Abstract
Objective
Connective tissue growth factor (CTGF) is a cysteine-rich secreted matricellular protein involved in wound healing and tissue repair. Enhanced and prolonged expression of CTGF has been associated with tissue fibrosis in humans. However, questions remain as to whether CTGF expression alone is sufficient to drive fibrosis. This study was undertaken to investigate whether CTGF alone is sufficient to cause fibrosis in intact animals and whether its effects are mediated through activation of transforming growth factor β (TGFβ) signaling or through distinct signal transduction pathways.
Methods
We generated mice overexpressing CTGF in fibroblasts under the control of the fibroblast-specific collagen α2(I) promoter enhancer. Tissues such as skin, lung, and kidney were harvested for histologic analysis. Mouse embryonic fibroblasts were prepared from embryos (14.5 days postcoitum) for biochemical analysis.
Results
Mice overexpressing CTGF in fibroblasts were susceptible to accelerated tissue fibrosis affecting the skin, lung, kidney, and vasculature, most notably the small arteries. We identified a marked expansion of the myofibroblast cell population in the dermis. RNA analysis of transgenic dermal fibroblasts revealed elevated expression of key matrix genes, consistent with a fibrogenic response. CTGF induced phosphorylation of p38, ERK-1/2, JNK, and Akt, but not Smad3, in transgenic mouse fibroblasts compared with wild-type mouse fibro-blasts. Transfection experiments showed significantly increased basal activity of the CTGF and serum response element promoters, and enhanced induction of the CTGF promoter in the presence of TGFβ.
Conclusion
These results demonstrate that selective expression of CTGF in fibroblasts alone causes tissue fibrosis in vivo through specific signaling pathways, integrating cues from the extracellular matrix into signal transduction pathways to orchestrate pivotal biologic responses relevant to tissue repair and fibrosis.
Connective tissue growth factor (CTGF) belongs to the CCN family of proteins, which share a similar modular structure (1–3). Depending on the cell type, CTGF has key biologic functions ranging from regulation of cell growth to tissue remodeling (4). Expression of CTGF is observed in the mesenchyme during embryonic development, and studies involving disruption of the CTGF gene indicate that it is required for the coordination of chondrogenesis (5) and angiogenesis during development of the skeleton, and also lung development (4). There is little or no detectable CTGF in normal adult skin tissue; however, during normal wound healing, CTGF expression is activated at the wound site and then switched off once the repair process is complete (6). CTGF levels are increased in fibrotic tissue, and its levels have been shown to correlate with disease severity, e.g., in scleroderma (7). The precise mechanism by which CTGF activates cellular changes and the triggers that elicit CTGF expression in fibrotic lesions are poorly understood.
Recently, activation of transforming growth factor β (TGFβ) signaling has been implicated as playing a major role in fibrosis (8). To directly assess the role of sustained TGFβ signaling, we have previously generated mice that specifically express genetically modified TGFβ receptors in fibroblasts, thus modulating TGFβ signaling (9,10). For example, expression of constitutively active TGFβ receptor I in fibroblasts postnatally recapitulated major clinical, biochemical, and histologic features of the fibrotic pathology in the skin and small blood vessels. Transgenic mouse fibroblasts displayed high levels of CTGF expression, suggesting that both TGFβ and CTGF may have an important role in a sustained chronic fibrotic outcome (9). A similar conclusion was reached from the analysis of different human fibroproliferative diseases. Interestingly, high levels of CTGF expression have been detected within fibrotic lesions in scleroderma patients even in the absence of elevated TGFβ levels (7).
The purpose of this study was to investigate whether CTGF alone causes fibrosis in intact animals and whether the effects of CTGF are mediated through activation of TGFβ signaling or through other signaling pathways. For this purpose, we generated mice in which overexpression of CTGF was targeted to fibroblasts. We report that these mice spontaneously developed a multiorgan fibrotic phenotype affecting skin, lung, and kidney and including vascular remodeling of small blood vessels. The resulting phenotype, at least in the skin and lung, had features of tissue fibrosis similar to those observed in scleroderma. Molecular and cell biology studies of isolated primary mesenchymal connective tissue cells revealed that forced expression of CTGF in fibro-blasts did not involve the canonical TGFβ pathway, but promoted the activation of several other signaling pathways and downstream transcriptional programs, resulting in disruption of connective tissue architecture that is replaced by increased extracellular matrix (ECM). These results suggest that CTGF and its downstream pathways could be targets for antifibrotic therapy.
MATERIALS AND METHODS
Generation of Col1a2-CTGF–transgenic mice
Transgenic mice were produced by the standard pronuclear injection of DNA into fertilized (C57BL/10 × CBA/J)F1 mouse eggs. Murine CTGF (Fisp-12) was cloned in the plasmid pCD3 containing the 6-kb enhancer of the Col1a2 gene (11). Integration of the transgenes was assessed by genotyping of mouse tail DNA with lacZ primers. All experiments performed with the mice were in compliance with the standards of care approved by the M. D. Anderson Cancer Center Institutional Animal Care and Use Committee.
Whole-mount LacZ staining
Mouse embryos at 15.5 days postcoitum were fixed, and whole mounts were stained in X-Gal staining solution. X-Gal–labeled embryos were embedded in paraffin, sectioned in several planes, and observed for LacZ staining. (Details of the procedure are described in the supplementary material, available in the online version of this article at http://www3.interscience.wiley.com/journal/76509746/home).
Genotyping of Col1a2-CTGF–transgenic mice and LacZ copy number
Genomic DNA was extracted, and real-time polymerase chain reaction was performed for lacZ genotyping. Details on genotyping and lacZ copy number are available in the supplementary material.
