Abstract
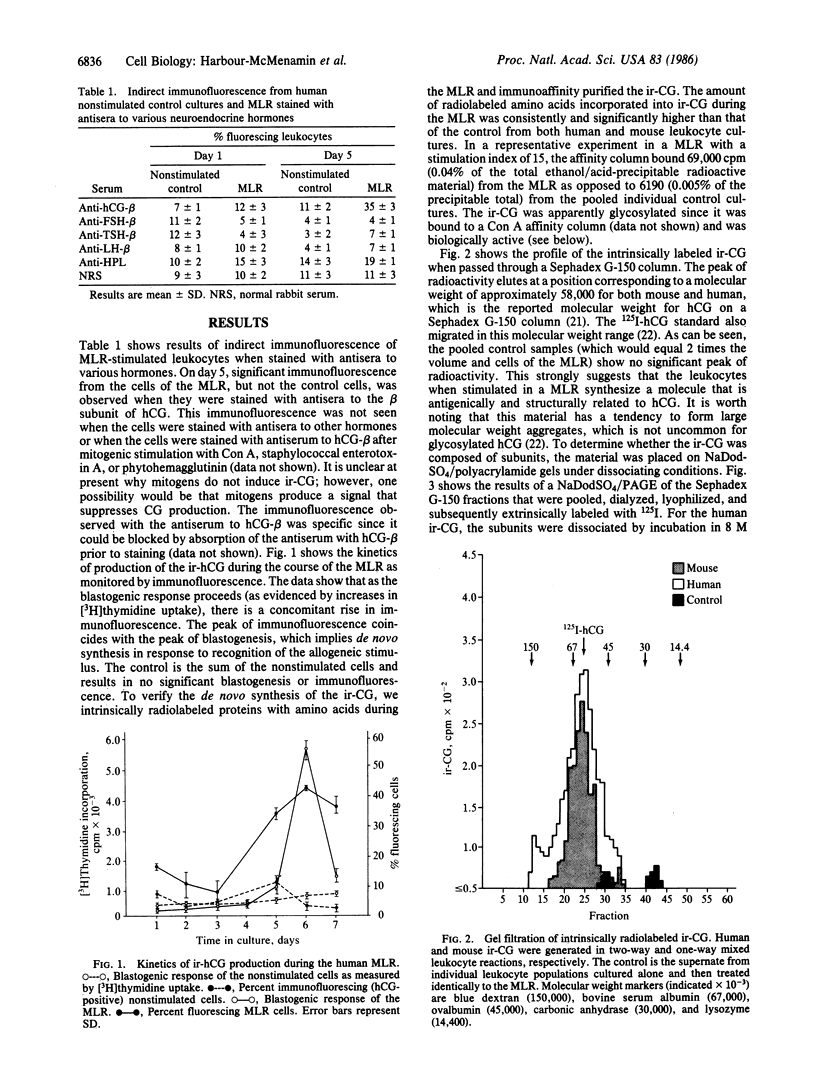
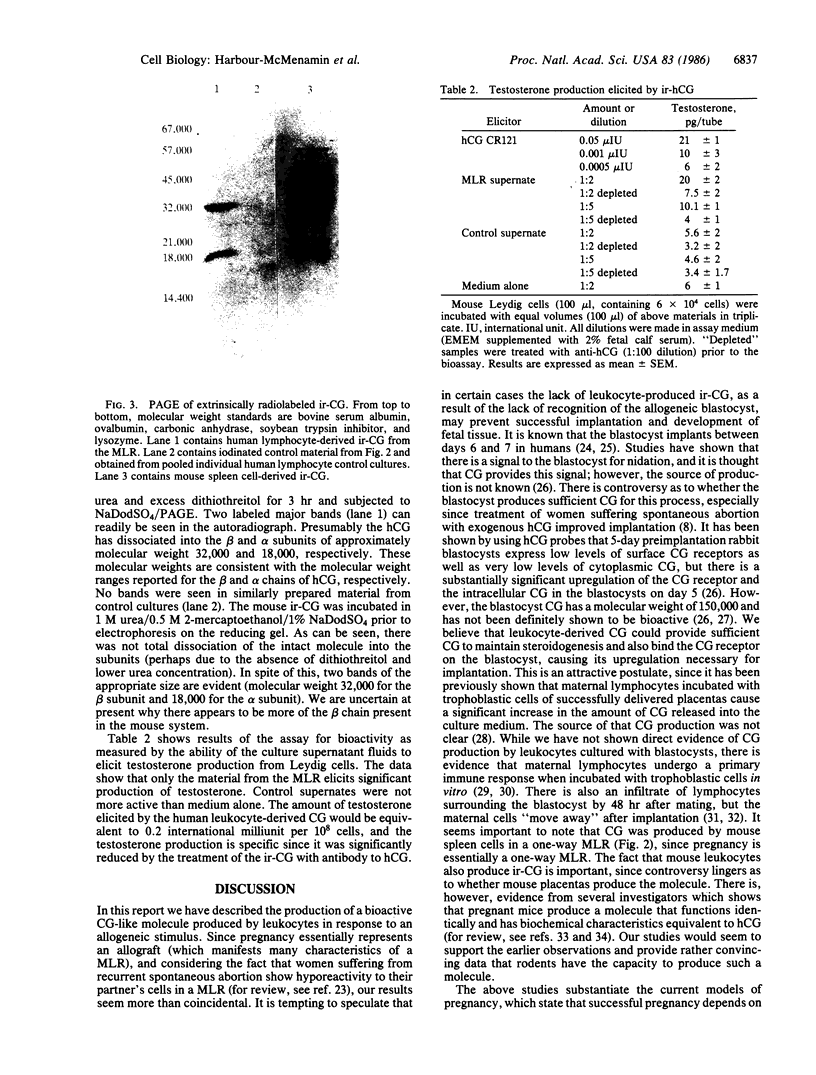
Maternal immunologic recognition of the blastocyst as well as the hormone chorionic gonadotropin (CG) has been shown to be a requirement for successful implantation of the blastocyst and maintenance of the fetal graft. On the basis of these observations, we tested whether lymphocytes could produce chorionic gonadotropic-like hormones during a mixed lymphocyte reaction (MLR). Here we described the production of immunoreactive chorionic gonadotropin (ir-CG) as a result of an allogeneic stimulus but not other mitogenic stimuli. The kinetics of production, as monitored by immunofluorescence with antibody specific to the beta chain of the human CG (hCG) molecule, paralleled the blastogenic response. Gel filtration of the de novo-synthesized ir-CG showed the molecular weight of the intact molecule to be approximately 58,000. This molecule bound to a concanavalin A affinity column and also dissociated into two subunits of approximate molecular weights 32,000 and 18,000. These molecular weights are consistent with reported molecular weights for glycosylated intact hCG, as well as the beta and alpha chains of hCG, respectively. The material from the MLR but not the control cultures elicited significant testosterone production from Leydig cells, and this activity could be blocked by antiserum to hCG. The results described here, together with the fact that the blastocyst is implanted in a lymphocyte infiltrate, lend support to our postulate that lymphocyte-derived CG enhances successful implantation of the allogeneic fetus, thus providing a mechanism for selection of genetic diversity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alanen A., Lassila O. Deficient natural killer cell function in preeclampsia. Obstet Gynecol. 1982 Nov;60(5):631–634. [PubMed] [Google Scholar]
- Bahl O. P. Human chorionic gonadotropin. I. Purification and physicochemical properties. J Biol Chem. 1969 Feb 25;244(4):567–574. [PubMed] [Google Scholar]
