Abstract
Objectives
To determine recurrence rates of lower extremity radicular pain after nonsurgical treatment of acute symptomatic lumbar disk herniation (LDH), and identify predictors of recurrence.
Design
Prospective inception cohort.
Setting
Outpatient spine clinic.
Participants
Patients (N=79) reporting resolution of radicular pain after MRI-confirmed LDH.
Interventions
Individualized nonsurgical treatment tailored to the patient. All patients received education, but other treatments varied depending on the individual.
Measurements
Resolution of radicular pain was defined as a pain-free period of ≥1 month. Patients who reported resolution of radicular pain within 1 year after seeking care for acute LDH were asked whether pain had recurred at 1 year after seeking care, and were also reassessed 1 year after the time of resolution of radicular pain, and 2 years after seeking care. Patients reported on recurrence, and the date of recurrence if any. We evaluated the 1-year incidence of recurrence, using Kaplan-Meier survival plots. We examined predictors of recurrence using bivariate and multivariate Cox proportional hazards models. We examined the secondary outcome of back pain recurrence using identical methods.
Results
Twenty five percent (95% confidence interval [CI], 15-35%) of individuals with resolution of radicular pain for at least one month reported subsequent recurrence of pain within 1 year after resolution. The only factor independently associated with radicular pain recurrence was the number of months prior to resolution of pain (Hazard ratio per month [95% CI] 1.24 [1.13-1.37]; p <0.0001). The 1-year incidence of back pain recurrence was 43% (95% CI, 30-56%), and older age decreased the hazard of recurrence.
Conclusions
Recurrence of radicular pain is relatively common after nonsurgical treatment of LDH, and is predicted by longer time to initial resolution of pain.
Keywords: Herniation, Intervertebral Disk Displacement, Outcome assessment (health care), Rehabilitation
INTRODUCTION
Acute symptomatic lumbar disk herniation (LDH) is a common cause of lower extremity radicular pain (or ‘sciatica’) in adults. The natural history of acute LDH is favorable for the majority of individuals1-3. Although it is clear that most patients with acute LDH experience resolution of their initial radicular pain symptoms without surgery, specific rates of subsequent symptom recurrence in patients who have had pain resolution with nonsurgical treatment are unknown. Weber reported that 24% of patients receiving nonsurgical treatment for LDH experienced ‘relapse’ within 4 years, but did not specify the symptoms or measures used to define either initial resolution or subsequent recurrence, and did not examine factors associated with recurrence2. All other studies of LDH recurrence have examined recurrence after surgery4. Since the probability of future recurrence is a common concern for patients who have had resolution of radicular pain with nonsurgical treatment, this paucity of empirical data on recurrence represents a deficiency in the knowledge base with which physicians are able to educate patients embarking on a course of nonsurgical treatment.
Recent systematic reviews and expert consensus definitions of recurrence after acute low back pain (LBP) have drawn attention to the potential difficulties in studying recurrence, and in accurately conveying this information to clinicians, patients, and researchers.5-9 The presence of a true recurrence requires that 1) an individual has had resolution of their original pain symptoms for a period of time (sometimes called ‘recovery’6, but defined as ‘resolution’ for the purposes of this article), and 2) subsequently experiences a separate episode of the same pain symptoms (recurrence).6 Reports of prevalent pain at a time after the onset of pain therefore do not capture true recurrence, since they include individuals with persistent pain who never originally recovered, and would therefore never have been eligible to have a recurrence. In addition, recurrences that had resolved prior to the time of reassessment would not be detected. These flaws are noted not only for LBP studies, but also for studies of radicular pain recurrence10. Rates of true recurrence furthermore are highly dependent on the definitions applied for the presence of pain, resolution, and recurrence.6,9 Because explicit definitions of symptom resolution and recurrence are often not stated, or have varied widely between different studies of LBP6 and LDH11, this poses a major limitation to the interpretability of study findings and the comparability of reports between different studies.
We conducted an inception cohort study to determine accurate estimates of pain recurrence after nonsurgical treatment of acute symptomatic LDH, using clear definitions of resolution and recurrence6. We examined 1) rates of recurrence of lower extremity radicular pain over two years of follow-up, and 2) factors associated with recurrence. We also examined recurrence of low back pain as a secondary outcome. We applied survival analysis techniques, which have been previously recommended for use in studies of LDH recurrence, but are rarely utilized for this purpose12. Survival analysis has advantages over other analytic approaches for studies of recurrence, because it takes into account both time-to-event and censored observations in situations where duration of follow-up is variable (an unavoidable circumstance in this instance since symptom resolution and subsequent recurrence necessarily occur at different times for all individuals after LDH as described above).