Histologic analysis
Histologic analysis was performed on 10 Col1a2-CTGF–transgenic mice and 10 wild-type (WT) littermates; representative findings are shown. Tissue samples were processed by standard procedures and embedded in paraffin (details of the procedure are included in the supplementary material, available at http://www3.interscience.wiley.com/journal/76509746/home). Sections were stained with hematoxylin and eosin and with Masson's trichrome. Type I collagen (1:100; Millipore) and bromodeoxyuridine (BrdU) antibodies (Santa Cruz Biotechnology) were used for immunohistochemical analysis. Slides were washed, and antibody staining was visualized with a peroxidase–3,3′-diaminobenzidine system (Vectastain ABC kit; Vector). Normal IgG (Sigma) was used as a control immunostain.
Immunofluorescence analysis of tissue sections was performed with the following antibodies: α-smooth muscle actin (α-SMA) (1:100; Sigma), type IV collagen (1:500; Chemicon), phosphorylated histone 3 (1:500; Upstate Biotechnology), CTGF, and CD31 (1:100; Santa Cruz Biotechnology). The secondary antibodies used were goat anti-mouse IgG conjugated to Alexa Fluor 488 or goat anti-rabbit IgG conjugated to Alexa Fluor 555 (Molecular Probes); nuclei were counterstained with Topro and sections were mounted in ProLong Gold (Molecular Probes). Image stacks were collected using a Zeiss LSM 510 confocal microscope.
Floating collagen gel cultures and quantitation of gel contraction
Gel contraction experiments were performed as described elsewhere (12). Approximately 80,000 cells/1.2 mg/ml of collagen were plated. After polymerization, the gel was detached from the wells by adding 1 ml of MCDB medium. Contraction of the gel was quantified based on loss of gel weight and decrease in gel diameter over a 24-hour period.
In vitro wound healing (“scratch”) assay
Scratch assay was performed using confluent cultured fibroblasts obtained from WT and Col1a2-CTGF–transgenic mice. Additional details are available in the supplementary material, at http://www3.interscience.wiley.com/journal/76509746/home.
Collagen quantitation
Collagen content was measured on picrosirius red–stained lung sections by digital image microscopy with polarized light (details available in the supplementary material). The percent positively stained area per slide (n = 4 per group) was determined.
Western blotting
Whole cell extracts were prepared, and Western blotting was performed as described earlier (9). The following antibodies were used for Western blotting: type I collagen (Chemicon), CTGF, α-SMA, GAPDH (Sigma-Aldrich), p-Smad3 (Abcam), β-actin, vinculin (Invitrogen), p38, anti–p-p38, TGFβ-activated kinase 1 (TAK-1), p–TAK-1, ERK-1/2, p–ERK-1/2, JNK, p-JNK, Akt, and p-Akt (all from Cell Signaling Technology).
Cell culture and transient transfections
Experiments were performed with mouse embryonic fibroblasts (MEFs) between passages 2 and 5 that were prepared from embryos (14.5 days postcoitum) from at least 6 different litters as described earlier (9). The CTGF intact promoter reporter and Smad deletion mutant vector were generated using an 800-bp construct in pGL3 (13). Promoter with 3 tandem repeats of serum response element binding sites (pSRE) reporter vector was obtained from Invitrogen. Cells were transfected with FuGene 6 according to the instructions of the manufacturer (Roche). Cells were treated with 2 ng/ml TGFβ the day after transfection. Reporter gene expression was determined using the luciferase reporter (Promega) or SEAP reporter (Sigma) assay system.
Statistical analysis
Mean ± SD values were determined. Statistical analysis was performed using Student's un-paired t-test. P values less than 0.05 were considered significant.
RESULTS
Generation of transgenic mice overexpressing CTGF specifically in fibroblasts
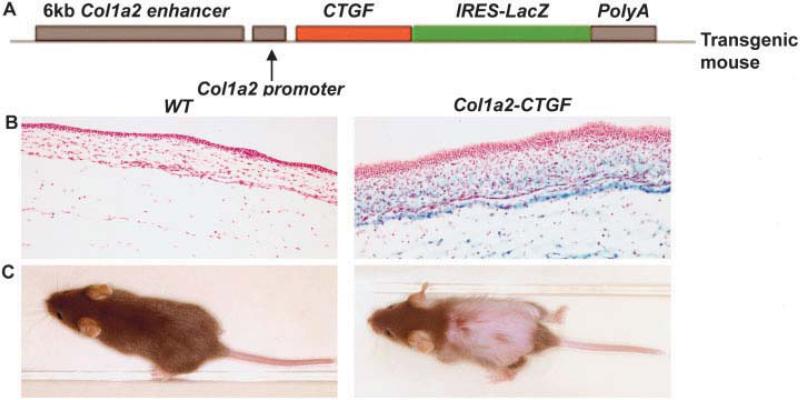
In transgenic mice, overexpression of the mouse homolog of the CTGF gene, Fisp-12, in fibroblasts was achieved using a fibroblast-specific 6-kb enhancer and 400-bp minimal promoter from the Col1a2 gene (11) (Figure 1A). All analyses were performed with mice homozygous for the CTGF transgene, designated Col1a2-CTGF–transgenic, and age- and sex-matched littermate controls. Extensive transgene expression was observed throughout the dermis of the embryos, as shown by expression of an IRES-lacZ cassette cloned downstream of the CTGF complementary DNA (Figure 1B). Around 3 weeks of age, Col1a2-CTGF–transgenic mice exhibited extensive hair loss (Figure 1C) and scaly patches affecting the skin in the lower dorsal region and in the upper ventral region, around the edges of the ear, and on the tail. Compared with WT littermates, adult Col1a2-CTGF–transgenic mice weighed 29.5% less and were 18.8% shorter in length, were not able to breed, and had a shortened lifespan, dying between ages 2 months and 6 months. Preliminary results indicated that 3 founder lines (155, 132, and L6) showed high levels of β-galactosidase in the dermis and had a similar phenotype; we used line 155 for further studies.
