial for understanding synaptic transmission, and also relevant for other types of Ca2+-regulated exocytosis, such as hormone secretion, mast cell exocytosis, and oocyte fertilization. Moreover, synapses exhibit diverse properties, and synaptic transmission is not a digital yes-or-no event but plastic. Thus, understanding how Ca2+ controls release additionally pertains to how neural circuits operate.
How are Ca2+-influx and vesicle docking co-localized to presynaptic release sites, and how are they coordinated with priming of vesicles for Ca2+-triggered release? The last millisecond in the life of a synaptic vesicle depends on tight co-localization of primed vesicles with Ca2+-channels – only their close proximity at release sites enables precise coupling of an action potential to release.
These three questions lie at the heart of a molecular understanding of synaptic transmission. As described below, we now have a plausible framework of answers to these questions, although much remains to be done.
In the following, I will first provide a brief broad outline of the general release machinery (Fig. 1), and then discuss in greater detail selected questions that in my personal view are particularly interesting. Due to space constraints, I do not aim to provide a comprehensive discussion of the field, and I apologize for the many omissions I am bound to commit. Moreover, owing to the same space constraints I will focus on physiological studies. In particular, I am unable to give appropriate consideration to the many elegant liposome fusion studies that have recently been performed; for a more complete treatment of this subject, please see Brunger et al. (2009) and Marsden et al. (2011).
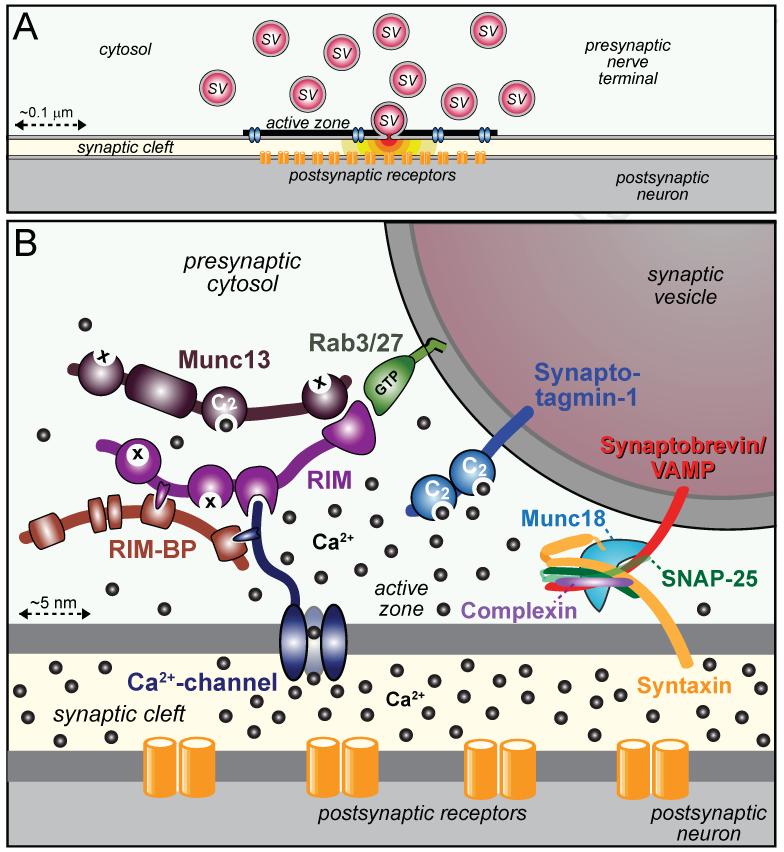
Figure 1. Organization of the presynaptic release machinery.
A. Drawing of a synapse with synaptic vesicles (SV), an active zone containing Ca2+-channels (blue), and a postsynaptic cluster of receptors (orange). One vesicle in the active zone is depicted in the process of fusing, with red neurotransmitters emitting from the fusion pore.
B. Schematic of the molecular machinery mediating Ca2+-triggered vesicle fusion. The drawing depicts a segment of a docked synaptic vesicle on the top right, and the presynaptic active zone in the middle. The three functional elements of the neurotransmitter release machinery are depicted from right to left. On the right, the core fusion machine composed of the SNARE/SM protein complex is shown; this machine comprises the SNARE proteins synaptobrevin/VAMP, syntaxin-1, and SNAP-25 and the SM protein Munc18-1. The Ca2+-sensor synaptotagmin-1 is depicted in the middle; it is composed of a short intravesicular sequence, a single transmembrane region, and two cytoplasmic C2-domains that bind Ca2+, and it functions using complexin (bound to the SNARE complex) as an assistant. The active zone protein complex containing RIM, Munc13, and RIM-BP, and a Ca2+-channel in the presynaptic plasma membrane is shown on the left. In this protein complex, RIM binding to specific target proteins coordinates all three functions of the active zone: RIM binding to vesicular rab proteins (Rab3 and Rab27 isoforms) mediates vesicle docking; RIM binding to the central priming factor Munc13 activates vesicle priming; and RIM binding to the Ca2+-channel, both directly and indirectly via RIM-BP, recruits the Ca2+-channels within 100 nm of the docked vesicles for fast excitation-secretion coupling. The overall design of the neurotransmitter release machinery depicted here enables in a single nanodevice fast and efficient triggering of release in response to an action potential by combining a fusion machine with a Ca2+-trigger and an active zone protein complex that positions all elements into appropriate proximity (modified from Kaeser et al., 2011).
A MOLECULAR FRAMEWORK FOR RELEASE
Work over the lifetime of Neuron – two and a half decades! – has produced a general framework for understanding neurotransmitter release that will be briefly summarized below (Fig. 1; see also reviews by Rizo and Rosenmund, 2008; Kochubey et al., 2011; Mohrmann and Sørensen, 2012).
Fusion
Intracellular membrane fusion is generally mediated by SNARE proteins (for ‘soluble NSF attachment receptor proteins’) and SM proteins (for ‘Sec1/Munc18-like proteins’) that undergo a cycle of association and dissociation during the fusion reaction (Fig. 2). At the synapse, the vesicular SNARE protein synaptobrevin (a.k.a. VAMP) forms a complex with the plasma membrane SNARE proteins syntaxin-1 and SNAP-25 (Söllner et al., 1993a). Prior to SNARE-complex formation, syntaxin-1 is present in a closed conformation that cannot engage in SNARE-complex formation; syntaxin-1 has to open for SNARE-complex assembly to proceed (Dulubova et al., 1999; Misura et al., 2000). The SM protein Munc18-1 initially binds to the closed conformation of syntaxin-1 (Hata et al., 1993; Dulubova et al., 1999). When the closed conformation of syntaxin-1 ‘opens’ in preparation to fusion and SNARE complexes form, Munc18-1 remains attached to syntaxin-1 in the assembling SNARE complex, but switches its binding mode to an interaction with the SNARE complex (Dulubova et al., 2007). Assembly of these SNARE/SM complexes mediates fusion, whereas disassembly of these complexes recycles SNARE and SM proteins for further use (Fig. 2; reviewed in Südhof and Rothman, 2009). The continued association of Munc18-1 to SNARE complexes throughout their assembly/disassembly cycle is essential for fusion (Khvotchev et al., 2007; Zhou et al., 2013a). SNARE/SM-complex assembly is maintained by chaperones whose dysfunction causes neurodegeneration (CSPs and synucleins; Burre et al., 2010; Sharma et al., 2011), whereas disassembly is mediated by an evolutionarily conserved specialized ATPase (NSF) and its adaptors (SNAPs; Söllner et al., 1993b).
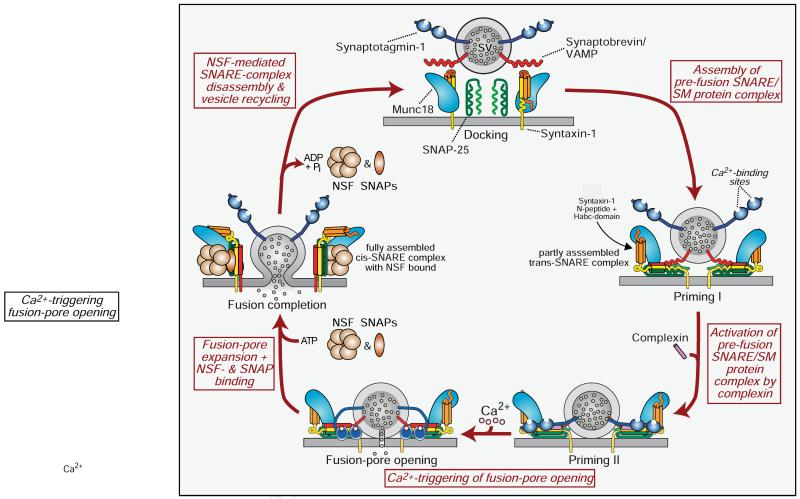
Figure 2. Schematic of the SNARE/SM protein cycle mediating fusion, and the role of synaptotagmin and complexin in Ca2+-triggering of fusion.
SNARE and SM proteins undergo a cycle of assembly and disassembly, such that the vesicular SNARE protein synaptobrevin assembles during priming into a trans-SNARE complex with the plasma membrane SNARE proteins syntaxin-1 and SNAP-25. Prior to SNARE-complex assembly, syntaxin-1 is present in a closed conformation in which its Habc-domain folds back onto its SNARE motif; this conformation precludes SNARE-complex assembly, and syntaxin-1 has to ‘open’ for SNARE-complex assembly to initiate. Prior to SNARE-complex assembly, Munc18-1 is associated with monomeric syntaxin-1 when syntaxin-1 is in a closed conformation; as syntaxin-1 opens during SNARE-complex assembly, Munc18-1 alters the mode of its binding to syntaxin-1 by binding to assembling trans-SNARE complexes via interacting with the syntaxin-1 N-peptide. Once SNARE complexes are partially assembled, complexin binds to further increase their priming. The ‘superprimed’ SNARE/SM protein complexes are then substrate for Ca2+-triggered fusion pore opening by Ca2+-binding to synaptotagmin, which causes an interaction of synaptotagmin with SNAREs and phospholipids. However, even before Ca2+-triggering synaptotagmin likely at least partly interacts with the fusion machinery as evidenced by the unclamping of spontaneous mini release in Syt1 knockout neurons. After fusion-pore opening, the resulting cis-SNARE complexes are disassembled by the NSF/SNAP ATPases, and vesicles are recycled, refilled with neurotransmitters, and reused for release (modified from Südhof, 2013).
The underlying principle of SNARE and SM protein function is simple (Fig. 2): SNARE proteins embedded in the two fusing membranes form a trans-complex that involves a progressive zippering of the four-helical SNARE complex in an N- to C-terminal direction (Hanson et al., 1997). Zippering of trans-SNARE complexes forces the fusing membranes into close proximity, destabilizing their hydrophilic surfaces. Assembly of the full trans-SNARE complex together with the action of the SM protein opens the fusion pore. Fusion-pore expansion transforms the initial ‘trans’-SNARE complexes into ‘cis’-SNARE complexes that are then dissociated by NSF (that binds to SNARE complexes via SNAP adapter proteins), completing the cycle.
In a physiological context, SNARE-complex assembly alone does not mediate fusion. We initially proposed that the SM protein Munc18-1 is an essential component of the core fusion machinery (Hata et al., 1993). This proposal was supported by pioneering work from the Novick laboratory demonstrating that the yeast SM protein Sec1p (the Munc18-1 homolog in yeast exocytosis) binds to assembled SNARE complexes (Carr et al., 1999). Moreover Novick and colleagues showed that Sec1p mutations completely block fusion but do not impair SNARE-complex assembly (Grote et al., 2000). However, the proposal that Munc18-1 activates fusion met resistance because Munc18-1 binding to closed syntaxin-1 appeared to suggest that Munc18-1 is an inhibitor of fusion (Yang et al., 2000), despite the fact that Munc18-1 is absolutely required for synaptic fusion (Verhage et al., 2000). This issue was only resolved over the last decade when studies revealed that Munc18-1 remains associated during fusion with SNARE proteins throughout their assembly/disassembly cycle, thereby removing the notion of an inhibitor (Dulubova et al., 2007; Shen et al., 2007). Subsequent data showed that Munc18-1 binding to assembling SNARE complexes is essential for synaptic vesicle fusion, whereas Munc18-1 binding to closed syntaxin-1 is not (Khvotchev et al., 2007; Gerber et al., 2008; Deák et al., 2009; Zhou et al., 2013a).