METHODS
Study Participants
One hundred fifty-four consecutive adults with acute lower extremity radicular pain were recruited from an outpatient spine clinic for this prospective study of symptomatic LDH. Magnetic resonance imaging (MRI) confirmed LDH corresponding with the clinical presentation for all participants. Inclusion and exclusion criteria for this study are described in detail elsewhere1. All participants received nonsurgical treatment tailored to the individual. This included education on the natural history of LDH and advice regarding gradual return to normal daily activities for all participants, but other treatments varied depending on the individual. Other treatments employed included use of oral medications, physical therapy, and lumbar interlaminar or transforaminal corticosteroid injections. Some participants were referred for surgical consultation based on various considerations including intractable pain not responsive to nonsurgical treatment, the presence of progressive motor/sensory deficits, and by patient request. The Institutional Review Board of New England Baptist Hospital approved the conduct of this study.
Sociodemographic and Clinical Characteristics
Baseline data collected at the time of recruitment included participant age, gender, medical comorbidity burden, prior history of low back pain, tobacco use, and worker’s compensation status. Medical comorbidity burden was measured using the Self-Administered Comorbidity Questionnaire (SACQ)13. Disk herniation level was classified as midlumbar (L1-L2, L2-L3, or L3-L4) or low lumbar (L4-L5 or L5-S1). Herniation morphology was classified as protrusion, extrusion, or sequestration14. Herniation location was classified as central (central, paracentral, or lateral recess location) or foraminal (foraminal or extraforaminal location)14. Straight leg raise (SLR) testing and femoral stretch testing were evaluated at the baseline clinical examination in a standardized manner that has been described elsewhere15.
Patient-reported pain intensity and disability information was collected at the baseline clinic visit, and by mailed questionnaires at 1-year and 2-year follow-up. Lower extremity radicular pain and back pain intensities were measured using a 0-10 visual analogue scale (VAS)16. Back-related disability was measured using the Oswestry Disability Index (ODI)17.
Defining Pain Resolution and Recurrence
At both the 1- and 2-year questionnaires, participants were prompted to recall the characteristic leg pain associated with their index LDH episode, and were asked if they had experienced resolution of that leg pain. Resolution of leg pain was defined as ‘a continuous period of at least 1 month duration where you experienced no pain, or essentially no pain, in your leg’. This definition is consistent with recent expert consensus recommendations for defining resolution of LBP6. Participants who reported having resolution of their initial leg pain specified the first day of the month-long period during which they had sustained resolution of pain. Participants were then asked if they had experienced a subsequent recurrence of leg pain after the one-month period when they were free of pain, and if so, when this recurrence began. The number of consecutive months during which they were free of pain was calculated as the difference between the date of resolution and the date of recurrence. Only first recurrences were specified and tracked in this study. In addition to the scheduled 1- and 2-year questionnaires, participants reporting resolution of leg pain at 1 year also received another questionnaire 1 year after the date when their month-long period without leg pain began (that is, between the scheduled 1- and 2- year questionnaires). This additional questionnaire was specifically designed to assess 1-year recurrence rates, and provide another timepoint for assessment. In follow-up questionnaires after a participant had reported resolution, the previously reported resolution date was printed for the participant, in order to minimize potential errors in recall. Similar questions were also asked for resolution and recurrence of the secondary outcome of back pain. All questionnaires were administered by mail. In case of non-response, questionnaires were re-sent.
Statistical Analysis
We used descriptive statistics to characterize the study population, including means and standard deviations (SD) for continuous variables, and frequencies and proportions for categorical variables. We used graphic plots to examine the distributions of variables and consider outliers. We used the chi-square test for categorical variables, and Student’s t-test for continuous variables, to compare characteristics of participants with and without resolution of leg pain. We calculated the 1-year cumulative incidence of leg pain recurrence as a simple proportion using data from those questionnaires sent 1 year after the date when leg pain was reported to have resolved. We plotted Kaplan-Meier survival curves for leg pain recurrence using the product-limit method for those individuals who had resolution of leg pain and were therefore eligible to have a recurrence. We tested the assumption of proportional hazards for predictor variables by calculating Schoenfeld residuals and examining correlations of residuals with failure time (recurrence time); we concluded that the proportional hazards assumption was reasonable if we failed to reject the null hypothesis that residuals are uncorrelated with time. We then used bivariate Cox proportional hazards regression models to examine associations between predictor variables and recurrence of leg pain. Statistical significance was determined using the convention of p=0.05. Although the relatively low frequency of the recurrent leg pain outcome prohibited the use of multivariate modeling with numerous predictor variables simultaneously, we utilized a multivariate analytic approach to include the covariates that were most strongly associated with recurrent leg pain, in an iterative series of parsimonious models including two predictor variables at a time. To do this, we examined associations between each predictor variable with a p-value ≤ 0.10 in combination with other predictor variables meeting this criterion, and examined whether significant bivariate associations remained significant after adjusting for other covariates. We assessed for multicollinearity between predictor variables by examining variance inflation factors (VIF), and considering values of VIF above 5 as a cause for concern. This analytic process above was also repeated for the secondary outcome of back pain recurrence. All analyses were performed using SAS software, version 9.2 (SAS Institute., Cary, NC).