Figure 1.
Fibroblast-directed overexpression of the connective tissue growth factor (CTGF) gene. A, Schematic representation of the construct in which the coding sequence for the mouse homolog of CTGF, known as Fisp-12, is directed by a fibroblast-specific enhancer and a minimal promoter of the Col1a2 gene. A viral internal ribosome entry site (IRES) sequence–linked lacZ reporter gene and a poly(A) sequence downstream of the CTGF cDNA direct coexpression of this marker from a dicistronic mRNA for identification of transgene expression. B, X-Gal staining of embryos from a Col1a2-CTGF–transgenic mouse and littermate wild-type (WT) control at 15.5 days postcoitum. Intense blue staining of skin was evident only in Col1a2-CTGF–transgenic mouse embryos. C, Gross appearance of 3-week-old WT and Col1a2-CTGF–transgenic mice. The transgenic mouse exhibits severe hair loss from the middle to lower dorsal and upper ventral areas of the body.
Sustained fibroblast-specific overexpression of CTGF causes skin fibrosis in vivo
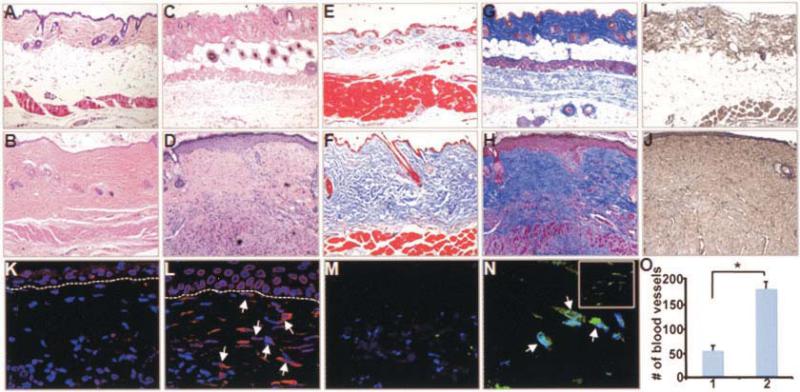
Dermal biopsy specimens from 4- and 8-week-old Col1a2-CTGF–transgenic mice and WT littermates were obtained. Histologic analysis revealed hallmarks of fibrotic skin, i.e., a dramatic thickening of the dermis that was progressive with age and focal hypertrophy of keratinocytes at the dermal–epidermal junction. The subcutaneous fat layer, visible in the WT mouse skin, was essentially replaced in the Col1a2-CTGF–transgenic mice by an expansion of the mesenchymal compartment and excessive connective tissue deposition (Figures 2A–D). The organization of the subdermal muscular layer, well-defined in the WT mice, was irregular in Col1a2-CTGF–transgenic mice, with infiltrations of ECM consisting mainly of collagen between muscle fibers. Mice heterozygous for the trans-gene exhibited pronounced skin fibrosis much later, at ~6 months of age (results not shown), suggesting that progression of fibrogenesis may depend on CTGF levels and may be slower at lower levels of CTGF expression.
Figure 2.
Extensive dermal fibrosis in adult Col1a2-CTGF–transgenic mice. A–N, Skin biopsy samples from WT littermate controls (A, C, E, G, I, K, and M) compared with those from adult Col1a2-CTGF–transgenic mice (B, D, F, H, J, L, and N) at 4 weeks of age (A, B, E, and F) and 8 weeks of age (C, D, G–O). Histologic results are representative of skin biopsy samples from 10 mice per group. Compared with WT mice, Col1a2-CTGF–transgenic mice showed pronounced and progressive dermal fibrosis with focal thickening of the epidermis, by hematoxylin and eosin staining (A–D) and Masson's trichrome staining (E–H). Immunochemistry analysis revealed increased accumulation of type I collagen in sections from Col1a2-CTGF–transgenic mice compared with those from WT mice (I and J). Immunofluorescence with CTGF antibody revealed increased CTGF-expressing fibroblasts in Col1a2-CTGF–transgenic mouse skin sections (arrows) compared with WT mouse skin sections (K and L). Increased numbers of myofibroblasts were observed in the papillary and reticular dermis (N and inset in N, respectively) of Col1a2-CTGF–transgenic mouse skin (arrows) compared with WT mouse skin (M). O, Angiogenesis in WT mice (column 1) and Col1a2-CTGF–transgenic mice (column 2), quantified based on numbers of blood vessels. Values are the mean and SD. * = P < 0.001. See Figure 1 for definitions.
The organization of dermal collagen exhibited dense accumulation of collagen in the dermis of Col1a2-CTGF–transgenic mice compared with WT mice, as shown by Masson's trichrome staining (Figures 2E–H), specifically type I collagen as determined by immunostaining (Figures 2I and J). Increased staining of CTGF was observed in dermal fibroblasts of transgenic mice compared with WT mice (Figures 2K and L), confirming specific targeting of the transgene expression to fibro-blasts.