- Bartocci A., Papademetriou V., Schlick E., Nisula B. C., Chirigos M. A. Effect of crude and purified human chorionic gonadotropin on murine delayed-type hypersensitivity: a role for prostaglandins. Cell Immunol. 1982 Aug;71(2):326–333. doi: 10.1016/0008-8749(82)90266-0. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Bost K. L., Smith E. M. Neuroendocrine peptide hormones and their receptors in the immune system. Production, processing and action. J Neuroimmunol. 1985 Nov;10(1):31–40. doi: 10.1016/0165-5728(85)90032-3. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- Casper R. F., Wilson E., Collins J. A., Brown S. F., Parker J. A. Enhancement of human implantation by exogenous chorionic gonadotropin. Lancet. 1983 Nov 19;2(8360):1191–1191. doi: 10.1016/s0140-6736(83)91233-3. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Dickman W. J., Cauchi M. N. Lymphocyte induced stimulation of human chorionic gonadotrophin production by trophoblastic cells in vitro. Nature. 1978 Jan 26;271(5643):377–378. doi: 10.1038/271377a0. [DOI] [PubMed] [Google Scholar]
- Fuchs T., Hammarström L., Smith C. I., Brundin J. Sex-dependent induction of human suppressor T cells by chorionic gonadotropin. J Reprod Immunol. 1982 Aug;4(4):185–190. doi: 10.1016/0165-0378(82)90025-0. [DOI] [PubMed] [Google Scholar]
- Gill T. J., 3rd Immunogenetics of spontaneous abortions in humans. Transplantation. 1983 Jan;35(1):1–6. [PubMed] [Google Scholar]
- Goodfellow C. F. Maternal lymphocyte responses during normal and abnormal pregnancies, measured in vitro using composite trophoblast antigens and phytohaemagglutinin. Immunol Rev. 1983;75:61–85. doi: 10.1111/j.1600-065x.1983.tb01091.x. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Holt J. A., Heise W. F., Wilson S. M., Keyes P. L. Lack of gonadotropic activity in the rabbit blastocyst prior to implantation. Endocrinology. 1976 Apr;98(4):904–909. doi: 10.1210/endo-98-4-904. [DOI] [PubMed] [Google Scholar]
- Johnson P. M., Barnes R. M., Hart C. A., Francis W. J. Determinants of immunological responsiveness in recurrent spontaneous abortion. Transplantation. 1984 Sep;38(3):280–284. doi: 10.1097/00007890-198409000-00016. [DOI] [PubMed] [Google Scholar]
- Joshi N. J., Nandedkar T. D. Effects of intrauterine instillation of antiserum to hCG during early pregnancy in mice. Acta Endocrinol (Copenh) 1984 Oct;107(2):268–274. doi: 10.1530/acta.0.1070268. [DOI] [PubMed] [Google Scholar]