In every SNARE-dependent fusion reaction studied, an SM protein participates and is essential for that fusion reaction. Although SM proteins always appear to bind to SNARE complexes, the molecular basis of their interactions varies. For example, several SM proteins (Munc18-1, Vps45, and Sly1) bind to the N-terminal ‘N-peptide’ of their cognate syntaxins (Yamaguchi et al., 2003; Dulubova et al., 2003), but the yeast SM protein Sec1p binds to assembled SNARE complexes independent of the syntaxin-1 N-peptide (Carr et al., 1999).
The role of NSF and SNAPs in dissociating and activating SNARE proteins prior to fusion was recognized early on (Söllner et al., 1993b; Mayer et al., 1996). More recently, two chaperones that maintain SNARE-protein function during multiple association/dissociation cycles were identified. CSPs are evolutionarily conserved co-chaperones containing a DNA-J domain that form a catalytically active complex with Hsc70 and SGT (for ‘small glutamate-rich tetratricopeptide repeat protein’). The CSPα/Hsc70/SGT complex prevents misfolding of monomeric SNAP-25, thereby enabling SNAP-25 to engage in SNARE complexes (Sharma et al., 2011a). Synucleins are small soluble vertebrate proteins that increase SNARE-complex assembly by a non-classical chaperone activity (Burre et al., 2010). Loss of CSPα in mice causes fulminant neurodegeneration in neurons because of SNAP-25 misfolding and impaired SNARE-complex formation (Sharma et al., 2011b). This neurodegeneration can be fully rescued by modest increases in α-synuclein levels which indirectly enhance SNARE-complex assembly and thereby rescue the CSPα knockout phenotype (Chandra et al., 2005).
Ca2+-triggering of fusion-pore opening
Synaptotagmins are evolutionarily conserved transmembrane proteins with two cytoplasmic C2-domains (Perin et al., 1990 and 1992; Fig. 2) that bind Ca2+ (Brose et al., 1992). C2-domains were initially defined in protein-kinase C isozymes as a conserved sequence of unknown function. Studies on synaptotagmin-1 (Syt1) showed that C2-domains constitute autonomously folding Ca2+/phospholipid-binding domains (Perin et al., 1990; Davletov and Südhof, 1993; Sutton et al., 1995). In addition, C2-domains constitute protein-interaction domains, and in the case of Syt1 bind to syntaxin-1 and to SNARE-complexes (Bennett et al., 1992; Söllner et al., 1993b; Li et al., 1995).
Although Syt1 was proposed to constitute Katz’s long-sought Ca2+-sensor for fast neurotransmitter release when it was cloned (Perin et al., 1990), initial experiments in C. elegans and Drosophila disappointingly indicated otherwise (Nonet et al., 1993; Di Antonio et al., 1993; Littleton et al., 1993). Electrophysiological analyses of Syt1 knockout mice, however, revealed that Syt1 is selectively essential for fast Ca2+-triggered release in forebrain neurons, but is not required for fusion as such (Geppert et al., 1994).
The Syt1 KO analysis supported the ‘synaptotagmin Ca2+-sensor hypothesis’, but did not exclude the possibility that Syt1 positions vesicles next to voltage-gated Ca2+-channels (a function now known to be mediated by RIMs and RIM-BPs, see below), with Ca2+-binding to Syt1 performing an unrelated role (Neher and Penner, 1994). Experiments with knock-in mice, however, proved that Ca2+-binding to Syt1 triggers neurotransmitter release (Fernandez-Chacon et al., 2001; Sørensen et al., 2003; Pang et al., 2006). Introduction into the endogenous mouse Syt1 gene of a point mutation that decreased the Syt1 Ca2+-binding affinity ~2-fold also decreased the Ca2+-affinity of neurotransmitter release ~2-fold. In addition to mediating Ca2+-triggering of release, Syt1 clamps mini release (Littleton et al., 1993; Xu et al., 2009), thus serving as an essential mediator of the speed and precision of release by association with SNARE complexes.
Sixteen synaptotagmins are expressed in brain, 8 or which bind Ca2+. All initial functional studies were carried out with Syt1, but further analyses revealed that Syt2 and Syt9 also act as Ca2+-sensors for synchronous synaptic vesicle exocytosis, albeit with different kinetics that correspond to the synapses in which these synaptotagmins are expressed (Xu et al., 2007). For example, Syt2 as the fastest synaptotagmin is expressed in the neurons mediating sound localization which requires extremely fast synaptic responses (Sun et al., 2007), whereas Syt9 is the slowest synaptotagmin that is primarily expressed in the limbic system mediating slower emotional responses (Xu et al., 2007).
Synaptotagmins do not act alone in fusion, but require complexin as a co-factor. Complexin was discovered by virtue of its tight binding to SNARE complexes (McMahon et al., 1995). Complexin-deficient neurons exhibit a milder phenocopy of Syt1-deficient neurons, with a selective suppression of fast synchronous exocytosis and an increase in spontaneous exocytosis (Reim et al., 2001). Complexin functions as a priming factor for SNARE complexes, as an activator of these SNARE complexes for subsequent synaptotagmin action, and as a clamp of spontaneous release (Giraudo et al., 2006; Tang et al., 2006; Xue et al., 2007; Maximov et al., 2009; Martin et al., 2011; Hobson et al., 2011; Kaeser-Woo et al., 2012; Jorquera et al., 2012).
Synaptotagmins also act as Ca2+-sensors for other Ca2+-dependent fusion reactions. For example, Syt1 and Syt7 are Ca2+-sensors for catecholamine and peptide hormone secretion (Schonn et al., 2008; Gustavsson et al., 2008 and 2009), and Syt2 is a Ca2+-sensor for mast cell exocytosis (Melicoff et al., 2009). Moreover, experiments in olfactory neurons uncovered a role for Syt10 as a Ca2+-sensor for IGF-1 exocytosis that differs from the Ca2+-sensor function of Syt1 in synaptic vesicle and neuropeptide vesicle exocytosis (Cao et al., 2011). Thus, even in a single neuron, different synaptotagmins can act as Ca2+-sensors for distinct Ca2+-triggered fusion reactions. All synaptotagmin-controlled fusion reactions appear to require complexin as a co-factor (e.g., see Reim et al., 2001; Cai et al., 2008; Jorquera et al., 2012; Cao et al., 2013). It seems likely that all Ca2+-triggered exocytosis depends on synaptotagmin Ca2+-sensors and complexins, and that different synaptotagmins contribute to the specificity of exocytosis pathways.
Spatial organization of the release machinery
Ultrafast neurotransmitter release in response to an action potential can only be achieved by tethering Ca2+-channels to docked and primed synaptic vesicles at the active zone. A large protein complex whose central components are three multidomain proteins called RIM, RIM-BP, and Munc13 mediate the docking and priming of synaptic vesicles at the active zone and recruit Ca2+-channels to the docked and primed vesicles (Kaeser et al., 2011). Thus, a single protein complex organizes release sites (Fig. 1; reviewed in Südhof, 2012).
RIM (for Rab3-interacting molecule; Wang et al., 1997) binds to small Rab3 and Rab27 GTP-binding proteins which are localized on synaptic vesicles, thereby docking the vesicles (Gracheva et al., 2008; Kaeser et al., 2011; Han et al., 2011; Fernández-Busnadiego et al., 2013). RIM also binds to Munc13 (no relation to Munc18; Brose et al., 1995; Betz et al., 2001), thereby activating Munc13 (Deng et al., 2011). Munc13 is a priming factor (Augustin et al., 1999) that catalyzes the conformational switch of syntaxin-1 from closed to open, promoting SNARE-complex assembly (Richmond et al., 2001; Ma et al., 2013). RIM-binding to both Munc13 and Rab3/27 is mediated by a composite N-terminal domain that contains a Munc13-binding zinc-finger surrounded by Rab3-binding α-helices (Dulubova et al., 2005; Lu et al., 2006).
Ca2+-channels need to be localized adjacent to docked and primed vesicles for fast coupling of an action potential to Ca2+-triggered exocytosis. Ca2+-channels are generally positioned less than 100 nm away from docked vesicles (Eggermann et al., 2011). RIM and the RIM-interacting molecule RIM-BP (Wang et al., 2000) both bind to Ca2+-channels in addition to binding to each other (Kaeser et al., 2011). Deletion of RIM in mice (Kaeser et al., 2011 and 2012; Han et al., 2011) and of RIM-BP in flies (Liu et al., 2011) causes a loss of Ca2+-channels from presynaptic active zones, and a decrease in Ca2+-influx. These data show that RIM and RIM-BP collaborate to recruit Ca2+-channels to release sites. Thus, in a parsimonious design a single protein complex that contains RIM as a central element mediates the co-localization of all critical proteins to the active zone. This protein complex localizes synaptic vesicles, Ca2+-channels, and vesicle priming factors next to release sites, thereby allowing fast coupling of an action potential to neurotransmitter release (Fig. 1C).
As sketched out above, we can now broadly account for the mechanism, speed, and regulation of neurotransmitter release. However, our understanding resembles an unfinished house with no plumbing and holes for windows, and raises major new questions. Below, I will briefly discuss those questions about fusion and Ca2+-triggering that seem most important to me personally, and apologize for the rather incomplete treatment of the issues. Although fascinating advances were recently made in understanding the active zone, space constraints prevent me from discussing these findings and the new questions that now arise in this subject.
SETTING UP MEMBRANE FUSION
To set the stage for fast Ca2+-triggering of release, the synaptic vesicle fusion machinery is primed into an energized, metastable state (Fig. 3A). Ca2+-binding to synaptotagmin then triggers fusion-pore opening by acting on the metastable primed fusion machinery. The nature of priming, however, and the mechanism of fusion remain debated.
Figure 3. Energy landscape of fusion and proposed role of the SM protein Munc18-1 in promoting fusion-pore opening.
A. Schematic diagram of the energy level of a vesicle that is docked, primed, and fused. The diagram illustrates that partial SNARE-complex assembly during priming is proposed to provide most of the energy required for fusion, such that Ca2+-triggering only adds a small amount of additional energy to induce fusion-pore opening.
B. Model of Munc18-1 function in fusion. Prior to priming of docked vesicles, Munc18-1 is bound to the closed conformation of syntaxin-1; this interaction is primarily regulatory to maintain a defined rate of entry into the fusion reaction, and additionally serves for the mutual stabilization of Munc18-1 and syntaxin-1 for each other (Gerber et al., 2008; Zhou et al., 2013a). Partial SNARE-complex assembly during priming (middle) is associated with a dramatic conformational change in syntaxin-1 which has to open, and in Munc18-1 whose binding changes from that to a closed syntaxin-1 conformation to binding to the open syntaxin-1 conformation via the N-peptide of syntaxin-1 (Dulubova et al., 2007). Full SNARE-complex assembly (right) produces fusion-pore opening. Munc18-1 is proposed to ride on the assembly SNARE complexes throughout the fusion reaction, and to couple the approximation of the membranes produced by the energy released by SNARE-complex assembly to fusion, possibly by mediating phospholipid mixing.
Partial SNARE-complex assembly may precede Ca2+-triggering of exocytosis
Elegant studies in neurons, chromaffin cells, and liposomes showed that the energy released by assembly of only 1-3 SNARE complexes is sufficient to drive fusion (Hua and Scheller, 2001; van den Bogaart et al., 2010; Mohrmann et al., 2010; Sinha et al., 2011; Shi et al., 2012). However, careful quantifications by Jahn and colleagues showed that SNARE proteins are very abundant, with approximately 70 synaptobrevin molecules per vesicle (Takamori et al., 2006), indicating that physiological fusion is effected by assembly of many SNARE complexes.