To determine whether the amount of skin fibrosis correlated with the extent of α-SMA expression, we analyzed α-SMA expression in skin sections as an indicator of putative myofibroblasts. Immunostaining showed large numbers of cells positive for α-SMA in Col1a2-CTGF–transgenic mouse skin sections in both the papillary and the reticular dermis (Figure 2N), suggesting that these were myofibroblasts; in WT mice, α-SMA–expressing cells were rare (Figure 2M). In addition, absence of macrophages was determined by immunostaining with F4/80 (results not shown).
Immunostaining of skin biopsy specimens using α-SMA antibody revealed a 3.2-fold increase in the number of blood vessels in fibrotic lesions from the skin of Col1a2-CTGF–transgenic mice compared with the number in WT mice (Figure 2O). This result suggested that constitutive activation of CTGF promotes angiogenesis in vivo.
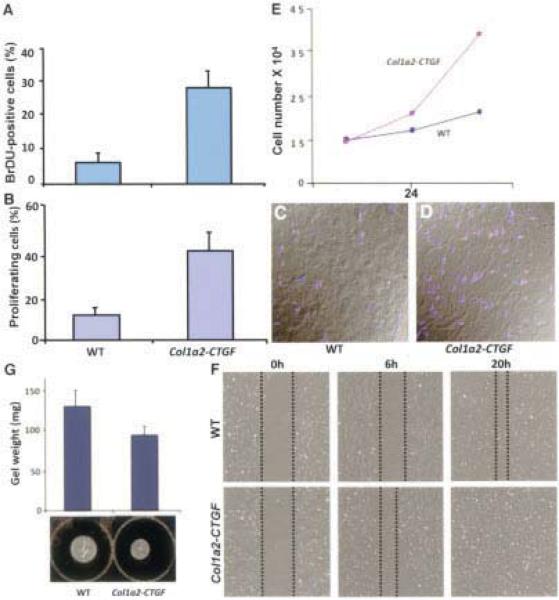
CTGF mediates increased proliferation of fibroblasts
The dermis of Col1a2-CTGF–transgenic mice showed a 3-fold increase in cells undergoing S phase entry compared with WT littermate controls, as revealed by an increase in BrdU-positive cells (Figure 3A). Consistent with this increase, we observed a 2–3-fold increase in phosphorylated histone 3–positive cells in transgenic mice relative to WT control mice (Figures 3B–D). In contrast, staining of skin sections for activated caspase 3 revealed no difference in apoptosis indices between Col1a2-CTGF–transgenic and WT mice (data not shown). These findings suggest that CTGF profoundly influences cell growth and differentiation of fibroblasts into myofibroblasts but has no adverse effect on their survival.
Figure 3.
Increased fibroblast proliferation in fibrotic skin of Col1a2-CTGF–transgenic mice. A 3-fold increase in bromodeoxyuridine (BrdU)–positive cells was observed in Col1a2-CTGF–transgenic mouse skin sections compared with sections from wild-type (WT) control mice (A). Phosphorylated histone 3 immunostaining of WT (C) and Col1a2-CTGF–transgenic (D) mouse skin sections for evidence of mitotic activity and quantitation of phosphorylated histone 3–positive cells revealed an increase in mitotic activity of cells in the dermis of Col1a2-CTGF–transgenic mice. Quantitative analysis showed that this increase was 2–3-fold (B). Col1a2-CTGF–transgenic mouse embryonic fibroblasts (MEFs) demonstrated 1.3- and 2.6-fold increases in cell number in the first 24 hours and 48 hours, respectively, compared with WT mouse cells (E). Under conditions of serum starvation, scratch assay of MEFs showed increased migration of Col1a2-CTGF–transgenic MEFs by 6 hours (F). Collagen gel contraction assay of WT and Col1a2-CTGF–transgenic MEFs showed increased contraction of the collagen lattice by Col1a2-CTGF–transgenic MEFs (G). Values in A, B, and G are the mean and SD; values in E are the mean.
Col1a2-CTGF–transgenic MEFs mirror the increased fibroblast proliferation observed in vivo
Consistent with the increase in BrdU- and phosphorylated histone 3–positive cells in vivo, Col1a2-CTGF–transgenic MEFs exhibited increased cellular proliferation relative to WT MEFs. In the presence of 10% serum, Col1a2-CTGF–transgenic MEFs underwent a 1.3-fold increase in cell number in 24 hours and a 2.6-fold increase at the end of 48 hours, compared with WT mouse cells (Figure 3E).
In addition to fibroblast proliferation and matrix production, CTGF promotes cell adhesion and migration in a variety of cell types. Since cell migration is a crucial event in normal wound healing and is often enhanced in fibrotic conditions, we tested the migratory properties of MEFs from control and Col1a2-CTGF–transgenic mouse embryos in gel contraction and scratch assays. MEFs were plated on gelatin-coated plates under normal growth conditions. After the cells reached confluence, they were serum starved for 24 hours. Six hours after scratch wounds were made, MEFs from Col1a2-CTGF–transgenic mouse embryos displayed substantial migration of cells toward the center in an attempt to heal the wounds, whereas MEFs from WT mouse embryos did not. By 20 hours, Col1a2-CTGF–transgenic MEFs had completely repaired the wounds, whereas WT MEFs required 24 hours or more to migrate into the area of the scratch (Figure 3F), suggesting that increased levels of CTGF enhanced the migratory ability of Col1a2-CTGF–transgenic MEFs. Similarly, in a gel contraction assay, MEFs from Col1a2-CTGF–transgenic mice showed greater ability to contract a collagen matrix than did control cells (Figure 3G). Cumulatively, these findings suggest that the differences observed in these in vitro fibrogenic assays, i.e., increased proliferation, migration, and contraction in Col1a2-CTGF–transgenic MEFs compared with WT control MEFs, are a consequence of the excess expression of CTGF in the transgenic mouse cells.