RESULTS
The flow of study participants is depicted in Figure 1. Of 154 participants with acute symptomatic LDH, 118 (77%) responded to follow-up questionnaires. Responders were slightly older than non-responders (54.0 ± 14.0 vs. 49.1 ± 10.5; p=0.07), but there were otherwise no material differences between responders and non-responders with respect to sociodemographics, pain intensity, or disability measures (data not shown). Of the responders, 21 participants underwent lumbar decompression surgery at some point during the 2-year follow-up. Of the 97 patients who did not receive surgery, 79 participants (81%) experienced resolution of leg pain, and were therefore eligible to have a recurrence of leg pain after nonsurgical treatment. The average time to resolution of leg pain was 6 months after the time of seeking care for symptomatic LDH.
Figure 1.
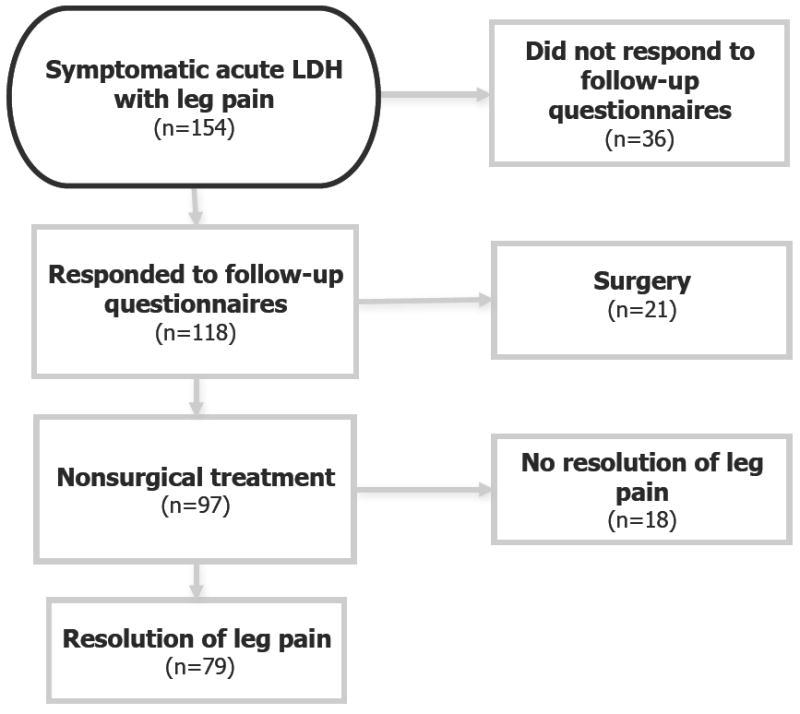
Flowchart of Study Participation
Table 1 presents the characteristics of participants who were eligible for leg pain recurrence. The 1-year cumulative incidence of leg pain recurrence was 25% (95% confidence interval [CI] 15-35%). Figure 2 depicts a Kaplan-Meier survival curve of the probability of leg pain recurrence. The survival curve depicts censoring at different timepoints over the 2-year follow-up, since different individuals experienced initial resolution of pain at different times, and were therefore followed for subsequent recurrence for different durations up to a maximum of 2 years. This figure shows that 53% of recurrences (9/17) occurred in the first 3 months after leg pain had resolved, 41% (7/17) occurred between 4-9 months, and only 6% (1/17) occurred after 9 months of follow-up. Bivariate associations between sociodemographic/clinical factors and leg pain recurrence using Cox proportional hazards models are presented in Table 2; formal tests supported the appropriateness of using proportional hazards models for these predictor variables. The only factor that was significantly associated with leg pain recurrence was the number of months prior to resolution of leg pain (Hazard ratio [HR] per month [95% CI] 1.24 [1.13-1.37]; p <0.0001). That is, for every month prior to complete resolution of leg pain, there was an almost 25% greater hazard of a subsequent recurrence once resolution occurs. In addition, the factors of prior spine surgery at a different spinal level (HR 4.02 [95% CI; 0.92-17.66) and greater medical comorbidity burden (HR 1.13 [95% CI; 0.99-1.27) demonstrated trends towards a greater hazard of recurrent leg pain, while opioid use (HR 0.40 [95% CI; 0.14-1.27]) demonstrated trends towards a lower hazard of recurrent leg pain. In multivariate models including also the covariates of prior spine surgery at a different spinal level, medical comorbidity (SACQ), or opioid use, time to resolution of leg pain remained significantly associated with recurrence (p <0.01 in all models; data not shown). When considering variables other than time to resolution of leg pain, only medical comorbidity was significantly associated with recurrence when another variable was included simultaneously in the model (p<0.05 in all models; data not shown). In the final multivariate model including both the predictor variables of time to resolution of leg pain (HR [95% CI] 1.21 [1.07-1.37]; p=0.003) and medical comorbidity (HR [95% CI] 1.03 [0.90-1.17]; p=0.72), only the former was significantly and independently associated with recurrence. The multivariate adjusted probability of leg pain recurrence was 20% (95% CI 9-30%) at both 1 year and 2 years.