A plausible model for priming posits that SNARE complexes are partially assembled to elevate a synaptic vesicle into an energized pre-fusion state (Fig. 3). This model is supported by significant evidence, but not proven (Jahn and Fasshauer, 2012). Complexin only binds to partly or fully assembled SNARE complexes (McMahon et al., 1995), and complexin binding to SNARE complexes is essential for priming and activating synaptic vesicle fusion (Cai et al., 2008; Maximov et al., 2009; Yang et al., 2010; Hobson et al., 2011). Thus, at least partly assembled SNARE complexes must be present prior to fusion to which complexin can bind. Moreover, Munc13 converts syntaxin-1 from a closed to an open conformation and is selectively required for synaptic vesicle priming upstream of fusion and of Ca2+-triggering of release (Augustin et al., 1999; Richmond et al., 2001; Varoqueaux et al., 2002; Ma et al., 2013), again suggesting that SNARE complexes are at least partly assembled prior to fusion. Furthermore, t-SNARE complexes composed of ‘open’ syntaxin-1 and SNAP-25 can be visualized in native axonal membranes, and thus exist before Ca2+ triggers neurotransmitter release (Pertsinidis et al., 2013). Finally, Ca2+ can trigger synaptic vesicle fusion in less than 100 microseconds (Sabatini and Regehr, 1996), a time period that appears insufficient to accommodate opening of syntaxin-1, formation of SNARE complexes, and Ca2+-triggering of fusion by synaptotagmin.
Of these four lines of evidence supporting patial assembly of SNARE complexes during priming, the time argument is the least compelling. Conceivably, Ca2+-binding to synaptotagmin and formation of SNARE complexes could occur from an undefined intermediate and may be very fast (Jahn and Fasshauer, 2012). However, the required functions of complexin and Munc13 in priming upstream of Ca2+-triggering are not easily explained by a model that postulates an action of Ca2+ upstream of SNARE-complex assembly, suggesting that SNARE complexes are at least partly pre-assembled prior to fusion.
How do SNAREs mediate fusion?
How precisely full SNARE-complex assembly induces fusion-pore opening is unclear, as is the role of SM proteins in fusion. Although only a few SNARE complexes are needed for fusion (Hua and Scheller, 2001; van den Bogaart et al., 2010; Mohrmann et al., 2010; Sinha et al., 2011; Shi et al., 2012), physiological synaptic vesicle fusion may involve tens of SNARE complexes. It seems likely that the number of SNARE complexes per vesicle has an effect on the speed and Ca2+-dependence of neurotransmitter release because synaptotagmin acts on assembling SNARE complexes, and mass action law predicts that this interaction depends on the concentration of the substrate. Thus, it would be interesting to probe the effect of changes in the number of SNARE complexes per vesicle on the properties of release. Indeed, we recently found that increasing SNARE-complex assembly by constitutively opening syntaxin-1 dramatically increases the speed of fusion-pore opening and decreases the amount of Ca2+ required to trigger fusion-pore opening (Acuna et al., submitted).
How does SNARE-complex assembly act on the membranes in which the SNAREs reside? Do SNARE proteins primarily pull membranes together, or is the force generated by SNARE-complex assembly transferred onto the SNARE transmembrane regions, such that the transmembrane regions mediate lipid mixing during fusion and/or form the fusion pore? Physiologically, increasing the distance between the SNARE motif and the transmembrane region within synaptobrevin impairs neurotransmitter release (Deak et al., 2006; Kesavan et al., 2007; Guzman et al., 2010). Similarly, adding only 3 residues to the linker separating the transmembrane region from the SNARE motif in syntaxin-1 severely impairs Ca2+-triggered fusion (Zhou et al., 2013b). Thus, transferring of the force generated by SNARE complex assembly onto the membrane is essential.
In a test of the role of the SNARE transmembrane regions in fusion at a synapse, we recently found that SNAREs lacking a transmembrane region on both the plasma membrane (syntaxin-1) and the synaptic vesicle (synaptobrevin) are still competent for fusion (Zhou et al., 2013b). Lipid-anchored SNAREs fully substituted for regular SNAREs containing a transmembrane region in spontaneous vesicle fusion, but were less efficient in mediating Ca2+-triggered fusion. Interestingly, although the transmembrane region was dispensable, the distance of the SNARE motif from the membrane anchor continued to be crucial in lipid-anchored syntaxin-1. A 3-residue insertion into lipid-anchored syntaxin-1 severely impaired Ca2+-triggered fusion, suggesting that the mechanism of fusion is the same for syntaxin-1 independent of whether it contains a lipid anchor or a transmembrane region (Zhou et al., 2013b).
Viewed together, these data suggest that SNARE transmembrane regions may not directly form a fusion pore, but serve as membrane anchors. These data simplify our view of how SNAREs work by reducing their activity to that of a force generator that pulls membranes together in a vertical but not horizontal direction with respect to the plane of the membranes.
What do SM proteins do in fusion?
Deletion of Munc18-1 completely blocks synaptic vesicle fusion during exocytosis, and neurons subsequently degenerate (Verhage et al., 2000). No other protein’s deletion (including deletion of any SNARE protein) produces a comparably severe block of fusion. Moreover, in yeast deletion of the SM protein that mediates exocytosis – Sec1p – also completely blocks fusion (Julius et al., 1984; Grote et al., 2000).
Several hypotheses have been advanced for SM protein function in fusion, which may be the most important unsolved question in understanding fusion. Here, I would like to propose a simple parsimonious hypothesis that arguably accounts for all available data and is consistent with the essential function of SM proteins in fusion (Fig. 3B). This hypothesis is suggested by the pioneering work of the Novick laboratory on yeast Sec1p (Carr et al., 1999; Grote et al., 2000), and proposes that SNARE proteins force fusing membranes into close proximity while SM proteins, riding on top of assembling SNARE complexes, enable lipid mixing between the fusing membranes (Fig. 3B).
The hypothesis that SM proteins mediate lipid mixing during fusion provides a parsimonious explanation for how fusion may work physiologically. It is consistent with the finding that fusion requires continuous association of Munc18-1 and Sec1p with SNAREs after SNARE-complex assembly has started (Khvotchev et al., 2007; Zhou et al., 2013a; Grote et al., 2000a), and agrees with the observation that SNARE transmembrane regions are not essential for fusion (Zhou et al., 2013b). An apparent contradiction to this hypothesis is the fact that Munc18-1 is not required for SNARE-mediated liposome fusion (Weber et al., 1997). However, the lack of a requirement for Munc18-1 in liposome fusion contradicts the universal necessity for SM proteins in physiological SNARE-dependent fusion reactions, and may be due to differences between biological membranes and liposomes. Biological membranes contain high concentrations of both intrinsic and peripheral membrane proteins, and may require an activator of lipid mixing for fusion beyond the proximity of the phospholipid membrane surfaces provided by SNARE-complex assembly. SM proteins may enable lipid mixing by organizing lipid patches adjacent to SNARE membrane anchors, such that the action of the SNAREs on the membrane allows exposed lipids to become destabilized for fusion, or may actually promote lipid mixing. Indeed, recent experiments uncovered a strong fusion-promoting role of SM proteins even for liposome fusion (Shen et al., 2007; Diao et al., 2010; Rathore et al., 2010; Schollmeier et al., 2011; Yu et al., 2013).
The proposal that SM proteins act in fusion by enabling phospholipid mixing, riding on the assembling SNARE complexes, is at present only that – a hypothesis. Alternative, not necessarily mutually exclusive hypotheses are also plausible. Early on, the notion that SM proteins primarily inhibit fusion obtained significant support (Yang et al., 2000; Wu et al., 2001), but more recent experiments with in vivo and in vitro fusion reactions have argued against this notion (e.g., see Schollmeier et al., 2011; Rathore et al., 2010). More recently, the idea that Munc18-1 and other SM proteins catalyze SNARE-complex assembly, possibly by nucleating it, has received significant attention. This idea accounts for the binding of SM proteins to SNARE complexes. However, a sole function of SM proteins in promoting SNARE-complex assembly is difficult to reconcile with the essential role of SM proteins in fusion. A decreased rate of SNARE-complex assembly in the absence of an SM protein should decrease the rate of fusion, but not block fusion. Moreover, deletion of Sec1p in yeast blocks fusion but not SNARE complex assembly (Grote et al., 2000). Finally, other proteins are known to promote SNARE-complex assembly (e.g., Munc13 [Ma et al., 2013] and synucleins [Burre et al., 2010]). Although these data argue against a primary function of SM proteins as SNARE-complex assembly catalysts, it is quite possible that SM proteins also act to promote SNARE-complex assembly.
Besides the notion that SM proteins mediate fusion and/or catalyze SNARE complex assembly, a third plausible hypothesis for how SM proteins activate fusion is that they spatially organize assembled SNARE complexes around the fusion site (Rizo et al., 2006). This esthetically pleasing hypothesis also accounts for the binding of SM proteins to assembled SNARE complexes, and could be related to a lipid-mixing activity of SM proteins. However, fusion mediated by only 1-3 SNARE complexes (van den Bogaart et al., 2010; Mohrmann et al., 2010) presumably still requires Munc18-1, and thus according to this hypothesis an SM protein would organize isolated SNARE complexes for fusion.
Differentiating between these hypotheses and testing them will require novel assays that monitor lipid mixing with a high temporal resolution in an SM protein-dependent manner. Thus, answering the question of SM protein function in fusion remains a major challenge whose resolution requires not only a reductionist approach using liposome fusion, but also physiological tests of normal fusion reactions in a living cell.
HOW DOES Ca2+ TRIGGER FUSION IN MICROSECONDS?
As outlined above, the Ca2+-sensor synaptotagmin and its assistant complexin transduce the presynaptic Ca2+-signal for release. These proteins likely act on a primed fusion machinery that is ready to go. However, the precise interplay of these two key players is incompletely understood.
Asynchronous neurotransmitter release
Although deletion of Syt1, Syt2, and Syt9 in vertebrate neurons, and of Syt1 in Drosophila neurons, blocks synchronous release, not all Ca2+-dependent release is ablated (Geppert et al., 1994; Yoshihara and Littleton, 2002; Maximov and Südhof, 2005; Sun et al., 2007). In most synapses, the remaining Ca2+-stimulated release is dramatically facilitated during action-potential bursts in vitro and in vivo (Xu et al., 2012). This remaining release is often referred to as ‘asynchronous’ because it lags after synchronous release and is not tightly coupled to an action potential.
Asynchronous release exhibits distinct properties in different types of neurons, and likely comprises multiple processes. Hippocampal Syt1 knockout neurons exhibit significant asynchronous release that is amplified by facilitation during action-potential trains (Maximov and Südhof, 2005), so much so that the total amount of asynchronous release in Syt1 knockout neurons becomes identical to that observed in wild-type neurons (Yoshihara and Littleton, 2002; Nishiki and Augustine, 2004; Maximov and Südhof, 2005; Xu et al., 2012)! In contrast, Syt2 knockout synapses in the calyx of Held display relatively little asynchronous release that exhibits only modest facilitation during high-frequency stimulus trains (Sun et al., 2007). In yet another example for a difference between synapses, some neurons such as cholecystokinin-containing interneurons in the hippocampus use a facilitating type of asynchronous release as the dominant form of release even in wild-type conditions (Hefft and Jonas, 2005; Daw et al., 2009; Karson et al., 2009). These observations prompted the question, what is asynchronous release, and what Ca2+-sensor mediates asynchronous release?
