Dear Editor,
Locally advanced melanoma is clinically challenging; melanomas are considered radioresistant (Harwood & Cummings, 1981). Recent reports, however, show variable radiosensitivity in patients and established cell lines (Stevens & McKay, 2006) (Sambade et al, 2011). Our purpose was to confirm this in early passage, patient-derived melanoma cultures and study radiosensitizing potential of commonly used melanoma drugs: temozolomide, carboplatin, paclitaxel and vemurafenib.
We studied 14 cultures with/without BRAF/NRAS mutations and PTEN loss. Seven efficiently formed colonies in vitro. We determined that the less cumbersome, luminescence-based CellTiter-Glo assay (Promega) was a good surrogate for colony forming assays. Radiation inhibitory doses 50 (ID50) from colony-forming and CellTiter-Glo assays correlated well (R=0.8) (sFigure 1). ID50s by CellTiter-Glo were established using ascending doses (2Gy, 5Gy, 8Gy), representing common conventional fractionation and hypofractionation doses (Table 1). We detected a range of radiation sensitivities (Figure 1); YUROL, YUTICA and YUMUT were 45-60% viable with 8Gy; growth was <20% in YUSIT1, YUGASP, and YUSAC cells. YUROL and YUSAC differed in radiosensitivity by >7-fold at 8Gy. All were relatively radioresistant at low ionizing radiation (IR) doses. The surviving fraction at 2Gy (SF2) is a common measure of intrinsic radiosensitivity; SF2≥0.5 is considered radioresistant (Fertil & Malaise, 1981). Our SF2 range was 0.52-0.88, mean-0.73±0.088. Although the number of cultures is small, when dichotomizing ID50s by their median, PTEN loss was associated with high ID50s (χ2=5, P=0.026). No association was found between BRAF/NRAS mutations and ID50.
Table 1.
Radiation inhibitory doses 50 (ID50) and inhibitory concentrations 50 (IC50) for cytotoxic drugs used to treat melanoma in clinic.
|
Cell
culture |
BRAF | NRAS | PTEN | irradiation | TMZ | Carbo | Paclitax | vemurafenib |
|---|---|---|---|---|---|---|---|---|
| Mutation | mutation | expression | ID50 (Gy) | IC50 (μM) |
IC50 (μM) |
IC50 (nM) |
IC50 (nM) | |
| YUCOT | V600E | WT | + | 5.4 | NR* | 26.7 | 4.3 | 94.0 |
| YUGANK | WT | Q61K | + | 5.0 | NR | 57.6 | 4.3 | NT** |
| YUGASP | WT | Q61L | + | 3.2 | 100 | 12.7 | 4.1 | NT |
| YUHOIN | WT | WT | + | 2.2 | NR | 33.1 | 4.0 | NT |
| YUKIM | WT | Q61R/WT | + | 3.6 | NR | 3.1 | 1.0 | NT |
| YUKOLI | V600E/WT | WT | + | 4.2 | NR | 14.4 | 2.9 | 138.0 |
| YUMAC | V600K | WT | + | 4.3 | 70 | 4.6 | 2.4 | 18.0 |
| YUMUL | WT | WT | − | 5.8 | NR | 11.3 | 2.5 | NT |
| YUMUT | V600E/WT | WT | − | 8.0 | NR | 46.1 | 1.3 | 160.0 |
| YURIF | V600K | WT | + | 4.8 | NR | 5.0 | 3.8 | 132.0 |
| YUROL | WT | WT | − | NR>8 | NR | 27.9 | 61.7 | NT |
| YUSAC | V600E | WT | + | 2.7 | NR | 7.0 | 4.9 | 22.0 |
| YUSIT1 | V600K/WT | WT | + | 3.7 | 15 | 6.0 | 4.1 | 21.0 |
| YUTICA | WT | Q61R/WT | + | 7.0 | NR | 10.3 | 4.2 | NT |
NR-Not reached, NT-Not tested, temozolomide is denoted by TMZ, carboplatin by Carbo, paclitaxel by Paclitax,
Figure 1.
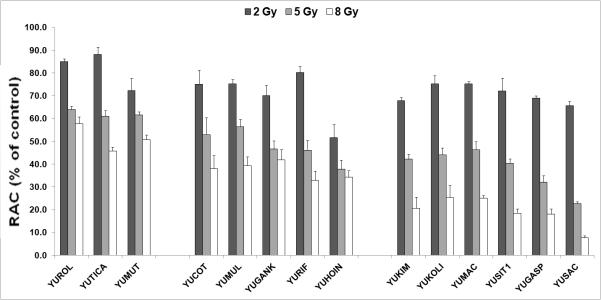
Sensitivity of primary melanoma cultures to increasing doses of x-ray irradiation. The Y axis depicts the relative ATP content (RAC).