Increased expression of CTGF in lung fibroblasts induces lung fibrosis in vivo
The respiratory rates of Col1a2-CTGF–transgenic mice revealed dyspnea of varying severity, with a pattern of labored breathing particularly evident during rest. Histologic analysis revealed extensive fibrosis and focal fibrotic lesions within the lung parenchyma. This fibrosis was evidenced by markedly increased Masson's trichrome staining (Figures 4A and B) and immunostaining of type I collagen (Figures 4C and D) compared with WT controls. Alveolar spaces in the area of the lesions were reduced and often completely obliterated, replaced with matrix. Col1a2-CTGF–transgenic mice had a 4-fold increase in lung collagen content compared with WT littermates (Figure 4E). Thus, CTGF expression in fibroblastic cells caused extensive fibrosis in the lungs. No evidence of plexiform lesions was observed in the pulmonary arteries of the lungs of Col1a2-CTGF–transgenic mice.
Figure 4.
Pulmonary fibrosis in adult Col1a2-CTGF–transgenic mice. A–D, Lung sections from representative wild-type (WT) mice (A and C) and Col1a2-CTGF–transgenic mice (B and D) exhibited increased staining with Masson's trichrome (A and B) and immunostaining for type I collagen deposition in the alveolar spaces and septa (C and D) in the Col1a2-CTGF–transgenic mice. E, A 4-fold increase in collagen content was observed in Col1a2-CTGF–transgenic mice compared with WT littermates. Values are the mean and SD (n = 5 per group). * = P < 0.05 versus WT mice.
Increased CTGF expression causes focal glomerulosclerosis, basement membrane thickening, and vascular defects in the kidney
Histologic sections of kidney from 8-week-old Col1a2-CTGF–transgenic mice showed increased Masson's trichrome staining in the periphery of the glomeruli as well as around the renal tubules. The lesions, which included smaller glomerular structures, suggested focal glomerulosclerosis and tubulointerstitial fibrosis (Figures 5A and B). Enhanced levels of type IV collagen revealed abnormalities of basement membrane (Figures 5C and D). In addition, CTGF expression was increased in the glomeruli (Figures 5E and F) and in basement membranes surrounding the tubules (results not shown) in Col1a2-CTGF–transgenic mice compared with WT mice.
Figure 5.
Focal interstitial glomerulosclerosis in Col1a2-CTGF–transgenic mouse kidneys. A–J, Kidney tissue specimens from WT littermate controls (A, C, E, G, and I) compared with those from adult Col1a2-CTGF–transgenic mice (B, D, F, H, and J). Masson's trichrome staining was increased in the basement membrane surrounding the glomeruli of Col1a2-CTGF–transgenic mouse kidneys (A and B). Immunostaining with type IV collagen antibody demonstrated that the accumulated collagen in the interstitium of Col1a2-CTGF–transgenic mice consisted of type IV collagen (C and D). Immunostaining with CTGF antibody showed increased CTGF expression in the glomeruli, surrounding interstitium, in Col1a2-CTGF–transgenic compared with WT mice (E and F). Small blood vessels in the kidney sections stained with Masson's trichrome showed increased collagen deposition around the blood vessels and in the intima in Col1a2-CTGF–transgenic mice compared with WT littermates (G and H). Immunostaining with CD31, a marker for endothelial cells, revealed increased proliferation of endothelial cells in small blood vessels of Col1a2-CTGF–transgenic mice and normal distribution of endothelial cells in WT mice (I and J). K and L, Differential interference contrast overlays of I and J, respectively. See Figure 1 for definitions.
Masson's trichrome staining of kidney tissue sections revealed increased matrix deposition around affected blood vessels and accumulation of matrix surrounding the intimal layer in Col1a2-CTGF–transgenic mice compared with similar-sized arteries in kidney sections from WT mice (Figures 5G and H). Thickening of the vessel walls of small arteries was also increased. As a result of the increased matrix accumulation, the lumens of many affected small arteries in the kidneys of Col1a2-CTGF–transgenic mice appeared occluded (Figure 5H). Expansion of endothelial cells in small arteries was also evidenced by the increased staining of the endothelial-specific marker CD31 (Figures 5I and J). Differential interference contrast images revealed the inner and outer boundaries of the blood vessel and the increased number of endothelial cells in the intima of Col1a2-CTGF–transgenic mice (Figures 5K and L), suggesting that the lumen of blood vessels in transgenic mice was obliterated. These remodeling processes were seen only in small arteries.
These findings indicate that CTGF causes fibrotic lesions associated with abnormal collagen deposition in the interstitial tissues surrounding glomeruli and renal tubules, as well as in the basement membranes of glomeruli. They further show that CTGF is associated with vascular remodeling in the kidney.
Expression of key fibrotic genes at increased levels in Col1a2-CTGF–transgenic mouse fibroblasts
To examine the effect of overexpression of CTGF in fibroblastic cells, Northern blotting was performed with RNA from MEFs of 6 Col1a2-CTGF–transgenic mice and 6 WT control mice. The results showed markedly higher expression of matrix genes, including the genes for procollagen α1(I), tissue inhibitor of metalloproteinases 1 (TIMP-1), TIMP-3, fibronectin, and α-SMA, in Col1a2-CTGF–transgenic MEFs (Figure 6A). Similar results were obtained with adult skin fibroblasts (results not shown). The level of endogenous CTGF RNA (2.4 kb) was also highly elevated in Col1a2-CTGF–transgenic MEFs (Figure 6B), translating to markedly higher CTGF and type I collagen protein levels in Col1a2-CTGF–transgenic MEFs than in WT mouse cells. Increased expression of α-SMA (Figure 6A) also correlated with high levels of collagen protein (Figure 6B).