Studies in chromaffin cells provided the first clue to answering these questions. Earlier experiments had shown that deletion of Syt1 in chromaffin cells produced a small but significant decrease in Ca2+-stimulated exocytosis and a delay in the rate of exocytosis (Sørensen et al., 2003). In a pivotal study, Schonn et al. (2008) then demonstrated that deletion of only Syt7, a Ca2+-binding synaptotagmin that had previously been implicated as a Ca2+-sensor in exocytosis in PC12 cells (Sugita et al., 2001; Fukuda et al., 2004) also produced a small decrease in Ca2+-stimulated exocytosis in chromaffin cells. However, the double deletion of both Syt1 and Syt7 caused a dramatic ablation of nearly all Ca2+-induced exocytosis (Schonn et al., 2008). This finding suggested that at least in chromaffin cells, Syt1 and Syt7 are redundant Ca2+-sensors for exocytosis with distinct response kinetics.
Syt7 is also expressed at high levels in brain – even higher than Syt1 – and is localized to synapses (Sugita et al., 2001). However, initial studies to uncover a role for Syt7 in synaptic exocytosis using constitutive Syt1 and Syt7 knockout mice were disappointingly unsuccessful (Maximov et al., 2008). This situation changed with acute ablation of Syt1 or Syt7 on the background of constitutive deletions of Syt7 or Syt1, respectively (Bacaj et al., 2013). Such acute manipulations severely impaired not only synchronous but also asynchronous release in hippocampal neurons (Fig. 4). Consistent with the studies in PC12 and primary chromaffin cells, this result suggests that effectively all Ca2+-triggered neurotransmitter release is mediated by a synaptotagmin. Moreover, this result agrees with studies indicating that Syt7 functions as a Ca2+-sensor in neuroendocrine secretion and in lysosome exocytosis (Shin et al., 2002; Chakrabarti et al., 2003; Fukuda et al., 2004; Tsuboi and Fukuda, 2007; Schonn et al., 2008; Gustavsson et al., 2008 and 2009; Li et al., 2009; Segovia et al., 2010). Finally, a role for Syt7 as a Ca2+-sensor in synaptic exocytosis agrees well with the similar Ca2+-binding properties and Ca2+-dependent phospholipid- and syntaxin-binding properties of Syt1 and Syt7 (Li et al., 1995; Sugita et al., 2002).
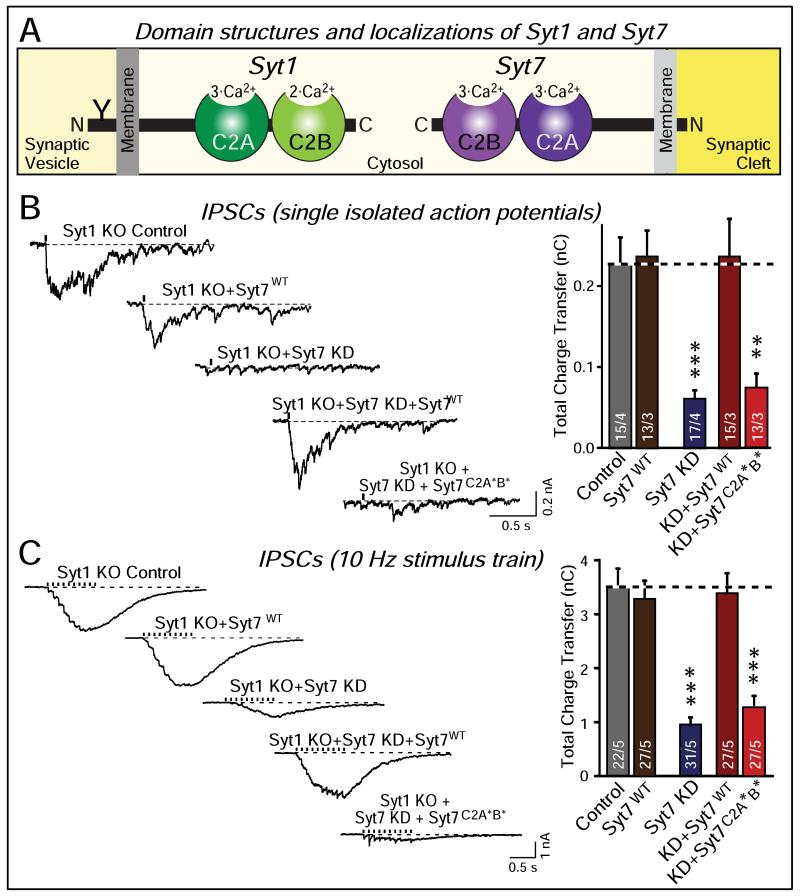
Figure 4. Complementary roles of synaptotagmin-1 (Syt1) and -7 (Syt7) Ca2+-sensors in triggering synchronous and asynchronous neurotransmitter release.
A. Domain structures and localizations of Syt1 as the paradigmatic fast synaptotagmin isoform, and of Syt7 as the slow synaptotagmin isoform. Syt1 and Syt7 have identical domain structures, except that Syt1 contains an N-terminal N-glycosylation site in the vesicle that is lacking from Syt7, and that Syt1 is primarily localized to synaptic vesicles where Syt7 is primarily absent from synaptic vesicles.
B. Inhibitory postsynaptic currents (IPSCs) measured in response to isolated action potentials in cultured hippocampal neurons from Syt1 knockout mice that were either infected with a control lentivirus, a lentivirus overexpressing wild-type Syt7, a lentivirus expressing a Syt7 shRNA that blocks most Syt7 expression, a lentivirus co-expressing the Syt7 shRNA together with wild-type Syt7, or a lentivirus co-expressing the Syt7 shRNA with a mutant Syt7 in which the C2A- and C2B-domain Ca2+-binding sites were mutated. Representative traces are shown on the left, and summary graphs on the right. Data shown are means ± SEM; numbers in the bars indicate the numbers of neurons/cultures analyzed. Statistical significance was assessed by one-way ANOVA comparing all test conditions to the control (**, p<0.01; ***, p<0.001).
C. Same as B, except that IPSCs were measured in response to a 10 Hz 1 sec stimulus train.
Data and figure were adapted from Bacaj et al. (2013).
However, Syt7 exhibits two puzzling properties. First, in neurons Syt7 is not dectable in synaptic vesicles, but at least partly localized to the plasma membrane (Sugita et al., 2001; Takamori et al., 2006). This is puzzling given the localization of Syt7 to secretory vesicles in non-neuronal cells (Chakrabarti et al., 2003; Fukuda et al., 2004; Schonn et al., 2008; Gustavsson et al., 2008 and 2009). Second, whereas in all vesicular synaptotagmins tested up to date, the C2B-domain Ca2+-binding sites are essential for Ca2+-stimulation of exocytosis and the C2A-domain Ca2+-binding sites only assist in Ca2+-triggering of exocytosis (e.g., see Mackler and Reist, 2001; Nishiki and Augustin, 2004; Shin et al., 2009; Cao et al., 2011; Lee et al., 2013), in Syt7 the C2A-domain Ca2+-binding sites were essential for asynchronous release and the C2B-domain Ca2+-binding sites were dispensable (Bacaj et al., 2013). The differences in the localization and the relative C2-domain functions between Syt1 and Syt7 may be related to each other, and the plasma membrane localization of Syt7 may also explain, at least in part, why Syt7 is generally less effective than Syt1 in triggering exocytosis. Alternatively, it is possible that a small amount of Syt7 is present on synaptic vesicles, and its relatively low Ca2+-triggering efficiency is due to its poor synaptic vesicle sorting efficiency. The recent Syt7 results suggest that different synaptotagmins collaborate and compete with each other as Ca2+-sensors for release, and expand the finding that Syt1 is also co-expressed with Syt2 or Syt9 in some synapses where these synaptotagmins also complement each other physiologically (Xu et al., 2007; Pang et al., 2007).
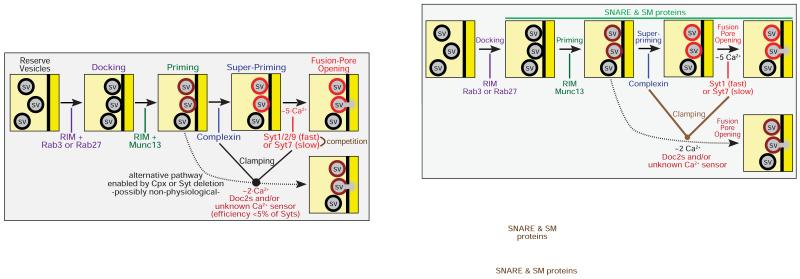
In the non-physiological situation of a Syt1 or Syt2 knockout synapse, the observed remaining Ca2+-dependent release may be more complex than simply allowing Syt7 function to become manifest (Fig. 5). This possibility is suggested by two observations. First, in Syt1 or Syt2 KO synapses, an approximately 10-fold increase of spontaneous miniature release is observed. The increased ‘minis’ in the Syt1 KO neurons are still largely Ca2+-dependent (>90%), just like normal minis, but exhibit a different Ca2+-cooperativity than normal minis (Xu et al., 2009; see below for a further discussion of ‘clamping’ of minis by synaptotagmin and complexin). These minis are thus driven by an unknown Ca2+-sensor that is not Syt7 because ablation of Syt7 expression does not affect these minis (Bacaj et al., 2013). Second, in Syt2 KO calyx synapses which do not exhibit the facilitating asynchronous release observed for hippocampal neurons, biophysical studies revealed that the remaining ‘asynchronous’ release has an apparent Ca2+-cooperativity of 1-2, whereas synaptotagmin-dependent release generally exhibits an apparent Ca2+-cooperativity of 4-5 (Sun et al., 2007; Kochubey and Schneggenburger, 2011). This finding suggests that non-facilitating asynchronous release observed in the Syt2 KO calyx, similar to the increased mini release in Syt1 KO hippocampus, is due to a non-synaptotagmin dependent mechanism. The relationship between physiological synaptotagmin-induced release and non-physiological Ca2+-induced release mediated by an as yet unknown Ca2+-sensor is illustrated in Fig. 5.
Figure 5. Schematic representation of different synaptic Ca2+-controlled neurotransmitter release pathways.
After docking and priming of synaptic vesicles mediated by the active zone protein complex containing RIM proteins as central elements, vesicles are further ‘superprimed’ by binding of complexin to the assembling SNARE complexes. The superprimed vesicles are the substrates of fast (Syt1/Syt2/Syt7) and slow (Syt7) synaptotagmins as synaptic Ca2+-sensors. Fast and slow synaptotagmins are differentially expressed, compete with each other, and are further regulated by alternative splicing and phosphorylation, such that synapses differ in the amount and short-term plasticity of Ca2+-induced release. In the absence of complexin or the fast synaptotagmin Ca2+-sensors, an alternative nonphysiological pathway becomes enabled (dotted line) whereby an as yet unidentified Ca2+-sensor with a Ca2+-cooperativity that differs from that of synaptotagmins mediates spontaneous ‘mini’ release. Key proteins are listed in the drawing at their points of action; SNARE and SM proteins are not shown for clarity sake, but are obviously vital components for the entire fusion reaction.
What synaptotagmin-independent Ca2+-sensor may mediate the increased mini release in Syt1 KO hippocampal neurons and the remaining release in Syt2 KO calyx synapses? Proteins like Doc2 and calmodulin were ruled out in loss-of-function experiments (Groffen et al., 2010; Pang et al., 2010 and 2011). It is striking that the priming factor Munc13 is activated by Ca2+. Munc13 contains at least three regulatory domains that are directly (the central C2-domain) or indirectly (the central C1-domain and the calmodulin-binding sequence) controlled by Ca2+ (Rhee et al., 2002; Junge et al., 2004; Shin et al., 2010). In the absence of the synaptotagmin/complexin clamp, Ca2+-stimulation of Munc13 may induce increased mini release in Syt1 and Syt2 KO neurons. However, this hypothesis implies that priming is rate-limiting in such neurons, i.e. that no reservoir of primed vesicles should be present, whereas the readily-releasable pool (RRP) of vesicles is not altered in Syt1 or Syt2 KO neurons (Geppert et al., 1994; Sun et al., 2007; Xu et al., 2007). These considerations suggest that the Ca2+-dependent pathway mediating the increased mini release in Syt1 KO neurons is downstream of priming and Munc13 (Fig. 5).