Table 1.
Characteristics of Participants with Resolution of Lower Extremity Pain (n=79)
| Mean (SD) or N(%) | |
|---|---|
| Sociodemographic Factors and Medical History | |
|
| |
| Age (yrs.) | 54.5 (13.1) |
| Female | 29 (26.7%) |
| SACQ (0-45) | 2.6 (3.2) |
| Prior back pain history | 58 (73.4%) |
| Prior spine surgery at a different spinal level | 3 (3.8%) |
| Tobacco use | 14 (17.7%) |
| Worker’s compensation | 5 (6.3%) |
|
| |
| Physical Examination | |
|
| |
| Positive Straight Leg Raise | 28 (35.4%) |
| Positive Femoral Stretch Test | 17 (21.5%) |
|
| |
| MRI Characteristics | |
|
| |
| Midlumbar Disk Herniation (L1-4 levels) | 24 (30.4%) |
| Foraminal/Extraforaminal Herniation | 26 (32.9%) |
| Disk Extrusion | 53 (67.1%) |
| Disk Sequestration | 8(10.1%) |
|
| |
| Severity of the Clinical Presentation | |
|
| |
| Oswestry Disability Index (0-100) | 50 (20) |
| Visual Analogue Scale Leg Pain (0-10) | 6.9 (2.3) |
| Visual Analogue Scale Back Pain (0-10) | 4.9 (3.0) |
Mean (standard deviation) or N (%)
Median (interquartile range)
Statistically significant (p ≤ 0.05)
Includes ‘unemployed’ and ‘student’ status
SACQ – Self-administered Comorbidity Questionnaire
Figure 2.
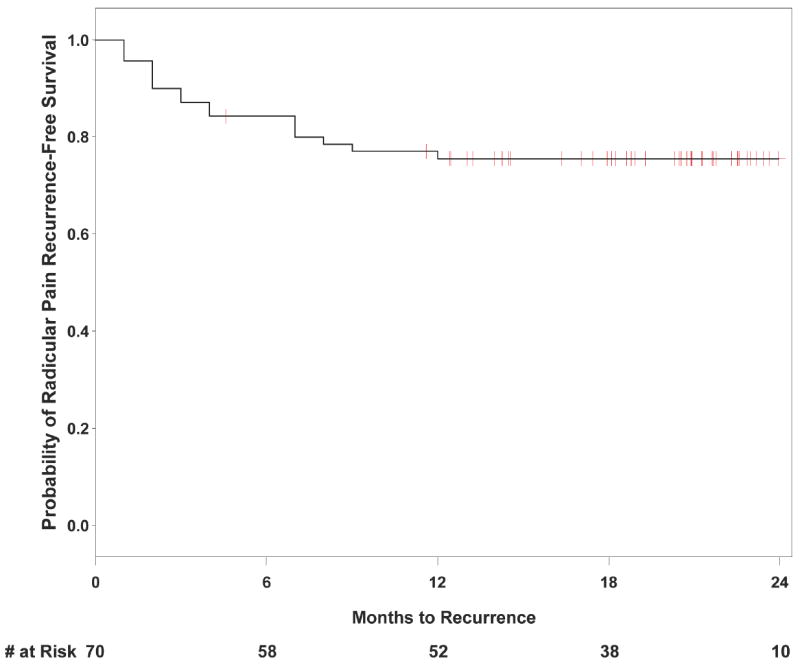
Kaplan-Meier Survival Curve for Recurrence of Radicular Pain (n=70)
Table 2.