Figure 6.
Overexpression of CTGF in fibroblasts causes increased expression of key matrix genes in Col1a2-CTGF–transgenic mouse embryonic fibroblasts (MEFs). A, Northern blotting revealed increased expression levels of genes for CTGF, procollagen α1(I), tissue inhibitors of metalloproteinases 1 and 3, fibronectin, and α-smooth muscle actin in Col1a2-CTGF–transgenic MEFs (lane 2 in A–D) compared with WT MEFs (lane 1 in A–D). B, Western blotting of MEFs showed a corresponding increase in CTGF and type I collagen protein levels in Col1a2-CTGF–transgenic MEFs. C, Western blotting with p-Smad3 antibody revealed no difference in p-Smad protein levels in Col1a2-CTGF–transgenic and WT mouse cells. D, In contrast, constitutive phosphorylation of p38, ERK-1/2, JNK, and Akt was observed in Col1a2-CTGF–transgenic MEFs only. GAPDH, β-actin, and vinculin for total cell extract were used as loading controls; promoter reporter constructs were transfected together with a β-galactosidase vector as an internal control. E, Basal activity levels of serum response element (SRE), CTGF, and CTGF(ΔSmad) promoters were all higher in Col1a2-CTGF–transgenic MEFs than in WT mouse cells. Upon addition of transforming growth factor β1 (TGFβ1), no further activity of the SRE promoter reporter was observed; however, a 3.5-fold increase in CTGF promoter activity occurred. The CTGF promoter with a mutated Smad site showed similar basal activity in the presence and absence of exogenous TGFβ. Values are the mean and SD adjusted level of luciferase (L) or secreted enhanced alkaline phosphatase (SEAP) expression. RLU = relative light units; RSU = relative SEAP units (see Figure 1 for other definitions).
The increase in CTGF expression by TGFβ has been shown to be associated with phosphorylation of Smad proteins (14). However, as in WT MEFs, little or no p-Smad3 was detected in MEFs from Col1a2-CTGF–transgenic mice; both cell types did display increased p-Smad3 after addition of TGFβ (Figure 6C). These results imply that the increase in endogenous CTGF, Col1a1, Timp1, Timp3, and other RNAs in Col1a2-CTGF–transgenic MEFs is not mediated by Smad phosphorylation. The virtual absence of p-Smad3 in Col1a2-CTGF–transgenic and WT mouse fibroblasts was further confirmed by immunofluorescence analysis of skin sections from Col1a2-CTGF–transgenic and WT mice (results not shown). Similar results were obtained with p-Smad2. In contrast to the lack of p-Smad3 in Col1a2-CTGF–transgenic MEFs, the CTGF-dependent activation of several key signaling pathways was evidenced by increased levels of phosphorylated p38, ERK-1/2, JNK, and Akt in Col1a2-CTGF–transgenic MEFs (Figure 6D).
Increased basal activity of SRE and CTGF promoters in Col1a2-CTGF–transgenic MEFs
Fibroblasts were transfected with luciferase reporter genes driven by a minimal promoter either without or with 3 tandem repeats of SRE binding sites (pSRE). In unstimulated Col1a2-CTGF–transgenic MEFs, the pSRE promoter exhibited 3.5-fold higher luciferase activity than in WT MEFs, consistent with the constitutive phosphorylation state of ERK-1/2 in these cells; pSRE promoter activity in either WT or Col1a2-CTGF–transgenic mouse cells was not further stimulated following addition of TGFβ (Figure 6E). Similarly, transfection of a CTGF promoter driving expression of secreted enhanced alkaline phosphatase showed higher basal activity in Col1a2-CTGF–transgenic mouse cells, and this was strongly further stimulated by TGFβ in both Col1a2-CTGF–transgenic and WT mouse cells (Figure 6E). Interestingly, the CTGF promoter with a mutation in the Smad binding site also showed increased basal expression in the Col1a2-CTGF–transgenic MEFs compared with WT mouse cells, with no further increase in promoter activity upon addition of TGFβ (Figure 6E). The increased basal levels of the mutant CTGF promoter that failed to respond to TGFβ indicate that the elevated basal level was not due to increased canonical TGFβ signaling.
DISCUSSION
The direct role of CTGF in tissue fibrosis remains controversial to date (15). To better understand its significance in normal connective tissue homeostasis and repair and the pathogenesis of fibrotic diseases (16–20), genetically defined mouse models are of considerable interest. Herein we report that in transgenic mice specifically expressing high levels of CTGF in fibroblastic cells, hallmarks of sustained multiorgan fibrosis affecting the skin, lung, kidney, and microvasculature were elicited. This is the first reported clear evidence of a causal link between high levels of CTGF in fibroblasts and irreversible chronic and progressive tissue fibrosis in vivo.