Activating vs. clamping functions of synaptotagmin
Deletion of Syt1 or Syt2 enhances the rate of spontaneous vesicle exocytosis approximately 10-fold to cause increased mini release (Littleton et al., 1993; Broadie et al., 1994; Maximov and Südhof, 2005; Sun et al., 2007; Xu et al., 2009). This is referred to as ‘unclamping’, with the notion that Syt1 and Syt2 normally clamp spontaneous mini release. The ‘clamping’ function of synaptotagmin is probably highly significant, but reflects an independent activity that is less efficacious than the Ca2+-triggering function of synaptotagmin, as suggested by the following considerations.
First, the enhancement in release induced by unclamping is relatively minor compared to the increase in the rate of synaptic vesicle exocytosis produced by Ca2+-binding to Syt1 or Syt2 (a ~10-fold increase in mini release vs. a ~1,000,000-fold increase produced by Ca2+; Sun et al., 2007). Per excitatory synapse, normal spontaneous fusion translates to an average rate of ~0.004 Hz, and even in the unclamped state an excitatory synapse will fire only once every 4 minutes or so, a very low rate that is not sufficient to deplete the readily-releasable pool of vesicles (Xu et al., 2009).
Second, unclamping is not observed in all synapses; for example, the Syt1 knockout does not produce an increase in mini release in autapses (Geppert et al., 1994).
Third, mutations that block Ca2+-binding to the Syt1 C2A-domain decrease Ca2+-triggered synchronous release in hippocampal neurons by approximately 50%, but do not ablate it, whereas mutations that block Ca2+-binding to the C2B-domain ablate fast synchronous release (Mackler and Reist, 2001; Nishiki and Augustin, 2004; Shin et al., 2009). In contrast, mutations of the Syt1 C2A- or C2B-domain equally abolish its clamping activity (Shin et al., 2009; Lee et al., 2013). Thus, activating and clamping functions of synaptotagmin are not obligatorily coupled. Interestingly, no Syt1 mutation is known that allows clamping but blocks Ca2+-triggering of release, although such mutations are described for complexin (see below).
Fourth, as mentioned above, the majority of spontaneous ‘mini’ release events both in wild-type and in Syt1-deficient synapses are Ca2+-dependent (Xu et al., 2009). However, their Ca2+-dependence exhibits a dramatically different Ca2+-cooperativity (~4 for normal minis vs. ~2 for minis in Syt1 knockout neurons; Xu et al., 2009), suggesting that the minis are carried by distinct Ca2+-sensors.
Viewed together, these results suggest that the activating and clamping functions of synaptotagmin are independent. Thus Ca2+-triggering of release by Ca2+-binding to synaptotagmin does not involve the removal of a clamp that prevents a primed, partially assembled SNARE complex from fully assembling and completing fusion. Moreover, these results suggest that the clamping functions of synaptotagmin are relatively minor, and may primarily ensure the precision of synaptic transmission, of fine-tuning the release process.
However, the fact that the synaptotagmin clamping function is relatively minor does not imply that it is not significant – clearly it is of utmost importance for synapses to be silent when not activated. Large numbers of whispering synapses would make a lot of noise. This computational noise would be detrimental for the ability of the brain to process real signals in neural circuits, and tightly regulating the amount of action-potential independent spontaneous release is likely an important mechanism of information processing.
The available clamping data also raise additional questions. It is striking that the clamping function of Syt1 requires the wild-type sequences of both its C2A- and its C2B-domain Ca2+-binding sites (Shin et al., 2009; Lee et al., 2013). Clamping itself cannot depend on Ca2+-binding to these domains because such Ca2+-binding triggers exocytosis (Fernandez-Chacon et al., 2001; Rhee et al., 2005; Pang et al., 2006; Xu et al., 2009). It is unlikely that the Ca2+-binding sites of the C2-domains are partially occupied in nerve terminals at rest given the high cooperativity of Ca2+-binding to C2-domains (Davletov and Südhof, 1993; Kohout et al., 2002). The most parsimonious interpretation is that prior to Ca2+-binding, the sequences of the C2-domain Ca2+-binding sites interact with an unidentified target in a Ca2+-independent manner, such that this interaction clamps mini release but is severed by Ca2+-binding (Fig. 2). Apart from prompting the question of the nature of this target, this interpretation implies that contrary to current models, Syt1 acts on the release process upstream of Ca2+-triggering, before the last millisecond in the lifetime of a vesicle, during the stage during which the fusion machinery is set up to prepare for the demise of the vesicle and the popping of its fusion pore (Fig. 2). Future experiments will have to explore the nature of this activity.
The complexin enigma
Complexin is a universal co-factor for synaptotagmin in all Ca2+-triggered fusion reactions that have been examined (e.g., see Reim et al., 2001; Tang et al., 2006; Cai et al., 2008; Jorquera et al., 2012; Cao et al., 2013). Three distinct changes caused by the loss-of-function of complexin have been defined: a decrease in Ca2+-triggering of release, an increase in spontaneous mini release, and a decrease in the size of the RRP. In two of these activities – the Ca2+-triggering of release and the clamping of mini release – complexin performs analogous roles to Syt1 and Syt2, but with considerably smaller effect sizes.
How does a small molecule like complexin, composed of only ~130 residues, act to activate and clamp synaptic vesicles for synaptotagmin action? Atomic structures revealed that complexin, when bound to assembled SNARE complexes, contains two short α-helices flanked by flexible sequences (Chen et al., 2001). The central, more C-terminal α-helix is bound to the SNARE complex, and is essential for all complexin function (Maximov et al., 2009). The accessory, more N-terminal α-helix is required only for the clamping but not for the activating function of complexin (Yang et al., 2010). The flexible N-terminal sequence of complexin, conversely, mediates only the activating but not the clamping function of complexin (Xue et al., 2007; Maximov et al.., 2009). The equally flexible C-terminal sequence, in turn, is required only for the clamping and priming activities of complexin, but not for its Ca2+-triggering activity (Kaeser-Woo et al., 2012). Thus, all three activities of complexin – clamping, priming, and activation of Ca2+-triggering – require distinct complexin sequences.
For complexin’s activity, its binding in the middle of the SNARE complex, close to the central ‘zero layer’, is crucial, as it implies that complexin can bind to partially assembled SNARE complexes prior to fusion-pore opening, consistent with its role in priming. Our current model is that complexin binding to SNAREs activates the SNARE/SM protein complex, and that at least part of complexin competes with synaptotagmin for SNARE-complex binding (Tang et al., 2006; Xu et al., 2013). Ca2+-activated synaptotagmin displaces this part of complexin (although not necessarily the entire complexin molecule), thereby triggering fusion-pore opening.
The conclusions made above for synaptotagmin function in clamping similarly apply to complexin: Complexin also does not primarily act as a clamp that prevents SNARE-complex assembly, and does not activate fast Ca2+-triggered release by being displaced. Apart from the fact that complexin clamping activity is variably observed in different contexts (e.g., see Reim et al., 2001, and Xue et al., 2008, vs. Huntwork and Littleton, 2007, and Maximov et al., 2009), complexin ‘poorclamp’ mutants with an inactive accessory α-helix fully support Ca2+-triggered fusion (Yang et al., 2010). As for synaptotagmin, the activation and clamping functions of complexin are not linked, and the cumulative evidence supports the notion that it is really the activation function of complexin that is most important, especially since that is also the only function observed in non-synaptic exocytosis (Cai et al., 2008; Cao et al., 2013).
How does complexin function? The clamping function is easier to address because it depends on the complexin accessory α-helix, suggesting that this accessory α-helix may insert into the partially assembled trans-SNARE complex to prevent full zippering (Giraudo et al., 2009). This hypothesis is supported by structural data showing that complexin can cross-link trans-SNARE complexes into a zigzag array (Kümmel et al., 2011). However, the relation of these observations to the activation functions of complexin is not clear. Moreover, these observations do not explain why the complexin C-terminus is required for clamping, even though it is not essential for Ca2+-triggering, and thus the loss of the accessory α-helix does not interfere with the localization or expression of complexin (Kaeser-Woo et al., 2012).
At present, no plausible hypothesis is available for how complexin activates Ca2+-triggering of release by synaptotagmin – possibly one of the most important questions in the field. Strikingly, such activation requires the N-terminal complexin sequences (Xue et al., 2007; Maximov et al., 2009), suggesting an as yet uncharacterized interaction, possibly with membrane phospholipids.
A contemporary model for synaptotagmin and complexin function
Based on the advances discussed above, we propose that fast (Syt1, Syt2, and Syt9) and slow (Syt7) synaptotagmin Ca2+-sensors normally compete with each other during Ca2+-stimulated neurotransmitter release (Fig. 5). Syt7 function is not readily apparent in a generic synapse in a cultured neuron because Syt1, Syt2, and Syt9 generally win the competition, but Syt7 function may be physiologically activated by extended stimulus trains, by alternative splicing of Syt7, and/or by phosphorylation which may inhibit Syt1, Syt2, or Syt9 or activate Syt7. Such physiological activation could account for the preponderance of asynchronous release in some synapses (Hefft and Jonas, 2005; Daw et al., 2009; Karson et al., 2009).
Mutagenesis experiments indicated that synaptotagmins induce fusion pore opening via Ca2+-stimulated binding to phospholipids and SNAREs (Fernandez-Chacon et al., 2001; Zhang et al., 2002; Pang et al., 2006), suggesting that synaptotagmins act on a primed fusion machinery via a simple Ca2+-induced interaction that may cause a mechanical push-and-pull, thereby opening the fusion pore. Consistent with this model, mutation of a conserved tryptophan-tryptophan sequence in the linker separating the SNARE motif from the membrane anchor of synaptobrevin did not block fusion as such, but ablated synchronous neurotransmitter release (Maximov et al., 2009). When the tryptophan-tryptophan motif was replaced by a double alanine motif, evoked release became desynchronized and spontaneous release was unclamped, suggesting that the clamping and activating functions of synaptotagmin and complexin act on the linker separating the SNARE motif from the membrane anchor. This hypothesis is further supported by experiments demonstrating that cleavage of the C-terminal residues of the second SNARE motif of SNAP-25 by botulinum toxin A impairs Ca2+-triggered fusion much more severely, in relative terms, than fusion as such (Xu et al., 1998; Sørensen et al., 2002; Zhang et al., 2002; Sakaba et al., 2005).
Clearly, Ca2+ does not trigger release by unclamping the SNARE complex, for example via a Ca2+-dependent displacement of complexin from SNARE complexes via synaptotagmin, although Ca2+-binding to Syt1 likely displaces at least part of complexin from SNARE complexes (Tang et al., 2006; Xu et al., 2013). If synaptotagmin is not the major clamp of fusion, what ‘clamps’ the SNARE complexes, i.e. what keeps partly zippered up complexes from completely zippering up and opening the fusion pore? This may be the wrong question – in a physiological system, a partly zippered-up complex may be perfectly stable, and simply require an additional push for completing the zippering process, a push that we propose is provided by synaptotagmin and complexin. Synaptotagmin and complexin may interact in the absence of Ca2+ with the partly zippered complex, thereby setting up the fast Ca2+-triggered reaction. Deletion of synaptotagmin and complexin may lead to a modest increase in spontaneous fusion that is still Ca2+-dependent by allowing a non-physiological Ca2+-dependent process – possibly a change in electrostatic properties at the site of fusion – to partly destabilize partly zippered SNARE complexes.