Bivariate Associations between Sociodemographic and Clinical Characteristics, and Radicular Pain Recurrence
| Hazard Ratio (95% Confidence Interval) | p-value | |
|---|---|---|
| Sociodemographic Factors and Medical History | ||
|
| ||
| Age (yrs.) | 0.98 (0.95-1.02) | 0.31 |
| Female Sex | 1.49 (0.57-3.91) | 0.42 |
| Prior back pain history | 0.53 (0.20-1.44) | 0.14 |
| Prior spine surgery at a different spinal level | 4.02 (0.92-17.66) | 0.07 |
| Medical Comorbidity [SACQ] (0-45) | 1.13 (0.99-1.27) | 0.06 |
| Number of months to resolution of radicular pain | 1.24 (1.13-1.37) | <0.0001† |
| Tobacco use | 0.73 (0.17-3.23) | 0.68 |
| Worker’s Compensation status | 0.77 (0.10-5.84) | 0.80 |
|
| ||
| Physical Examination | ||
|
| ||
| Positive Straight Leg Raise | 1.91 (0.74-4.96) | 0.18 |
| Positive Femoral Stretch Test | 1.03 (0.34-3.16) | 0.96 |
|
| ||
| Magnetic Resonance Imaging Findings | ||
|
| ||
| Midlumbar or High Lumbar Disk Herniation (L1-L2, L2-L3, L3-L4 levels) | 0.61 (0.20-1.87) | 0.76 |
| Foraminal or Extraforaminal Herniation | 1.07 (0.40-2.90) | 0.89 |
| Disk Extrusion | 0.52 (0.19-6.26) | 0.71 |
| Disk Sequestration | 0.37 (0.04-3.07) | 0.36 |
|
| ||
| Severity of Initial Clinical Presentation | ||
|
| ||
| Oswestry Disability Index (0-100) | 0.53 (0.04-8.08) | 0.65 |
| Visual Analogue Scale Leg Pain (0-10) | 0.94 (0.77-1.16) | 0.59 |
|
| ||
| Specific Treatments Received | ||
|
| ||
| Oral corticosteroids | 1.16 (0.38-3.57) | 0.79 |
| Non-steroidal anti-inflammatory medication | 1.08 (0.42-2.80) | 0.88 |
| Opioids | 0.40 (0.14-1.13) | 0.08 |
| Physical therapy | 0.56 (0.21-1.44) | 0.23 |
| Interlaminar Epidural Steroid Injection | 1.08 (0.42-2.80 | 0.88 |
| Transforaminal Epidural Steroid Injection | 0.48 (0.14-1.68) | 0.25 |
Mean (standard deviation) or N (%)
Statistically significant (p ≤ 0.05)
SACQ – Self-administered Comorbidity Questionnaire
For the surgical cases who were not included in the analysis above, the 1-year cumulative incidence of recurrence after surgical decompression was 43%. In survival analyses using bivariate Cox proportional hazards models, surgical treatment was not significantly associated with leg pain recurrence (HR [95% CI] 1.99 [0.78-5.05]; p=0.15).
Sixty three participants had both back pain with their initial episode, as well as resolution of back pain with nonsurgical treatment, and were therefore eligible to have a recurrence of back pain. The 1-year cumulative incidence of back pain recurrence was 43% (95% CI 30-56%). Kaplan-Meier survival analysis of back pain recurrence demonstrated that recurrence times were evenly distributed over the first 12 months after resolution, and were infrequent thereafter (data not shown). In examining associations between specific factors and back pain recurrence, age in years was associated with a lower hazard of recurrence (HR per year 0.95 [95% CI 0.92-0.99]; p=0.006), and a positive straight leg raise was significantly associated with a greater hazard of recurrence (HR 3.27 [95% CI 1.46-7.36]; p=0.004). Other factors were not significantly associated with recurrence, and no trends towards such an association were seen (data not shown). In the final multivariate model including both age and the straight leg raise test, age remained independently associated with the hazard of back pain recurrence (HR per year 0.96 [95% CI 0.93-0.99]; p=0.02), but straight leg raise was not (HR 0.91 [95% CI 0.29-2.92]; p=0.88). The multivariate adjusted probability of back pain recurrence was 43% (95% CI 27-55%) at 1 year and 51% (95% CI 32-65%) at 2 years. For surgical cases not included in the analysis above, the 1-year cumulative incidence of recurrence after surgical decompression was also 43%. In survival analyses using bivariate Cox proportional hazards models, back pain recurrence did not differ according to whether a participant received surgical treatment (HR [95% CI] 0.91 [0.21-4.03]; p=0.90).