- Khan-Dawood F. S., Dawood M. Y. Chorionic gonadotropin receptors and immunoreactive chorionic gonadotropin in implantation of the rabbit blastocyst. Am J Obstet Gynecol. 1984 Feb 15;148(4):359–365. doi: 10.1016/0002-9378(84)90707-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence R., Church J. A., Richards W., Borzy M. Immunological mechanisms in the maintenance of pregnancy. Ann Allergy. 1980 Mar;44(3):166–173. [PubMed] [Google Scholar]
- McIntyre J. A., Faulk W. P., Verhulst S. J., Colliver J. A. Human trophoblast-lymphocyte cross-reactive (TLX) antigens define a new alloantigen system. Science. 1983 Dec 9;222(4628):1135–1137. doi: 10.1126/science.6648525. [DOI] [PubMed] [Google Scholar]
- Moore P. D. Exploitation of animal mobility. 1985 Sep 26-Oct 2Nature. 317(6035):288–289. doi: 10.1038/317288a0. [DOI] [PubMed] [Google Scholar]
- Morgan F. J., Canfield R. E. Nature of the subunits of human chorionic gonadotropin. Endocrinology. 1971 Apr;88(4):1045–1053. doi: 10.1210/endo-88-4-1045. [DOI] [PubMed] [Google Scholar]
- Redman C. W. HLA-DR antigen on human trophoblast: a review. Am J Reprod Immunol. 1983 Jun;3(4):175–177. doi: 10.1111/j.1600-0897.1983.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Ricketts R. M., Jones D. B. Differential effect of human chorionic gonadotrophin on lymphocyte proliferation induced by mitogens. J Reprod Immunol. 1985 May;7(3):225–232. doi: 10.1016/0165-0378(85)90053-1. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Blalock J. E. Human lymphocyte production of corticotropin and endorphin-like substances: association with leukocyte interferon. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7530–7534. doi: 10.1073/pnas.78.12.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C., Faulk W. P. Prevention of recurrent abortion with leucocyte transfusions. Lancet. 1981 Jul 11;2(8237):68–70. doi: 10.1016/s0140-6736(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Taylor P. V., Hancock K. W. Antigenicity of trophoblast and possible antigen-masking effects during pregnancy. Immunology. 1975 May;28(5):973–982. [PMC free article] [PubMed] [Google Scholar]
- Van Damme M. P., Robertson D. M., Diczfalusy E. An improved in vitro bioassay method for measuring luteinizing hormone (LH) activity using mouse Leydig cell preparations. Acta Endocrinol (Copenh) 1974 Dec;77(4):655–671. doi: 10.1530/acta.0.0770655. [DOI] [PubMed] [Google Scholar]