In this study, fibrosis of the skin was generalized, but fibrosis was less diffuse in the lungs and clearly focal in the kidneys. In the dermis of the Col1a2-CTGF–transgenic mice, the excessive accumulation of ECM was likely due to increased fibroblast proliferation, enhanced collagen synthesis by increasing numbers of myofibroblasts, and decreased breakdown of the ECM owing to elevated expression of TIMPs in fibroblasts. Fibroblasts also displayed increased mobility, as suggested by enhanced activity in gel contraction and in vitro scratch assays. In addition, features of de novo angiogenesis were observed in the skin. It has been widely reported that CTGF has potent proangiogenic activity as assessed in specific assays, including in a number of in vitro angiogenic assays such as the Matrigel endothelial tube–forming assay and also in in vivo angiogenic assays including those involving chicken chorioallantoic membrane and rat cornea (21–23). Fibrotic lesions in the lung of Col1a2-CTGF–transgenic mice exhibited features resembling human interstitial pulmonary fibrosis.
Focal changes in the kidneys of these mice were reflected by increased Masson's trichrome staining around glomerular and tubular structures. Enhanced deposition of type IV collagen in basement membranes of glomeruli and tubules implied interstitial remodeling of the basement membranes in these tissues. However, the kidney lesions did not cause proteinuria or increased serum creatinine or urea levels, suggesting no major functional consequence in the kidney at the time the animals were killed. Recently, transgenic mice in which CTGF was targeted to the kidney using a podocyte-specific nephrin promoter were shown to be essentially normal unless challenged with streptozotocin (24).
To examine possible molecular pathways that would account for the fibrotic phenotype, MEFs of Col1a2-CTGF–transgenic and WT mice were used. Our findings of high basal activity of the CTGF promoter and a lack of detectable p-Smad3 in Col1a2-CTGF–transgenic MEFs indicate that the in vitro fibrotic phenotype is not mediated through the canonical TGFβ signaling pathway. In contrast to the lack of p-Smad3, phosphorylation of both p38 and ERK-1/2 in Col1a2-CTGF–transgenic mouse fibroblasts was markedly increased, and their activation could account for the higher basal activity of the CTGF and SRE promoters in Col1a2-CTGF–transgenic MEFs. The CTGF promoter construct was, however, also highly responsive to TGFβ in Col1a2-CTGF–transgenic MEFs, suggesting that in these cells CTGF and TGFβ signaling have the potential to synergize with one another. Lack of increased phosphorylation of TAK-1, an upstream component of the p38 signaling pathway, suggests that p38 phosphorylation may occur via a non–TAK-1–dependent mechanism.
We speculate that the activation of multiple cellular signaling pathways could be a reflection of the multiple effects of CTGF in fibroblasts of Col1a2-CTGF–transgenic mouse embryos, including increased adhesion, migration, contraction, and proliferation and an increase in profibrotic messenger RNAs and proteins. Despite intensive efforts by investigators at a number of laboratories (25), no specific receptor for CTGF has been identified. Hence, it is possible that CTGF, through different elements of its modular structure, interacts with multiple cell surface receptors or coreceptors (3). For example, syndecan 4 has been shown to bind to CTGF at the surface of fibroblasts and to activate ERK-1/2 (26). Similar experiments have demonstrated interactions of CTGF with betaglycan, a TGFβ coreceptor and activator of the p38 MAPK pathway (27).
In conclusion, our results demonstrate for the first time that constitutive overexpression of CTGF in fibroblasts in vivo is sufficient to cause marked fibrotic disease of major organs such as skin and lung. This model provides direct evidence that, as previously established for TGFβ, constitutive overexpression of CTGF is capable of inducing a fibrotic event. These transgenic mice should serve as a useful model with which to study features of fibrogenesis, some of which may resemble those seen in human scleroderma. In addition we observed other aspects of CTGF biology, such as increased angiogenesis and fibroblast proliferation, that are not characteristic features of scleroderma. Targeting the TGFβ pathway for therapeutic intervention is difficult owing to the many important functions of TGFβ during development as well as in the adult. Interestingly, after embryonic development, CTGF expression in the adult is observed during wound healing, but aside from this its expression is either very low or undetectable. However, in most fibrotic conditions, the level of CTGF appears to be elevated far beyond its physiologic requirement.
Taken together, our current findings with this mouse model have demonstrated CTGF as an important mediator of fibrosis. The transgenic mice will be a useful model for studying the key processes that may affect the fibrotic features of scleroderma, although we do not consider these mice an optimal model of the entire pathogenic process in the human disease. In addition, these mice will serve as an excellent platform for testing antifibrotic therapies for attenuating chronic persistent dermal and systemic fibrosis.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to Dr. Hank Adams for help with confocal microscopy, to Dimariss Carr for technical assistance, and to Sunita Patterson for editing the manuscript.
Supported by the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases, Center of Research Translation grant 1-P50-AR-054144), the Arthritis Research Campaign, UK, the Welton Foundation, National Cancer Institute Cancer Center Core Grant CA-16672-29, the Canadian Institute of Health Research, and the Ontario Thoracic Society. Dr. Leask is a New Investigator of the Arthritis Society (Scleroderma Society of Ontario), recipient of an Early Researcher Award from the Ontario Ministry of Research and Innovation, and a member of the Canadian Scleroderma Research Group New Emerging Team.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Sonnylal had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Sonnylal, Abraham, de Crombrugghe. Acquisition of data. Sonnylal, Shi-wen, Leoni, Naff, Van Pelt, Nakamura.
Analysis and interpretation of data. Sonnylal, Van Pelt, Leask, Bou-Gharios, de Crombrugghe.