DISCUSSION
The primary finding of this study was that recurrent lower extremity radicular pain after nonsurgical treatment of acute LDH was relatively common, occurring in 25% of participants over 1 year following resolution of leg pain. The only factor that was strongly predictive of radicular pain recurrence was the duration of time to initial radicular pain resolution: participants who recovered more slowly were more likely to have a recurrence. Recurrent back pain was more common (43%) than recurrent radicular pain, and was predicted by younger age.
Recent work by Stanton and colleagues has drawn attention to major limitations in the literature on resolution of LBP and the measurement of LBP recurrence.5-7 These flaws noted by Stanton are also pertinent to studies of recurrence after LDH. Stanton found that the vast majority of studies examining LBP recurrence did not specify the minimum conditions needed to define resolution of pain, and did not use explicit definitions for what constitutes recurrence. Both these limitations apply to the only prior report of recurrence after nonsurgical LDH, contained in Weber’s seminal natural history study of LDH2. Weber reported a 24% incidence of ‘relapse’ within 4 years of an acute LDH episode, but did not define ‘relapse’, or specify whether this involved leg pain, back pain, or some other combination of subjective report or objective signs. Furthermore, Weber did not specify requirements for defining resolution of symptoms prior to their recurrence, which makes it impossible to distinguish persistent pain from a true recurrence. Nevertheless, our finding of a 25% 1-year cumulative incidence of recurrent lower extremity radicular pain is generally consistent with Weber’s estimate when chance is taken into account (our 95% CI ranges from 15-35%), and when considering that no radicular pain recurrences were noted after the first 12 months of follow-up (despite a total follow-up duration as long as 26 months). Our estimates of recurrence are not directly comparable with measures of frequency reported in an often-cited work by Tubach and colleagues, which found that 55% of industrial workers with sciatica had prevalent persistent and/or recurrent sciatica after 2 years. Factors in the study by Tubach and colleagues that limit comparability with our results include their use of a combined persistent/recurrent pain outcome that does not allow one to distinguish true recurrence from persistent pain, inclusion of both LBP and radiating lower extremity pain in the definition of sciatica used, and sampling irrespective of chronicity (our study was limited to individuals with acute symptomatic MR-confirmed LDH). In contrast, our study design has a number of methodologic strengths including 1) use of a specific definition of recovery such that only patients with known symptom resolution were included in the group ‘at-risk’ for recurrence, 2) specification of the symptoms which constituted recurrence, i.e. leg pain vs. back pain, and 3) the use of patient-centered self-reporting of symptom resolution and recurrence, rather than global assessments by clinicians. The findings of our study provide the only accurate estimates to date regarding the likelihood of pain recurrence after resolution of symptomatic LDH managed nonsurgically.
To our knowledge, this is also the first study to examine factors that are predictive of symptom recurrence after nonsurgical treatment of LDH. The only factor that was independently predictive of recurrent radicular pain was duration (in months) to initial resolution of radicular pain. This means that patients who took longer for their pain to resolve were also more likely to have their pain return; participants who did not have recurrence of leg pain within 6 months were unlikely to have recurrence later. This association between time to symptom resolution and likelihood of recurrence has never been noted in prior studies of symptom recurrence after surgical management of LDH. A variety of reasons may explain this. First, this may be in part due to the survival analysis methods used in the current study, which have been advocated for use in studies of LDH recurrence12, but have largely not been used. Survival analysis incorporates important information such as when symptoms resolve or recur, which is not captured in studies that report recurrence only in terms of cumulative incidence of a combined persistent/recurrent pain outcome11. Second, a link between time to resolution and leg pain recurrence may not have been noted in prior surgical studies of recurrence because some have defined recovery to be a 6-month pain-free period after surgery4,18; our data suggest that this definition of resolution would exclude the vast majority of individuals from being eligible for recurrence, and decrease the apparent frequency of recurrent symptoms. Third, time to symptom resolution without surgery may be fundamentally different from time to symptom resolution with surgery. That is, surgery itself may obscure measurement of this risk factor for symptom recurrence. Our finding that most clinical characteristics were not associated with recurrent symptoms is consistent with some reports from the surgical literature19,20.
Recurrent back pain after LDH was more common than recurrent leg pain. Time to back pain resolution was not associated with back pain recurrence in the same manner as with leg pain recurrence. Greater patient age decreased the hazard of back pain recurrence, and explained the association of a positive SLR at baseline with subsequent back pain recurrence. In other words, the association of a positive SLR with back pain recurrence was explained by confounding due to age. This is likely explained by a tendency to fewer positive SLR tests in older adults, due to a higher frequency of midlumbar/high lumbar disk herniations in older adults, and possibly to less neural tension.
