Activating mutations in the FMS-like tyrosine kinase-3 (FLT3), a tyrosine kinase receptor important in haematopoiesis, are among the most common molecular aberrations in acute myeloid leukaemia (AML), occurring in 30% of adult patients (Levis & Small 2003). Common FLT3-activating mutations include FLT3 internal tandem duplications (FLT3-ITDs), detected in about 23% of AML patients, and point mutations within the tyrosine kinase domain, found in about 8% (Levis & Small 2003). These mutations result in a constitutively active FLT3 receptor, leading to growth factor–independent proliferation and survival of leukaemic cells and conferring poor prognosis (Levis & Small 2003).
Clinical studies of single-agent first-generation FLT3 inhibitors have demonstrated clinical activity, with responses that are typically short-lived and mostly partial or complete responses with incomplete haematopoietic recovery. This may be due to suboptimal potency and/or pharmacokinetics, leading to insufficient or transient target inhibition, or concomitant c-kit inhibition (Knapper 2011). Recently, high potency second-generation FLT3 inhibitors (eg, quizartinib) have shown substantial efficacy as monotherapy, suggesting a potency threshold for clinical benefit (Knapper 2011). The validation of FLT3-ITD as a therapeutic target has rekindled interest in developing and testing new potent FLT3 inhibitors in AML patients with FLT3-ITD mutations (Smith et al, 2012).
Ponatinib is a novel, orally administered tyrosine kinase inhibitor (TKI) and a potent pan–BCR-ABL1 inhibitor (O’Hare et al, 2009). Based on results in patients with chronic myeloid leukaemia (CML) and Philadelphia chromosome–positive acute lymphoblastic leukaemia (Ph+ ALL) in phase 1 and phase 2 clinical trials (Cortes et al 2012a, Cortes et al (2012b), ponatinib (45 mg once daily) has been approved in the United States for the treatment of patients with CML and Ph+ ALL that is resistant or intolerant to prior TKI therapy. Preclinical studies revealed that ponatinib also potently inhibits FLT3, leading to apoptosis of leukaemic cell lines carrying the FLT3-ITD mutation and tumour regression in xenograft models, suggesting the potential for activity in patients with AML (Gozgit et al, 2011). Additionally, ponatinib appears to retain activity against the clinically-relevant quizartinib-resistant mutant FLT3-ITD F691L (Smith et al, 2013). Here we report the first clinical experience with ponatinib in 12 AML patients included in the phase 1 study. Methods are described in the on-line supporting information.
The median age of these patients was 49 (30-72) years. The median time from diagnosis to treatment was 1 year. Patients received a median of 3 (1-7) prior therapies; 58% had received 3 or more prior therapies (Table I and Table S1). Mutational analysis in a central laboratory confirmed the presence of FLT3-ITD in 7 patients (58%). Three additional patients did not have an adequate DNA sample at study entry; however, they had a history of FLT3-ITD mutation—as reported by the investigator—and they are included in the FLT3-ITD mutation– positive group for these analyses. Three patients (all FLT3-ITD mutation positive) were previously treated with one or more FLT3 inhibitors (sorafenib, quizartinib, and/or IMC-EB10); one patient progressed on IMC-EB10 and had a partial response to sorafenib, one patient had a complete response to sorafenib and a partial response to quizartinib, and one patient had a partial response to quizartinib. Seven patients (70%) with FLT3-ITD mutation were FLT3 inhibitor– naïve (Table I). The median treatment duration was 52 (10-173) days. At the time of analysis, all patients had discontinued ponatinib: 5 (42%) due to death (all unrelated to ponatinib), 3 (25%) due to adverse events (AEs: unrelated central nervous system [CNS] haemorrhage, possibly related acute pancreatitis, unrelated graft vs host disease), 2 (17%) due to progressive disease (PD), and 2 (17%) due to investigator decision (Table I).
Table 1.