For radiation sensitization, we determined inhibitory concentration 50 (IC50) for individual drugs (Table 1). Cells were treated with concentrations at or below the average IC50 and irradiated 4 hours later. Combination indices (CIs) were calculated using CalcuSyn software, incorporating the Chou and Talalay method; CI <0.9 is synergistic, 0.9-1.1 additive and >1.1 antagonistic.
All cultures but one were resistant to temozolomide. We focused our analysis on 2Gy and 5Gy, conventional irradiation doses. Temozolamide at 100μM was synergistic with 2Gy in six cultures and with 5Gy in four, although growth remained >50% in many (sTable 1, Figure 2A). Temozolomide induces guanine methylation, repaired by O6-methylguanine-DNA methyltransferase, which can be hypermethylated in melanoma (Carlson et al, 2009). It is unclear that this synergizes with IR-induced double-strand breaks (DSBs) (Wedge et al, 1997).
Figure 2.
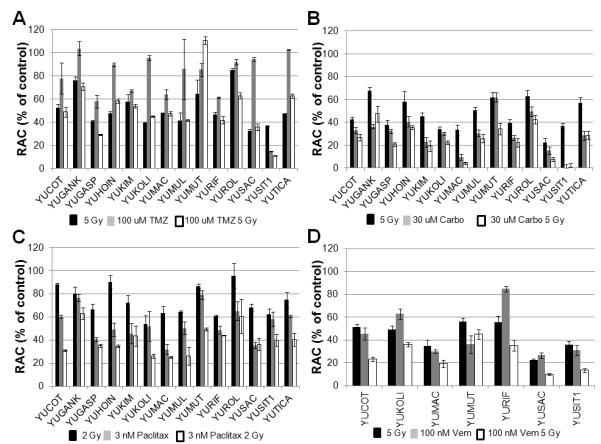
Combined effects of cytotoxic drugs and x-ray irradiation. The more effective combinations are shown for the four drugs studied; temozolomide (A), carboplatin (B), paclitaxel (C) and vemurafenib (D). Standard errors were calculated based on three independent experiments, each done in triplicate. The Y axis depicts the relative ATP content (RAC).
IC50s for carboplatin were 4.6-57.6μM. There was some synergism between 2Gy and 3μM carboplatin in two cultures, but not with 30μM. Synergistic effects were more abundant in carboplatin-treated cultures (30μM) with 5Gy; seven of 14 showed synergism and five additivity, with <50% growth (sTable 2, Figure 2B). Although cultures are fairly sensitive to 30μM carboplatin, adding radiation clearly enhances growth inhibition. Carboplatin creates inter- and intra-strand adducts, synergizes with IR-induced DNA damage to create DSBs (Yang et al, 1995). Additional mechanisms include non-homologus end-joining repair inhibition, hypoxia, and cell-cycle dysregulation.
IC50s for paclitaxel were 1-62nM (3 day exposure). 3nM and 2Gy were synergistic in eight cultures, additive in five (sTable 3, Figure 2C). 10nM was synergistic with 5Gy in two cultures, suggesting that paclitaxel radiosensitizes primarily at lower radiation doses. Microtubule stabilizing agents block cells in the radiosensitive G2/M cell division phase (Liebmann et al, 1994).
IC50s for vemurafenib in mutated BRAF cultures were 18-160nM, lower than in repeatedly passaged, older cultures used by Sambade et al, but similar to those in our early passage cultures in prior studies (Halaban et al, 2010; Sambade et al, 2011). 100nM vemurafanib was synergistic with 5Gy in six cultures (sTable 4, Fig 2D), confirming other studies (Sambade et al, 2011).
Our data demonstrate that radiation sensitivity is variable in short term melanoma cultures, which may be better surrogates for in vivo response patterns than established cultures, many being radio-sensitive, particularly at higher doses. This supports previous reports that melanomas have low alpha-beta ratios, predicting enhanced sensitivity to hypofractionation (Overgaard et al, 1986). PTEN loss might be associated with radioresistance, as demonstrated in gliomas (Inaba et al, 2011). Carboplatin and paclitaxel, used as radiosensitzers in other diseases (Groen et al, 2004), and vemurafenib appear to be more effective than temozolomide. The greatest growth inhibition was seen with higher carboplatin concentrations and 5Gy; this combination might be appropriate in anatomic sites that tolerate higher doses of radiation. Paclitaxel and carboplatin can be administered in single, high doses, and pre-treating patients with one or both prior to irradiation might improve local tumor control. In vivo studies are needed to assess toxicity and optimal dosing schedules of newer drugs such as vemurafenib, when used together with irradiation. Studies are underway to identify additional biomarkers (other than PTEN loss) that predict radiosensitivity in melanomas.