There are some limitations to this research. First, the relatively small sample size of our study limited our statistical power, and our ability to control for many factors simultaneously. This was unfortunately a consequence of the fact that with this inception cohort, a large percentage of patients underwent surgery and/or did not report full recovery, and were therefore never eligible for assessment of recurrence after nonsurgical treatment. Based on our results, we expect that future studies examining nonsurgical LDH recurrence will need to recruit roughly twice as many subjects presenting with acute symptomatic LDH as the planned sample size of individuals followed for nonsurgical recurrence. It should be noted however that despite a relatively small sample size, a significant and robust association was noted between time to resolution of radicular pain and radicular pain recurrence. Second, although our response rate of 77% is within the range of what is generally considered acceptable for prospective epidemiological studies21, non-response bias is a possibility, despite the fact that responders and non-responders were similar in a wide range of characteristics. For these two reasons, future studies are needed to confirm our findings in a larger sample. Third, it should be noted that we examined leg and back pain symptom recurrence, and not anatomic LDH per se. We view this as appropriate, because disk herniations may often be present after LDH-related symptoms have resolved, and it is symptoms- not pathoanatomic changes on imaging- that distinguishes abnormal from normal22,23. Last, resolution of pain and recurrence of pain in our study was identified by patient self-report at up to three timepoints between 1- year and 2-years after seeking care for acute symptomatic LDH. As such, these events were identified by recall of past events, and may be affected by recall bias. We think it highly unlikely that participants would fail to remember resolution or recurrence entirely had these events occurred, since these events are generally quite noteworthy to patients in pain. Similarly, we think it unlikely that participants would remember events that in actuality did not happen. However, some degree of inaccuracy of the specific date when resolution or recurrence occurred is certainly possible. Although inaccuracy in measurement of these events should have been minimized by our frequent sampling frame, any resultant random error would be expected to result in bias towards the null; this therefore would not explain the fact that time to resolution was identified as an extremely strong predictor of leg pain recurrence in our study. Nevertheless, future observational studies are needed to replicate the findings presented here. These studies would benefit from a larger sample size inclusive of patients from more than one treatment facility, in contrast to our single-center study.
CONCLUSIONS
We present the first study of lower extremity radicular pain recurrence after recovery from acute LDH with nonsurgical treatment. This study reveals that radicular pain recurrence is common, and is more likely to happen in individuals who take longer to recover from the original episode. Recurrence is an important endpoint neglected in most prospective studies of nonsurgical LDH to date. Further research is warranted to study recurrence of LDH. Ideally, future studies should include comparative surgical and nonsurgical treatment groups, repeated serial assessment, and clear and appropriate definitions of recovery and recurrence.
Acknowledgments
The authors wish to thank the study participants for their time and effort.
Funding Sources:
Dr. Suri is funded by the Rehabilitation Medicine Scientist Training Program (RMSTP) and the National Institutes of Health (K12 HD001097-12). Dr Hunter is funded by an Australian Research Council Future Fellowship. Dr. Katz is funded in part by NIH/NIAMS P60 AR 047782.
The authors certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated, and that all financial and material support for this and work are clearly identified above.