Selected baseline characteristics, treatment duration, response, and reasons for discontinuation by individual patients with AML
| Patient ID | Selected baseline characteristics | Duration of ponatinib treatment (days) | Best response to ponatinib | Reason for discontinuation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Time from diagnosis to treatment (months) | History of FLT3 mutations | FLT3-ITD at study entry | Prior number of treatment regimens* | Prior transplant | Prior FLT3 inhibitors | Best response to prior therapy† | ||||
| 1 | 41 | 14.7 | + | ND‡ | 7 | N | Sorafenib, IMC-EB10‡ | CR | 15 | PD | Adverse event (unrelated CNS haemorrhage leading to death) |
| 2 | 52 | 11.4 | + | + | 3 | N | Sorafenib, quizartinib§ | CR | 21 | PD | Death (unrelated multiorgan failure) |
| 3 | 30 | 48.6 | + | + | 4 | Y | None | CR | 10 | PD | Death (unrelated multiorgan failure) |
| 4 | 57 | 6.8 | + | − | 3 | N | None | SD | 61 | SD | Death (unrelated disease progression) |
| 5 | 43 | 120.5 | + | + | 4 | Y | None | CR | 96 | SD | Death (unrelated disease progression) |
| 6 | 48 | 6.4 | + | + | 1 | N | None | CR | 150 | PR | Death (probably unrelated pneumonia and unrelated sepsis) |
| 7 | 49 | 7.1 | + | − | 2 | Y | None | CR | 90 | SD | Investigator decision |
| 8 | 63 | 17.5 | + | + | 2 | Y | None | CR | 90 | CRi | Disease progression |
| 9 | 39 | 13.4 | + | + | 5 | N | None | CR | 43 | PD | Disease progression |
| 10 | 72 | 9.7 | + | + | 2 | N | None | CR | 12 | NA | Adverse event (possibly related acute pancreatitis)∥ |
| 11 | 39 | 11.4 | + | ND¶ | 4 | Y | Quizartinib** | CR | 15 | PD | Adverse event (unrelated graft vs host disease) |
| 12 | 58 | 15.2 | + | ND¶ | 2 | Y | None | CR | 173 | CRi | Investigator decision |
FLT3, FMS-like tyrosine kinase-3; ITD, internal tandem duplication; ND, no DNA sample collected; N, no; CR, complete remission; PD, progressive disease; CNS, central nervous system; Y, yes; SD, stable disease; PR, partial remission; CRi, complete remission with incomplete blood count recovery; NA, not assessed.
Includes transplant regimen, if applicable.
The best response to any prior cancer therapy was collected as CR, PD, PR, or SD.
The best responses to IMC-EB10 and sorafenib were progression and partial remission, respectively.
The best responses to sorafenib and quizartinib were complete response and partial response, respectively.
This patient ultimately died due to unrelated disease progression.
FLT3-ITD–positive is defined as positive by history or at study entry, but absent negative assay.
The best response to quizartinib was partial response.
Nine patients experienced at least one treatment-related AE. The most common treatment-related AEs occurring in 2 or more patients were pancreatitis (n=3) and petechiae (n=2). Three patients experienced a treatment-related serious AE (SAE) of pancreatitis (all grade 2), which was a dose-limiting toxicity in this trial (Cortes et al, 2012a). Pancreatitis resolved in 2 patients after dose interruption, lasting 3 days in one patient and 8 days in the other. These 2 patients continued therapy at a reduced dose (30 mg) and were subsequently re-escalated to 45 mg without recurrence. The third patient discontinued therapy per investigator decision. Additional details regarding treatment-emergent AEs and SAEs can be found in Table S2. Seven patients died during the study for reasons not related to ponatinib: disease progression (n=3), multiorgan failure (n=2), pneumonia and sepsis (n=1), and CNS haemorrhage (n=1) (Table I). Ponatinib had an acceptable safety profile in this small group of patients with refractory AML, similar to that observed in patients with CML and Ph+ ALL. Few treatment-related AEs were reported; the most common was pancreatitis, which was manageable, and re-challenge with ponatinib was possible in most cases.
The geometric mean maximal concentration and area under the curve of single-dose ponatinib at day 1, cycle 1 in AML patients were 97 nM and 1441 nM*h, respectively, similar to findings across all 31 patients receiving 45 mg ponatinib (98.8 nM and 1360.1 nM*h).