We thus propose that Ca2+ triggers release in a two-stage reaction that involves a close collaboration between synaptotagmin and complexin. Prior to Ca2+-influx, both synaptotagmin and complexin interact with the fusion machinery composed of a partly assembled SNARE/SM protein complex to activate the complex and enable a fast response to Ca2+. Such interaction is indicated by the ‘clamping’ activity of synaptotagmin and complexin, and by the priming activity of complexin. When Ca2+-levels rise during an action potential, Ca2+-binding to synaptotagmin triggers a rearrangement of the overall fusion complex containing also complexin and synaptotagmin, such that part of complexin is displaced from the complex via the Ca2+-dependent SNARE-complex interaction of synaptotagmin, and the SNARE complex is moved with respect to the membrane via the Ca2+-dependent phospholipid interaction of synaptotagmin. This overall proposal is consistent with the available data, but far from proven – no direct evidence for an upstream activity of synaptotagmin apart from its clamping activity is available, and the atomic basis of the various interactions has not been elucidated.
ANOTHER QUARTER CENTURY IS COMING!
Given the detailed current understanding of how a presynaptic terminal converts a presynaptic action potential into a trans-synaptic neurotransmitter signal, and how the terminal not only translates an action potential into neurotransmitter release, but also computes the action potential signal dependent on the previous use of a synapse and on extrinsic inputs – given this detailed understanding, is there anything left to be done? This question is particularly pertinent because of current views that the molecular and computational mechanisms of synaptic transmission do not matter for an understanding of the brain, and that not only synapses, but even entire neurons can be dealt with as unitary entities in the large information processing machine that constitutes the brain.
At present, a widely shared opinion is that understanding the architecture of the brain will be sufficient for explaining how the brain works, maybe combined with a description about information flow, similar to the beautiful drawings of Cajal that have dominated neuroscientists’ vision for a century. However, understanding the brain is not like understanding a house where features like air ducts, electrical connections, and window locks are just details that you don’t really need to know in order to live in it. Instead of one house, a brain is rather like an assembly of billions of houses – the synapses – each of which has their own air ducts, electrical connections, and window locks. To carry this analogy further, understanding Shanghai will not be possible by looking at street maps, even if one knows how the traffic flows – it is necessary to know what is going on in individual buildings, and how such buildings work. Moreover, it is necessary to know how the houses change, as there is continuous construction activity, demolition and rebuilding of houses, or just renovations – a never ending series of changes. Thus, we would like to argue that until we understand how synapses work, how synapses differ from each other, and how synapses change as a function of use over milliseconds to years, we will not be able to understand how the brain works, no matter how many connections have been mapped and how many stimulated neurons have been shown to elicit a certain behavior. Among the key questions about neurotransmitter release that have not been addressed are questions such as how vesicles are made, how short- and long-term plasticity is effected, and how precisely complexin works at the atomic level.
Much of contemporary neuroscience and cell biology seems to believe that everything concerning molecules or purified proteins is a detail. The general perspective often is that the only attractive type of scientist corresponds to an architect who designs beautiful buildings but pays no attention to air ducts, electrical wiring, and window locks. The idea is that what counts is the overall design, and that the details are negligible. I hope that at least some of my readers have been convinced by my arguments that the molecules which make up a biological system are actually more than trivial details, but are the system, and that studying and understanding them is not just an unfortunate necessity but the only avenue to building the building in the first place.
Finally, increasing evidence implicates synapse dysfunction in neurological and psychiatric disorders. This evidence includes the observation that α-synuclein, which is centrally involved in multiple neurodegenerative disorders including Parkinson’s disease, is a SNARE-complex assembly chaperone (Burre et al., 2010), the finding that the SM protein Munc18-1 is frequently mutated in Ohtahara syndrome (Saitsu et al., 2008), and the discovery that many ‘synaptic’ genes are mutated in schizophrenia and autism (Südhof, 2008). However, we know very little about how the pathophysiological mechanisms underlying any of these diseases. Thus, unraveling not only the normal mechanisms of release but also the abnormal processes producing neurological disorders will be a major challenge for future work.
ACKNOWLEDGEMENTS
I thank all my lab members for their advice and comments, and my colleagues J. Rothman (Yale University) and J. Rizo (UTSW) for their invaluable input. Work on neurotransmitter release in my laboratory is supported by grants from the NIMH (P50 MH086403) and NINDS (R01 NS077906) as well as the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Augustin I, Rosenmund C, Südhof TC, Brose N. Munc-13 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- Bacaj T, Wu D, Yang X, Morishita W, Zhou P, Xu W, Malenka RC, Südhof TC. Synaptotagmin-1 and -7 Trigger Synchronous and Asynchronous Phases of Neurotransmitter Release. Neuron. 2013 doi: 10.1016/j.neuron.2013.10.026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Broadie K, Bellen HJ, DiAntonio A, Littleton JT, Schwarz TL. Absence of synaptotagmin disrupts excitation-secretion coupling during synaptic transmission. Proc Natl Acad Sci U S A. 1994;91:10727–10731. doi: 10.1073/pnas.91.22.10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N, Petrenko AG, Südhof TC, Jahn R. Synaptotagmin: A Ca2+ sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Brose N, Hofmann K, Hata Y, Südhof TC. Mammalian homologues of C. elegans unc-13 gene define novel family of C2-domain proteins. J Biol Chem. 1995;270:25273–25280. doi: 10.1074/jbc.270.42.25273. [DOI] [PubMed] [Google Scholar]
- Brunger AT, Weninger K, Bowen M, Chu S. Single-molecule studies of the neuronal SNARE fusion machinery. Annu Rev Biochem. 2009;78:903–928. doi: 10.1146/annurev.biochem.77.070306.103621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton M, Südhof TC. α-Synuclein Promotes SNARE-Complex Assembly in Vivo & in Vitro. Science. 2010;329:1664–1668. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Reim K, Varoqueaux F, Tapechum S, Hill K, Sørensen JB, Brose N, Chow RH. Complexin II plays a positive role in Ca2+-triggered exocytosis by facilitating vesicle priming. Proc Natl Acad Sci U S A. 2008;105:19538–19543. doi: 10.1073/pnas.0810232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CM, Grote E, Munson M, Hughson FM, Novick PJ. Sec1p binds to SNARE complexes & concentrates at sites of secretion. J Cell Biol. 1999;146:333–344. doi: 10.1083/jcb.146.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Maximov A, Südhof TC. Activity-dependent IGF-1 exocytosis is controlled by the Ca2+-sensor synaptotagmin-10. Cell. 2011;145:300–311. doi: 10.1016/j.cell.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Yang X, Südhof TC. Complexin activates exocytosis of distinct secretory vesicles controlled by different synaptotagmins. J Neurosci. 2013;33:1714–1727. doi: 10.1523/JNEUROSCI.4087-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Kobayashi KS, Flavell RA, Miyake K, Liston DR, Fowler KT, Gorelick FS, Andrews NW. Impaired membrane resealing & autoimmune myositis in synaptotagmin VII-deficient mice. J Cell Biol. 2003;162:543–549. doi: 10.1083/jcb.200305131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernández-Chacón R, Schlüter OM, Südhof TC. α-Synuclein Cooperates with CSPα in Preventing Neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Davletov BA, Südhof TC. A single C2-domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid-binding. J Biol Chem. 1993;268:26386–26390. [PubMed] [Google Scholar]
- Daw MI, Tricoire L, Erdelyi F, Szabo G, McBain CJ. Asynchronous transmitter release from cholecystokinin-containing inhibitory interneurons is widespread & target-cell independent. J Neurosci. 2009;29:11112–11122. doi: 10.1523/JNEUROSCI.5760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deák F, Shin OH, Kavalali ET, Südhof TC. Structural determinants of synaptobrevin 2 function in synaptic vesicle fusion. J Neurosci. 2006;26:6668–6676. doi: 10.1523/JNEUROSCI.5272-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deák F, Xu Y, Chang W-P, Dulubova I, Khvotchev M, Liu X, Südhof TC, Rizo J. Munc18-1 Binding to the Neuronal SNARE Complex Controls Synaptic Vesicle Priming. J Cell Biol. 2009;184:751–764. doi: 10.1083/jcb.200812026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Kaeser PS, Xu W, Südhof TC. RIM Proteins Activate Vesicle Priming by Reversing Auto-Inhibitory Homodimerization of Munc13. Neuron. 2011;69:317–331. doi: 10.1016/j.neuron.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao J, Su Z, Lu X, Yoon TY, Shin YK, Ha T. Single-Vesicle Fusion Assay Reveals Munc18-1 Binding to the SNARE Core Is Sufficient for Stimulating Membrane Fusion. ACS Chem Neurosci. 2010;1:168–174. doi: 10.1021/cn900034p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAntonio A, Parfitt KD, Schwarz TL. Synaptic transmission persists in synaptotagmin mutants of Drosophila. Cell. 1993;73:1281–1290. doi: 10.1016/0092-8674(93)90356-u. [DOI] [PubMed] [Google Scholar]
- Dodge FA, Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Südhof TC, Rizo J. A conformational switch in syntaxin during exocytosis. EMBO J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Yamaguchi T, Min SW, Gao Y, Südhof TC, Rizo J. How Tlg2p/syntaxin16 “snares” Vps45. EMBO J. 2002;21:3620–3631. doi: 10.1093/emboj/cdf381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Lou X, Lu J, Huryeva I, Alam A, Schneggenburger R, Südhof TC, Rizo J. A Munc13/RIM/Rab3 tripartite complex: from priming to plasticity? EMBO J. 2005;24:2839–2850. doi: 10.1038/sj.emboj.7600753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Khvotchev M, Südhof TC, Rizo J. Munc18-1 Binds Directly to the Neuronal SNARE Complex. Proc Natl Acad Sci USA. 2007;104:2697–2702. doi: 10.1073/pnas.0611318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann E, Bucurenciu I, Goswami SP, Jonas P. Nanodomain coupling between Ca2+ channels & sensors of exocytosis at fast mammalian synapses. Nat Rev Neurosci. 2011;13:7–21. doi: 10.1038/nrn3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Busnadiego R, Asano S, Oprisoreanu AM, Sakata E, Doengi M, Kochovski Z, Zürner M, Stein V, Schoch S, Baumeister W, Lucić V. Cryo-electron tomography reveals a critical role of RIM1α in synaptic vesicle tethering. J Cell Biol. 2013;201:725–740. doi: 10.1083/jcb.201206063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Chacon R, Königstorffer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Südhof TC. Synaptotagmin I functions as a Ca2+-regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Ogata Y, Saegusa C, Kanno E, Mikoshiba K. Alternative splicing isoforms of synaptotagmin VII in the mouse rat & human. Biochem J. 2002;365(Pt 1):173–180. doi: 10.1042/BJ20011877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Kanno E, Satoh M, Saegusa C, Yamamoto A. Synaptotagmin VII is targeted to dense-core vesicles & regulates their Ca2+-dependent exocytosis in PC12 cells. J Biol Chem. 2004;279:52677–52684. doi: 10.1074/jbc.M409241200. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Südhof TC. Synaptotagmin I: A major Ca2+-sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Gerber SH, Rah J-C, Min S-W, Liu X, de Wit H, Dulubova I, Meyer AC, Rizo J, Arancillo M, Hammer RE, Verhage M, Rosenmund C, Südhof TC. Conformational Switch of Syntaxin-1 Controls Synaptic Vesicle Fusion. Science. 2008;321:1507–1510. doi: 10.1126/science.1163174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudo CG, Eng WS, Melia TJ, Rothman JE. A clamping mechanism involved in SNARE-dependent exocytosis. Science. 2006;313:676–680. doi: 10.1126/science.1129450. [DOI] [PubMed] [Google Scholar]