REFERENCES
- 1.Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993;327:125–30. doi: 10.1016/0014-5793(93)80155-n. [DOI] [PubMed] [Google Scholar]
- 2.Perbal B. NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol Pathol. 2001;54:57–79. doi: 10.1136/mp.54.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–10. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 4.Friedrichsen S, Heuer H, Christ S, Winckler M, Brauer D, Bauer K, et al. CTGF expression during mouse embryonic development. Cell Tissue Res. 2003;312:175–88. doi: 10.1007/s00441-003-0712-6. [DOI] [PubMed] [Google Scholar]
- 5.Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, et al. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–91. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell. 1993;4:637–45. doi: 10.1091/mbc.4.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato S, Nagaoka T, Hasegawa M, Tamatani T, Nakanishi T, Takigawa M, et al. Serum levels of connective tissue growth factor are elevated in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. J Rheumatol. 2000;27:149–54. [PubMed] [Google Scholar]
- 8.Verrecchia F, Mauviel A. Transforming growth factor-β and fibrosis. World J Gastroenterol. 2007;13:3056–62. doi: 10.3748/wjg.v13.i22.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonnylal S, Denton CP, Zheng B, Keene DR, He R, Adams HP, et al. Postnatal induction of transforming growth factor β signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum. 2007;56:334–44. doi: 10.1002/art.22328. [DOI] [PubMed] [Google Scholar]
- 10.Denton CP, Zheng B, Evans LA, Shi-wen X, Ong VH, Fisher I, et al. Fibroblast-specific expression of a kinase-deficient type II transforming growth factor β (TGFβ) receptor leads to paradoxical activation of TGFβ signaling pathways with fibrosis in transgenic mice. J Biol Chem. 2003;278:25109–19. doi: 10.1074/jbc.M300636200. [DOI] [PubMed] [Google Scholar]
- 11.Bou-Gharios G, Garrett LA, Rossert J, Niederreither K, Eberspaecher H, Smith C, et al. A potent far-upstream enhancer in the mouse proα2(I) collagen gene regulates expression of reporter genes in transgenic mice. J Cell Biol. 1996;134:1333–44. doi: 10.1083/jcb.134.5.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Abraham DJ, Shi-wen X, Pearson JD, Black CM, Lyons KM, et al. CCN2 (connective tissue growth factor) promotes fibroblast adhesion to fibronectin. Mol Biol Cell. 2004;15:5635–46. doi: 10.1091/mbc.E04-06-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leask A, Holmes A, Black CM, Abraham DJ. Connective tissue growth factor gene regulation: requirements for its induction by transforming growth factor-β2 in fibroblasts. J Biol Chem. 2003;278:13008–15. doi: 10.1074/jbc.M210366200. [DOI] [PubMed] [Google Scholar]
- 14.Leask A, Sa S, Holmes A, Shiwen X, Black CM, Abraham DJ. The control of ccn2 (ctgf) gene expression in normal and scleroderma fibroblasts. Mol Pathol. 2001;54:180–3. doi: 10.1136/mp.54.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, et al. Role and interaction of connective tissue growth factor with transforming growth factor-β in persistent fibrosis: a mouse fibrosis model. J Cell Physiol. 1999;181:153–9. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 16.Ito Y, Aten J, Bende RJ, Oemar BS, Rabelink TJ, Weening JJ, et al. Expression of connective tissue growth factor in human renal fibrosis. Kidney Int. 1998;53:853–61. doi: 10.1111/j.1523-1755.1998.00820.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaminski N, Allard JD, Pittet JF, Zuo F, Griffiths MJ, Morris D, et al. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc Natl Acad Sci U S A. 2000;97:1778–83. doi: 10.1073/pnas.97.4.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gressner AM, Yagmur E, Lahme B, Gressner O, Stanzel S. Connective tissue growth factor in serum as a new candidate test for assessment of hepatic fibrosis. Clin Chem. 2006;52:1815–7. doi: 10.1373/clinchem.2006.070466. [DOI] [PubMed] [Google Scholar]
- 19.Leask A, Denton CP, Abraham DJ. Insights into the molecular mechanism of chronic fibrosis: the role of connective tissue growth factor in scleroderma. J Invest Dermatol. 2004;122:1–6. doi: 10.1046/j.0022-202X.2003.22133.x. [DOI] [PubMed] [Google Scholar]
- 20.Gressner OA, Gressner AM. Connective tissue growth factor: a fibrogenic master switch in fibrotic liver diseases. Liver Int. 2008;28:1065–79. doi: 10.1111/j.1478-3231.2008.01826.x. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem. 2002;277:36288–95. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
- 22.Shimo T, Nakanishi T, Nishida T, Asano M, Sasaki A, Kanyama M, et al. Involvement of CTGF, a hypertrophic chondrocyte-specific gene product, in tumor angiogenesis. Oncology. 2001;61:315–22. doi: 10.1159/000055339. [DOI] [PubMed] [Google Scholar]
- 23.Babic AM, Chen CC, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin αvβ3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol. 1999;19:2958–66. doi: 10.1128/mcb.19.4.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokoi H, Mukoyama M, Mori K, Kasahara M, Suganami T, Sawai K, et al. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int. 2008;73:446–55. doi: 10.1038/sj.ki.5002722. [DOI] [PubMed] [Google Scholar]
- 25.Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–83. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Shi-wen X, van Beek J, Kennedy L, McLeod M, Renzoni EA, et al. Matrix contraction by dermal fibroblasts requires transforming growth factor-β/activin-linked kinase 5, heparan sulfate-containing proteoglycans, and MEK/ERK: insights into pathological scarring in chronic fibrotic disease. Am J Pathol. 2005;167:1699–711. doi: 10.1016/s0002-9440(10)61252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santander C, Brandan E. Betaglycan induces TGF-β signaling in a ligand-independent manner, through activation of the p38 pathway. Cell Signal. 2006;18:1482–91. doi: 10.1016/j.cellsig.2005.11.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.