Supplementary Material
Acknowledgements
This publication was made possible by the CTSA grant ULI RR024139 from the National Center for Research Resources (NCCR) and the National Institutes of Health (NIH) roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCCR or NIH. The work was also supported by Yale Cancer Center Translational Targeted Area of Research Excellence Funds (to HMK) and by the Yale SPORE in Skin Cancer funded by the National Cancer Institute grant number 1 P50 CA121974 (R. Halaban, PI).
REFERENCES
- Carlson BL, Grogan PT, Mladek AC, Schroeder MA, Kitange GJ, Decker PA, Giannini C, Wu W, Ballman KA, James CD, Sarkaria JN. Radiosensitizing effects of temozolomide observed in vivo only in a subset of O6-methylguanine-DNA methyltransferase methylated glioblastoma multiforme xenografts. Int J Radiat Oncol Biol Phys. 2009;75(1):212–219. doi: 10.1016/j.ijrobp.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertil B, Malaise EP. Inherent cellular radiosensitivity as a basic concept for human tumor radiotherapy. Int J Radiat Oncol Biol Phys. 1981;7(5):621–629. doi: 10.1016/0360-3016(81)90377-1. [DOI] [PubMed] [Google Scholar]
- Groen HJ, van der Leest AH, Fokkema E, Timmer PR, Nossent GD, Smit WJ, Nabers J, Hoekstra HJ, Hermans J, Otter R, van Putten JW, de Vries EG, Mulder NH. Continuously infused carboplatin used as radiosensitizer in locally unresectable non-small-cell lung cancer: a multicenter phase III study. Ann Oncol. 2004;15(3):427–432. doi: 10.1093/annonc/mdh100. [DOI] [PubMed] [Google Scholar]
- Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S, Krauthammer M, McCusker JP, Kluger Y, Sznol M. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010;23(2):190–200. doi: 10.1111/j.1755-148X.2010.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood AR, Cummings BJ. Radiotherapy for malignant melanoma: a re-appraisal. Cancer Treat Rev. 1981;8(4):271–282. doi: 10.1016/s0305-7372(81)80011-4. [DOI] [PubMed] [Google Scholar]
- Inaba N, Kimura M, Fujioka K, Ikeda K, Somura H, Akiyoshi K, Inoue Y, Nomura M, Saito Y, Saito H, Manome Y. The effect of PTEN on proliferation and drug-, and radiosensitivity in malignant glioma cells. Anticancer Res. 2011;31(5):1653–1658. [PubMed] [Google Scholar]
- Liebmann J, Cook JA, Fisher J, Teague D, Mitchell JB. In vitro studies of Taxol as a radiation sensitizer in human tumor cells. Journal of the National Cancer Institute. 1994;86(6):441–446. doi: 10.1093/jnci/86.6.441. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Overgaard M, Hansen PV, von der Maase H. Some factors of importance in the radiation treatment of malignant melanoma. Radiother Oncol. 1986;5(3):183–192. doi: 10.1016/s0167-8140(86)80048-2. [DOI] [PubMed] [Google Scholar]
- Sambade MJ, Peters EC, Thomas NE, Kaufmann WK, Kimple RJ, Shields JM. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98(3):394–399. doi: 10.1016/j.radonc.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens G, McKay MJ. Dispelling the myths surrounding radiotherapy for treatment of cutaneous melanoma. Lancet Oncol. 2006;7(7):575–583. doi: 10.1016/S1470-2045(06)70758-6. [DOI] [PubMed] [Google Scholar]
- Wedge SR, Porteous JK, Glaser MG, Marcus K, Newlands ES. In vitro evaluation of temozolomide combined with X-irradiation. Anticancer Drugs. 1997;8(1):92–97. doi: 10.1097/00001813-199701000-00013. [DOI] [PubMed] [Google Scholar]
- Yang LX, Douple EB, O’Hara JA, Wang HJ. Production of DNA double-strand breaks by interactions between carboplatin and radiation: a potential mechanism for radiopotentiation. Radiat Res. 1995;143(3):309–315. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