- Giraudo CG, Garcia-Diaz A, Eng WS, Chen Y, Hendrickson WA, Melia TJ, Rothman JE. Alternative zippering as an on-off switch for SNARE-mediated fusion. Science. 2009;323:512–516. doi: 10.1126/science.1166500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracheva EO, Hadwiger G, Nonet ML, Richmond JE. Direct interactions between C elegans RAB-3 and Rim provide a mechanism to target vesicles to the presynaptic density. Neurosci Lett. 2008;444:137–142. doi: 10.1016/j.neulet.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen AJ, tens S, Diez Arazola R, Cornelisse LN, Lozovaya N, de Jong AP, Goriounova NA, Habets RL, Takai Y, Borst JG, et al. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science. 2010;327:1614–1618. doi: 10.1126/science.1183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote E, Carr CM, Novick PJ. Ordering the final events in yeast exocytosis. J Cell Biol. 2000;151:439–452. doi: 10.1083/jcb.151.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson N, Lao Y, Maximov A, Chuang JC, Kostromina E, Repa JJ, Li C, Radda GK, Südhof TC, Han W. Impaired insulin secretion & glucose intolerance in synaptotagmin-7 null mutant mice. Proc Natl Acad Sci U S A. 2008;105:3992–3997. doi: 10.1073/pnas.0711700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson N, Wei SH, Hoang DN, Lao Y, Zhang Q, Radda GK, Rorsman P, Südhof TC, Han W. Synaptotagmin-7 is a principal Ca2+ sensor for Ca2+-induced glucagon exocytosis in pancreas. J Physiol. 2009;587:1169–1178. doi: 10.1113/jphysiol.2008.168005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman RE, Schwarz YN, Rettig J, Bruns D. SNARE force synchronizes synaptic vesicle fusion & controls the kinetics of quantal synaptic transmission. J Neurosci. 2010;30:10272–10281. doi: 10.1523/JNEUROSCI.1551-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Kaeser PS, Südhof TC, Schneggenburger R. RIM determines Ca2+-channel density & vesicle docking at the presynaptic active zone. Neuron. 2011;69:304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE. Structure and conformational changes in NSF & its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- Hata Y, Slaughter CA, Südhof TC. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 1993;366:347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- Hobson RJ, Liu Q, Watanabe S, Jorgensen EM. Complexin maintains vesicles in the primed state in C elegans. Curr Biol. 2011;21:106–113. doi: 10.1016/j.cub.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Scheller RH. Three SNARE complexes cooperate to mediate membrane fusion. Proc Natl Acad Sci U S A. 2001;98:8065–8070. doi: 10.1073/pnas.131214798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntwork S, Littleton JT. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat Neurosci. 2007;10:1235–1237. doi: 10.1038/nn1980. [DOI] [PubMed] [Google Scholar]
- Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490:201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorquera RA, Huntwork-Rodriguez S, Akbergenova Y, Cho RW, Littleton JT. Complexin controls spontaneous & evoked neurotransmitter release by regulating the timing & properties of synaptotagmin activity. J Neurosci. 2012;32:18234–18245. doi: 10.1523/JNEUROSCI.3212-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Schekman R, Thorner J. Glycosylation and processing of prepro-alpha-factor through the yeast secretory pathway. Cell. 1984;36:309–318. doi: 10.1016/0092-8674(84)90224-1. [DOI] [PubMed] [Google Scholar]
- Junge HJ, Rhee JS, Jahn O, Varoqueaux F, Spiess J, Waxham MN, Rosenmund C, Brose N. Calmodulin and Munc13 form a Ca2+-sensor/effector complex that controls short-term synaptic plasticity. Cell. 2004;118:389–401. doi: 10.1016/j.cell.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Südhof TC. RIM proteins tether Ca2+-channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Fan M, Südhof TC. RIM Genes Differentially Contribute to Organizing Presynaptic Release Sites. Proc Natl Acad Sci USA. 2012;109:11830–11835. doi: 10.1073/pnas.1209318109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser-Woo YJ, Yang X, Südhof TC. C-terminal Complexin Sequence is Selectively Required for Clamping and Priming but Not for Ca2+-Triggering of Synaptic Exocytosis. J Neurosci. 2012;32:2877–2885. doi: 10.1523/JNEUROSCI.3360-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karson MA, Tang AH, Milner TA, Alger BE. Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J Neurosci. 2009;29:4140–4154. doi: 10.1523/JNEUROSCI.5264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The Release of Neural Transmitter Substances. Liverpool Univ Press; Liverpool: 1969. [Google Scholar]
- Kesavan J, Borisovska M, Bruns D. v-SNARE actions during Ca2+-triggered exocytosis. Cell. 2007;131:351–363. doi: 10.1016/j.cell.2007.09.025. [DOI] [PubMed] [Google Scholar]
- Khvotchev M, Dulubova I, Sun J, Dai H, Rizo J, Südhof TC. Dual Modes of Munc18-1/SNARE Interactions Are Coupled by Functionally Critical Binding to syntaxin-1 N-terminus. J Neurosci. 2007;27:12147–12155. doi: 10.1523/JNEUROSCI.3655-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochubey O, Lou X, Schneggenburger R. Regulation of transmitter release by Ca2+ and synaptotagmin: insights from a large CNS synapse. Trends Neurosci. 2011;34:237–246. doi: 10.1016/j.tins.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Kochubey O, Schneggenburger R. Synaptotagmin increases the dynamic range of synapses by driving Ca2+-evoked release & by clamping a near-linear remaining Ca2+ sensor. Neuron. 2011;69:736–748. doi: 10.1016/j.neuron.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Kohout SC, Corbalán-García S, Torrecillas A, Goméz-Fernandéz JC, Falke JJ. C2 domains of protein kinase C isoforms alpha, beta, and gamma: activation parameters and calcium stoichiometries of the membrane-bound state. Biochemistry. 2002;41:11411–11424. doi: 10.1021/bi026041k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümmel D, Krishnakumar SS, Radoff DT, Li F, Giraudo CG, Pincet F, Rothman JE, Reinisch KM. Complexin cross-links prefusion SNAREs into a zigzag array. Nat Struct Mol Biol. 2011;18:927–933. doi: 10.1038/nsmb.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Guan Z, Akbergenova Y, Littleton JT. Genetic analysis of synaptotagmin C2 domain specificity in regulating spontaneous and evoked neurotransmitter release. J Neurosci. 2013;33:187–200. doi: 10.1523/JNEUROSCI.3214-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ullrich B, Zhang ZZ, Anderson RGW, Brose N, Südhof TC. Ca2+-dependent and Ca2+-independent activities of neural & nonneural synaptotagmins. Nature. 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- Li J, Xiao Y, Zhou W, Wu Z, Zhang R, Xu T. Silence of Synaptotagmin VII inhibits release of dense core vesicles in PC12 cells. Sci China C Life Sci. 2009;52:1156–1163. doi: 10.1007/s11427-009-0160-y. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Stern M, Schulze K, Perin M, Bellen HJ. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca2+-activated neurotransmitter release. Cell. 1993;74:1125–1134. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- Liu KS, Siebert M, Mertel S, Knoche E, Wegener S, Wichmann C, Matkovic T, Muhammad K, Depner H, Mettke C, Bückers J, Hell SW, Müller M, Davis GW, Schmitz D, Sigrist SJ. RIM-binding protein a central part of the active zone is essential for neurotransmitter release. Science. 2011;334:1565–1569. doi: 10.1126/science.1212991. [DOI] [PubMed] [Google Scholar]
- Lu J, Machius M, Dulubova I, Dai H, Südhof TC, Tomchick D, Rizo J. Structural Basis for a Munc13-1 Homodimer - Munc13-1/RIM Heterodimer Switch: C2-domains as Versatile Protein-Protein Interaction Modules. PLOS Biology. 2006;4:e192. doi: 10.1371/journal.pbio.0040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Su L, Seven AB, Xu Y, Rizo J. Reconstitution of the vital functions of Munc18 & Munc13 in neurotransmitter release. Science. 2013;339:421–425. doi: 10.1126/science.1230473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackler JM, Reist NE. Mutations in the second C2 domain of synaptotagmin disrupt synaptic transmission at Drosophila neuromuscular junctions. J Comp Neurol. 2001;436:4–16. [PubMed] [Google Scholar]
- Mackler JM, Drummond JA, Loewen CA, Robinson IM, Reist NE. The C2B Ca2+-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature. 2002;418:340–344. doi: 10.1038/nature00846. [DOI] [PubMed] [Google Scholar]
- Marsden HR, Tomatsu I, Kros A. Model systems for membrane fusion. Chem Soc Rev. 2011;40:1572–1585. doi: 10.1039/c0cs00115e. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hu Z, Fenz KM, Fernandez J, Dittman JS. Complexin has opposite effects on two modes of synaptic vesicle fusion. Curr Biol. 2011;21:97–105. doi: 10.1016/j.cub.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov A, Südhof TC. Autonomous Function of Synaptotagmin 1 in Triggering Synchronous Release Independent of Asynchronous Release. Neuron. 2005;48:547–554. doi: 10.1016/j.neuron.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Maximov A, Tang J, Yang X, Pang ZP, Südhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–521. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Missler M, Li C, Südhof TC. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell. 1995;83:111–119. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- Melicoff E, Sansores-Garcia L, Gomez A, Moreira DC, Datta P, Thakur P, Petrova Y, Siddiqi T, Murthy JN, Dickey BF, Heidelberger R, Adachi R. Synaptotagmin-2 controls regulated exocytosis but not other secretory responses of mast cells. J Biol Chem. 2009;284:19445–19451. doi: 10.1074/jbc.M109.002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- Mohrmann R, Sørensen JB. SNARE requirements en route to exocytosis: from many to few. J Mol Neurosci. 2012;48:387–394. doi: 10.1007/s12031-012-9744-2. [DOI] [PubMed] [Google Scholar]
- Mohrmann R, de Wit H, Verhage M, Neher E, Sørensen JB. Fast vesicle fusion in living cells requires at least three SNARE complexes. Science. 2010;330:502–505. doi: 10.1126/science.1193134. [DOI] [PubMed] [Google Scholar]
- Neher E, Penner R. Mice sans synaptotagmin. Nature. 1994;372:316–317. doi: 10.1038/372316a0. [DOI] [PubMed] [Google Scholar]
- Nishiki T, Augustine GJ. Dual roles of the C2B domain of synaptotagmin I in synchronizing Ca2+-dependent neurotransmitter release. J Neurosci. 2004;24:8542–8550. doi: 10.1523/JNEUROSCI.2545-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet ML, Grundahl K, Meyer BJ, Rand JB. Synaptic function is impaired but not eliminated in C elegans mutants lacking synaptotagmin. Cell. 1993;73:1291–1305. doi: 10.1016/0092-8674(93)90357-v. [DOI] [PubMed] [Google Scholar]