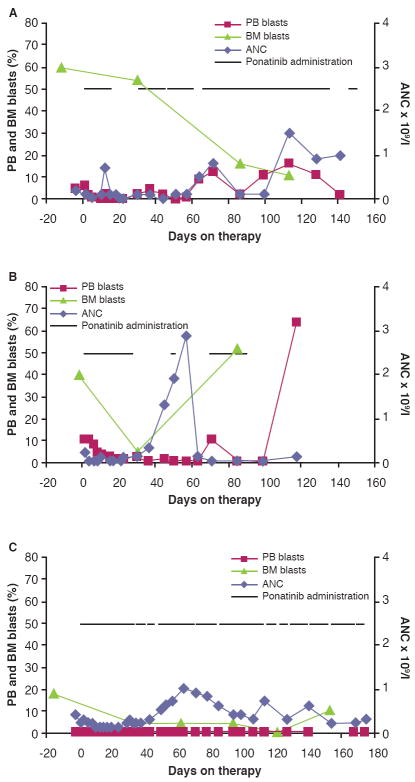
The overall response rate (RR, partial remission or better) was 3/12 (25%): 2 patients achieved complete remission with incomplete blood count recovery and one patient experienced partial remission (Table I, Fig 1). These 3 responders carried FLT3-ITD mutations and were all FLT3 inhibitor–naïve; the duration of ponatinib treatment in these patients was 3 to 6 months. Among 10 patients with FLT3-ITD mutations, RR was 3/10 (30%). Among 7 patients with FLT3-ITD mutations who were FLT3 inhibitor–naïve, RR was 3/7 (43%). Three patients (2 FLT3-ITD negative) had stable disease, as they did not meet criteria for complete/partial remission or PD; however, peripheral blood blasts in 2 of these patients decreased considerably (~60-90%) during the first treatment cycle. The RR reported with quizartinib in phase 1 testing was 30% (Cortes et al, 2009) and 10% with sorafenib (Borthakur et al, 2011). Although the sample size reported here is small, these results suggest that ponatinib has clinical activity in AML patients with FLT3-ITD, requiring confirmation in a larger cohort of patients and with additional focus on optimization of response (eg, combination therapy) and response durability.
Figure 1. Course of the disease in 3 responders during ponatinib treatment.
(A) Patient 6, who achieved partial remission. (B) Patient 8, who achieved complete remission with incomplete blood count recovery. (C) Patient 12, who achieved complete remission with incomplete blood count recovery. PB, peripheral blood; BM, bone marrow; ANC, absolute neutrophil count
Supplementary Material
Acknowledgments
This study was sponsored by ARIAD Pharmaceuticals, Inc., and was supported in part by MD Anderson’s Cancer Center Support Grant CA016672 and NCI grant P01 CA055164-20. We thank the patients, their caregivers, the investigators, and the study site research personnel for their participation in the trial. We also thank the members of the Ponatinib Phase I Study Team (ARIAD). NPS is a Leukemia & Lymphoma Society Scholar in Clinical Research. Professional medical writing assistance for this publication was provided by Francesca Balordi, PhD, Medicus International New York, and funded by ARIAD Pharmaceuticals, Inc.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Methods
Table S1. Patient characteristics
Table S2. Adverse events
Data from this study have previously been presented at the 2011 American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 3-7, 2011.
Conflict of interest
Neil P. Shah – Institution received funding for the current clinical trial from ARIAD Pharmaceuticals Inc.; paid member of the Phase II Molecular Steering Committee of ARIAD Pharmaceuticals; institution received funding for clinical trials with FLT3 inhibitors in AML patients from ARIAD Pharmaceuticals Inc., Ambit Biosciences, and Plexxikon. Moshe Talpaz – Institution received funding for the current clinical trial from ARIAD Pharmaceuticals, Inc. Michael W. N. Deininger – Institution received funding for the current clinical trial from ARIAD Pharmaceuticals, Inc., institution received funding from BMS, Celgene, Novartis, and Genzyme; received consulting fee/honorarium from BMS, ARIAD Pharmaceuticals Inc., and Novartis; received payment as member of advisory boards for BMS, ARIAD Pharmaceuticals Inc., and Novartis. For activities outside the submitted work: paid member of boards/advisory committees of BMS, ARIAD Pharmaceuticals Inc., and Novartis; employed by the University of Utah; paid consultant for BMS, ARIAD Pharmaceuticals Inc., and Novartis. Michael J. Mauro – For activities outside the submitted work: paid member of boards/advisory committees for Novartis and BMS; paid consultant for Novartis and BMS; received travel/accommodations/meeting expenses from Novartis and BMS. Ian W. Flinn – Institution received funding for the current clinical trial from ARIAD Pharmaceuticals, Inc. Dale Bixby – No competing financial interests. Stephanie Lustgarten, Joseph M. Gozgit, Tim Clackson, Christopher D. Turner, and Frank G. Haluska are employees of ARIAD Pharmaceuticals Inc. and own stock/stock options in ARIAD Pharmaceuticals, Inc. Hagop Kantarjian – Institution received funding for the current clinical trial from ARIAD Pharmaceuticals, Inc. Jorge E. Cortes – Institution received funding for the current clinical trial from ARIAD Pharmaceuticals Inc.; received consulting fee/honorarium from ARIAD Pharmaceuticals Inc. For activities outside the submitted work: paid consultant for Pfizer, and Teva; institution received funding from BMS, Novartis, Pfizer, Ambit, Astellas, Arog, and ChemGenex.