Abbreviations
The following is the list of abbreviations used in the text:
- CI
Confidence Interval
- HR
Hazard Ratio
- LBP
Low back pain
- LDH
Lumbar disk herniation
- SACQ
Self-Administered Comorbidity Questionnaire
- SLR
straight leg raise test
References
- 1.Suri P, Hunter DJ, Jouve C, et al. Nonsurgical treatment of lumbar disk herniation: are outcomes different in older adults? J Am Geriatr Soc. 59:423–9. doi: 10.1111/j.1532-5415.2011.03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine. 1983;8:131–40. [PubMed] [Google Scholar]
- 3.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. Jama. 2006;296:2451–9. doi: 10.1001/jama.296.20.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JK, Amorosa L, Cho SK, Weidenbaum M, Kim Y. Recurrent lumbar disk herniation. J Am Acad Orthop Surg. 2010;18:327–37. doi: 10.5435/00124635-201006000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Stanton TR, Henschke N, Maher CG, Refshauge KM, Latimer J, McAuley JH. After an episode of acute low back pain, recurrence is unpredictable and not as common as previously thought. Spine (Phila Pa 1976) 2008;33:2923–8. doi: 10.1097/BRS.0b013e31818a3167. [DOI] [PubMed] [Google Scholar]
- 6.Stanton TR, Latimer J, Maher CG, Hancock M. Definitions of recurrence of an episode of low back pain: a systematic review. Spine (Phila Pa 1976) 2009;34:E316–22. doi: 10.1097/BRS.0b013e318198d073. [DOI] [PubMed] [Google Scholar]
- 7.Stanton TR, Latimer J, Maher CG, Hancock MJ. A modified Delphi approach to standardize low back pain recurrence terminology. Eur Spine J. 2011;20:744–52. doi: 10.1007/s00586-010-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasiak R, Pransky G, Verma S, Webster B. Recurrence of low back pain: definition-sensitivity analysis using administrative data. Spine (Phila Pa 1976) 2003;28:2283–91. doi: 10.1097/01.BRS.0000085032.00663.83. [DOI] [PubMed] [Google Scholar]
- 9.Wasiak R, Young AE, Dunn KM, et al. Back pain recurrence: an evaluation of existing indicators and direction for future research. Spine (Phila Pa 1976) 2009;34:970–7. doi: 10.1097/BRS.0b013e3181a01b63. [DOI] [PubMed] [Google Scholar]
- 10.Tubach F, Beaute J, Leclerc A. Natural history and prognostic indicators of sciatica. J Clin Epidemiol. 2004;57:174–9. doi: 10.1016/S0895-4356(03)00257-9. [DOI] [PubMed] [Google Scholar]
- 11.McGirt MJ, Ambrossi GL, Datoo G, et al. Recurrent disc herniation and long-term back pain after primary lumbar discectomy: review of outcomes reported for limited versus aggressive disc removal. Neurosurgery. 2009;64:338–44. doi: 10.1227/01.NEU.0000337574.58662.E2. discussion 44-5. [DOI] [PubMed] [Google Scholar]
- 12.Gaston P, Marshall RW. Survival analysis is a better estimate of recurrent disc herniation. J Bone Joint Surg Br. 2003;85:535–7. doi: 10.1302/0301-620x.85b4.13813. [DOI] [PubMed] [Google Scholar]
- 13.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Medical care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Fardon DF. Nomenclature and classification of lumbar disc pathology. Spine. 2001;26:461–2. doi: 10.1097/00007632-200103010-00007. [DOI] [PubMed] [Google Scholar]
- 15.Suri P, Rainville J, Katz JN, et al. The Accuracy of the Physical Examination for the Diagnosis of Midlumbar and Low Lumbar Nerve Root Impingement. Spine (Phila Pa 1976) 2010 doi: 10.1097/BRS.0b013e3181c953cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain. 1997;72:95–7. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 17.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25:2940–52. doi: 10.1097/00007632-200011150-00017. discussion 52. [DOI] [PubMed] [Google Scholar]
- 18.Suk KS, Lee HM, Moon SH, Kim NH. Recurrent lumbar disc herniation: results of operative management. Spine (Phila Pa 1976) 2001;26:672–6. doi: 10.1097/00007632-200103150-00024. [DOI] [PubMed] [Google Scholar]
- 19.Carragee EJ, Han MY, Suen PW, Kim D. Clinical outcomes after lumbar discectomy for sciatica: the effects of fragment type and anular competence. The Journal of bone and joint surgery. 2003;85-A:102–8. [PubMed] [Google Scholar]
- 20.Quigley MR, Bost J, Maroon JC, Elrifai A, Panahandeh M. Outcome after microdiscectomy: results of a prospective single institutional study. Surgical neurology. 1998;49:263–7. doi: 10.1016/s0090-3019(97)00448-5. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 21.Fewtrell MS, Kennedy K, Singhal A, et al. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch Dis Child. 2008;93:458–61. doi: 10.1136/adc.2007.127316. [DOI] [PubMed] [Google Scholar]
- 22.Lebow RL, Adogwa O, Parker SL, Sharma A, Cheng J, McGirt MJ. Asymptomatic same-site recurrent disc herniation after lumbar discectomy: Results of a prospective longitudinal study with two-year serial imaging. Spine (Phila Pa 1976) 2011 doi: 10.1097/BRS.0b013e3182054595. [DOI] [PubMed] [Google Scholar]
- 23.van Rijn JC, Klemetso N, Reitsma JB, et al. Symptomatic and asymptomatic abnormalities in patients with lumbosacral radicular syndrome: Clinical examination compared with MRI. Clin Neurol Neurosurg. 2006;108:553–7. doi: 10.1016/j.clineuro.2005.10.003. [DOI] [PubMed] [Google Scholar]