- Palay SL, Palade GE. The fine structure of neurons. J Biophys Biochem Cytol. 1955;1:69–88. doi: 10.1083/jcb.1.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Shin O-H, Meyer AC, Rosenmund C, Südhof TC. A gain-of-function mutation in synaptotagmin-1 reveals a critical role of Ca2+-dependent SNARE-complex binding in synaptic exocytosis. J Neurosci. 2006;26:12556–12565. doi: 10.1523/JNEUROSCI.3804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Melicoff E, Padgett D, Liu Y, Teich AF, Dickey BF, Lin W, Adachi R, Südhof TC. Synaptotagmin-2 is Essential for Survival and Contributes to Ca2+-Triggering of Neurotransmitter Release in Central & Neuromuscular Synapses. J Neurosci. 2007;26:13493–13504. doi: 10.1523/JNEUROSCI.3519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Xu W, Cao P, Südhof TC. Calmodulin Controls Synaptic Strength via Presynaptic Activation of CaM Kinase II. J Neurosci. 2010;30:4132–4142. doi: 10.1523/JNEUROSCI.3129-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Bacaj T, Yang X, Zhou P, Xu W, Südhof TC. Doc2 supports spontaneous synaptic transmission by a Ca2+-independent mechanism. Neuron. 2011;70:244–251. doi: 10.1016/j.neuron.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin MS, Fried VA, Mignery GA, Jahn R, Südhof TC. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990;345:260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- Perin MS, Johnston PA, Özcelik T, Jahn R, Francke U, Südhof TC. Structural & functional conservation of synaptotagmin (p65) in Drosophila & humans. J Biol Chem. 1991;266:615–622. [PubMed] [Google Scholar]
- Pertsinidis A, Mukherjeec K, Sharma M, Pang ZP, Park SR, Zhang Y, Brungerc AT, Südhof TC, Chu S. Ultrahigh-resolution imaging reveals formation of neuronal SNARE/Munc18 complexes in situ. PNAS. 2013;110:E2812–E2820. doi: 10.1073/pnas.1310654110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore SS, Bend EG, Yu H, Hammarlund M, Jorgensen EM, Shen J. Syntaxin N-terminal peptide motif is an initiation factor for the assembly of the SNARE-Sec1/Munc18 membrane fusion complex. Proc Natl Acad Sci U S A. 2010;107:22399–22406. doi: 10.1073/pnas.1012997108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reim K, et al. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Rhee S-R, Betz A, Pyott S, Reim K, Varoqueaux F, Augustin I, Hesse D, Südhof TC, Takahashi M, Rosenmund C, Brose N. Phorbol ester- and diacylglycerol-induced augmentation of neurotransmitter release from hippocampal neurons is mediated by Munc13s and not by PKCs. Cell. 2002;108:121–133. doi: 10.1016/s0092-8674(01)00635-3. [DOI] [PubMed] [Google Scholar]
- Rhee J-S, Li L, Shin O-H, Rah J-C, Rizo J, Südhof TC, Rosenmund C. Augmenting neuro-transmitter release by enhancing the apparent Ca2+-affinity of synaptotagmin 1. Proc Natl Acad Sci USA. 2005;102:18664–18669. doi: 10.1073/pnas.0509153102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J, Chen X, Araç D. Unraveling the mechanisms of synaptotagmin & SNARE function in neurotransmitter release. Trends Cell Biol. 2006;16:339–350. doi: 10.1016/j.tcb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat Struct Mol Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Timing of neurotransmission at fast synapses in the mammalian brain. Nature. 1996;384:170–172. doi: 10.1038/384170a0. [DOI] [PubMed] [Google Scholar]
- Saitsu H, et al. De novo mutations in the gene encoding STXBP1 (MUNC18-1) cause early infantile epileptic encephalopathy. Nat Genet. 2008;40:782–788. doi: 10.1038/ng.150. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Stein A, Jahn R, Neher E. Distinct kinetic changes in neurotransmitter release after SNARE protein cleavage. Science. 2005;309:491–494. doi: 10.1126/science.1112645. [DOI] [PubMed] [Google Scholar]
- Schollmeier Y, Krause JM, Kreye S, Malsam J, Söllner TH. Resolving the function of distinct Munc18-1/SNARE protein interaction modes in a reconstituted membrane fusion assay. J Biol Chem. 2011;286:30582–30590. doi: 10.1074/jbc.M111.269886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonn JS, Maximov A, Lao Y, Südhof TC, Sorensen JB. Synaptotagmin-1 and -7 are functionally overlapping Ca2+ sensors for exocytosis in adrenal chromaffin cells. Proc Natl Acad Sci U S A. 2008;105:3998–4003. doi: 10.1073/pnas.0712373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia M, Ales E, Montes MA, Bonifas I, Jemal I, Lindau M, Maximov A, Südhof TC, Alvarez de Toledo G. Push-&-pull regulation of the fusion pore by synaptotagmin-7. Proc Natl Acad Sci U S A. 2010;107:19032–19037. doi: 10.1073/pnas.1014070107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Burré J, Südhof TC. CSPα Promotes SNARE-Complex Assembly by Chaperoning SNAP-25 during Synaptic Activity. Nature Cell Biol. 2011;13:30–39. doi: 10.1038/ncb2131. [DOI] [PubMed] [Google Scholar]
- Sharma M, Burré J, Bronk P, Zhang Y, Xu W, Südhof TC. CSPα Knockout Causes Neurodegeneration by Impairing SNAP-25 Function. EMBO J. 2011b;31:829–841. doi: 10.1038/emboj.2011.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Shi L, Shen QT, Kiel A, Wang J, Wang HW, Melia TJ, Rothman JE, Pincet F. SNARE proteins: one to fuse & three to keep the nascent fusion pore open. Science. 2012;335:1355–1359. doi: 10.1126/science.1214984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin OH, Rizo J, Südhof TC. Synaptotagmin function in dense core vesicle exocytosis studied in cracked PC12 cells. Nat Neurosci. 2002;5:649–656. doi: 10.1038/nn869. [DOI] [PubMed] [Google Scholar]
- Shin OH, Xu J, Rizo J, Südhof TC. Differential but convergent functions of Ca2+-binding to synaptotagmin-1 C2-domains mediate neurotransmitter release. Proc Natl Acad Sci USA. 2009;106:16469–16474. doi: 10.1073/pnas.0908798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin OH, Lu J, Rhee J-S, Tomchick DR, Pang ZP, Wojcik S, Camacho-Perez M, Brose N, Machius M, Rizo J, Rosenmund C, Südhof TC. Munc13 C2B-domain – an activity-dependent Ca2+-regulator of synaptic exocytosis. Nature Struct Mol Biol. 2010;17:280–288. doi: 10.1038/nsmb.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Ahmed S, Jahn R, Klingauf J. Two synaptobrevin molecules are sufficient for vesicle fusion in central nervous system synapses. Proc Natl Acad Sci USA. 2011;108:14318–14323. doi: 10.1073/pnas.1101818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993a;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking activation and fusion. Cell. 1993b;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Sørensen JB, Matti U, Wei SH, Nehring RB, Voets T, Ashery U, Binz T, Neher E, Rettig J. The SNARE protein SNAP-25 is linked to fast calcium triggering of exocytosis. Proc Natl Acad Sci U S A. 2002;99:1627–1632. doi: 10.1073/pnas.251673298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen JB, Fernández-Chacón R, Südhof TC, Neher E. Examining synaptotagmin 1 function in dense core vesicle exocytosis under direct control of Ca2+ J Gen Physiol. 2003;122:265–276. doi: 10.1085/jgp.200308855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. Neuroligins and Neurexins Link Synaptic Function to Cognitive Disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. A molecular machine for neurotransmitter release: Synaptotagmin and beyond. Nature Medicine. 2013 doi: 10.1038/nm.3338. in press. [DOI] [PubMed] [Google Scholar]
- Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE & SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Han W, Butz S, Liu X, Fernandez-Chacon R, Lao Y, Südhof TC. Synaptotagmin VII as a plasma membrane Ca2+ sensor in exocytosis. Neuron. 2001;30:459–473. doi: 10.1016/s0896-6273(01)00290-2. [DOI] [PubMed] [Google Scholar]
- Sugita S, Shin OH, Han W, Lao Y, Südhof TC. Synaptotagmins form hierarchy of exocytotic Ca2+-sensors with distinct Ca2+-affinities. EMBO J. 2002;21:270–280. doi: 10.1093/emboj/21.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Südhof TC. A Dual Ca2+-Sensor Model for Neuro-transmitter Release in a Central Synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton AB, Davletov BA, Berghuis AM, Südhof TC, Sprang SR. Structure of the first C2-domain of synaptotagmin I: A novel Ca2+/phospholipid binding fold. Cell. 1995;80:929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Tang J, et al. A complexin/ synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Tsuboi T, Fukuda M. Synaptotagmin VII modulates the kinetics of dense-core vesicle exocytosis in PC12 cells. Genes Cells. 2007;12:511–519. doi: 10.1111/j.1365-2443.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- van den Bogaart G, Holt MG, Bunt G, Riedel D, Wouters FS, Jahn R. One SNARE complex is sufficient for membrane fusion. Nat Struct Mol Biol. 2010;17:358–364. doi: 10.1038/nsmb.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Okamoto M, Schmitz F, Hofmann K, Südhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sugita S, Südhof TC. The RIM/NIM family of neuronal C2 domain proteins Interactions with Rab3 & a new class of Src homology 3 domain proteins. J Biol Chem. 2000;275:20033–20044. doi: 10.1074/jbc.M909008199. [DOI] [PubMed] [Google Scholar]
- Wu MN, Schulze KL, Lloyd TE, Bellen HJ. The ROP-syntaxin interaction inhibits neurotransmitter release. Eur J Cell Biol. 2001;80:196–199. doi: 10.1078/0171-9335-00143. [DOI] [PubMed] [Google Scholar]
- Xu T, Binz T, Niemann H, Neher E. Multiple kinetic components of exocytosis distinguished by neurotoxin sensitivity. Nat Neurosci. 1998;1:192–200. doi: 10.1038/642. [DOI] [PubMed] [Google Scholar]
- Xu J, Mashimo T, Südhof TC. Synaptotagmin-1 -2 & -9: Ca2+-sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Xu J, Pang ZP, Shin OH, Südhof TC. Synaptotagmin-1 functions as a Ca2+ sensor for spontaneous release. Nature Neurosci. 2009;12:759–766. doi: 10.1038/nn.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Morishita W, Buckmaster PS, Pang ZP, Malenka RC, Südhof TC. Distinct Neuronal Coding Schemes in Memory Revealed by Selective Erasure of Fast Synchronous Synaptic Transmission. Neuron. 2012;73:990–1001. doi: 10.1016/j.neuron.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Brewer KD, Perez-Castillejos R, Rizo J. Subtle Interplay between Synaptotagmin & Complexin Binding to the SNARE Complex. J Mol Biol. 2013;425:3461–3475. doi: 10.1016/j.jmb.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, et al. Distinct domains of complexin I differentially regulate neurotransmitter release. Nat Struct Mol Biol. 2007;14:949–958. doi: 10.1038/nsmb1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Stradomska A, Chen H, Brose N, Zhang W, Rosenmund C, Reim K. Complexins facilitate neurotransmitter release at excitatory & inhibitory synapses in mammalian central nervous system. Proc Natl Acad Sci U S A. 2008;105:7875–7880. doi: 10.1073/pnas.0803012105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Dulubova I, Min SW, Chen X, Rizo J, Südhof TC. Sly1 binds to Golgi and ER syntaxins via a conserved N-terminal peptide motif. Developmental Cell. 2002;2:295–305. doi: 10.1016/s1534-5807(02)00125-9. [DOI] [PubMed] [Google Scholar]
- Yang B, Steegmaier M, Gonzalez LC, Jr, Scheller RH. nSec1 binds a closed conformation of syntaxin1A. J Cell Biol. 2000;148:247–252. doi: 10.1083/jcb.148.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Kaeser Woo YJ, Pang ZP, Xu W, Südhof TC. Complexin Clamps Asynchronous Release by Blocking a Secondary Ca2+-Sensor via its Accessory α-Helix. Neuron. 2010;68:907–920. doi: 10.1016/j.neuron.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara M, Littleton JT. Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron. 2002;36:897–908. doi: 10.1016/s0896-6273(02)01065-6. [DOI] [PubMed] [Google Scholar]
- Yu H, Rathore SS, Lopez JA, Davis EM, James DE, Shen J. Comparative studies of Munc18c & Munc18-1 reveal conserved & divergent mechanisms of Sec1/Munc18 proteins. Proc Natl Acad Sci U S A. 2013;110:E3271–3280. doi: 10.1073/pnas.1311232110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kim-Miller MJ, Fukuda M, Kowalchyk JA, Martin TF. Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron. 2002;34:599–611. doi: 10.1016/s0896-6273(02)00671-2. [DOI] [PubMed] [Google Scholar]
- Zhou P, Pang ZP, Yang X, Zhang Y, Rosenmund C, Bacaj T, Südhof TC. Syntaxin-1 N-Peptide and Habc-Domain Perform Distinct Essential Functions in Synaptic Vesicle Fusion. EMBO J. 2013a;32:159–171. doi: 10.1038/emboj.2012.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Bacaj T, Yang X, Pang ZP, Südhof TC. Lipid-Anchored SNARE Lacking Transmembrane Regions Support Membrane Fusion During Neurotransmitter Release. Neuron. 2013b doi: 10.1016/j.neuron.2013.09.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]