Professional medical writing assistance for this publication was provided by Francesca Balordi, PhD, Medicus International New York, and funded by ARIAD Pharmaceuticals, Inc.
References
- Borthakur G, Kantarjian H, Ravandi F, Zhang W, Konopleva M, Wright JJ, Faderl S, Verstovsek S, Mathews S, Andreeff M, Cortes JE. Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica. 2011;96:62–68. doi: 10.3324/haematol.2010.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes J, Foran J, Ghirdaladze D, DeVetten MP, Zodelava M, Holman P, Levis MJ, Kantarjian HM, Borthakur G, James J, Zarringkar PP, Gunawardane RN, Armstrong RC, Padre NM, Wierenga W, Corringham R, Trikha M. AC220, a potent, selective, second generation FLT3 receptor tyrosine kinase (RTK) inhibitor, in a first-in-human (FIH) phase 1 AML study. Blood. 2009;114 Abstract 636. [Google Scholar]
- Cortes JE, Kantarjian H, Shah NP, Bixby D, Mauro MJ, Flinn IW, O’Hare T, Haluska FG, Druker BJ, Deininger MW, Talpaz M. Ponatinib in refractory Philadelphia chromosome-positive leukemias. New England Journal of Medicine. 2012a;367:2075–2088. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, Nicolini FE, Apperley JF, Khoury HJ, Talpaz M, DiPersio JF, DeAngelo DJ, Abruzzese E, Rea D, Baccarani M, Müller MC, Gambacorti-Passerini C, Wong S, Lustgarten S, Rivera VM, Clackson T, Turner CD, Haluska FG, Guilhot F, Deininger MW, Hochhaus A, Hughes T, Goldman JM, Shah N, Kantarjian HM. A pivotal phase 2 trial of ponatinib in patients with chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) resistant or intolerant to dasatinib or nilotinib, or with the T315I BCR-ABL mutation: 12-month follow-up of the PACE trial. Blood (ASH Annual Meeting Abstracts) 2012b;120 Abstract 163. [Google Scholar]
- Gozgit JM, Wong MJ, Wardwell S, Tyner JW, Loriaux MM, Mohemmad QK, Narasimhan NI, Shakespeare WC, Wang F, Druker BJ, Clackson T, Rivera VM. Potent activity of ponatinib (AP24534) in models of FLT3-driven acute myeloid leukemia and other hematologic malignancies. Molecular Cancer Therapeutics. 2011;10:1028–1035. doi: 10.1158/1535-7163.MCT-10-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapper S. The clinical development of FLT3 inhibitors in acute myeloid leukemia. Expert Opinion in Investigational Drugs. 2011;20:1377–1395. doi: 10.1517/13543784.2011.611802. [DOI] [PubMed] [Google Scholar]
- Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17:1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- O’Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, Adrian LT, Zhou T, Huang WS, Xu Q, Metcalf CA, 3rd, Tyner JW, Loriaux MM, Corbin AS, Wardwell S, Ning Y, Keats JA, Wang Y, Sundaramoorthi R, Thomas M, Zhou D, Snodgrass J, Commodore L, Sawyer TK, Dalgarno DC, Deininger MW, Druker BJ, Clackson T. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Wang Q, Chin CS, Salerno S, Damon LE, Levis MJ, Perl AE, Travers KJ, Wang S, Hunt JP, Zarrinkar PP, Schadt EE, Kasarskis A, Kuriyan J, Shah NP. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485:260–263. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Lasater EA, Zhu X, Lin KC, Stewart WK, Damon LE, Salerno S, Shah NP. Activity of ponatinib against clinically-relevant AC220-resistant kinase domain mutants of FLT3-ITD. Blood. 2013 doi: 10.1182/blood-2012-07-442871. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.