Abstract
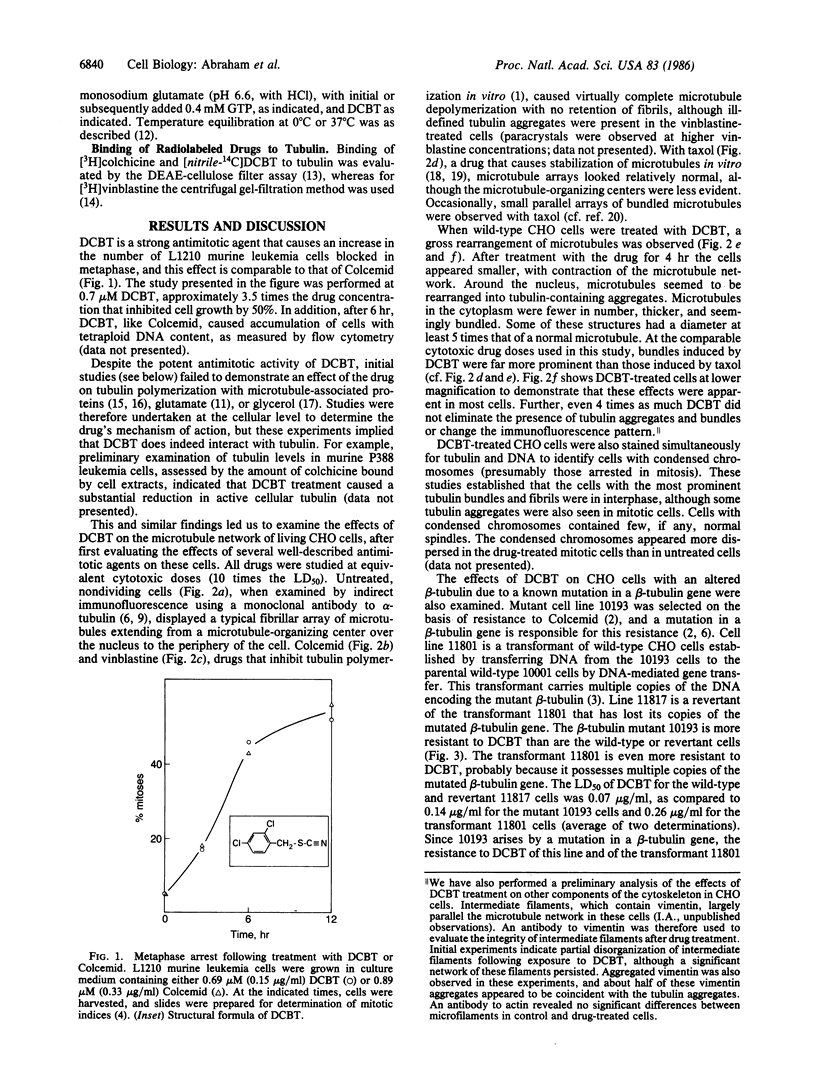
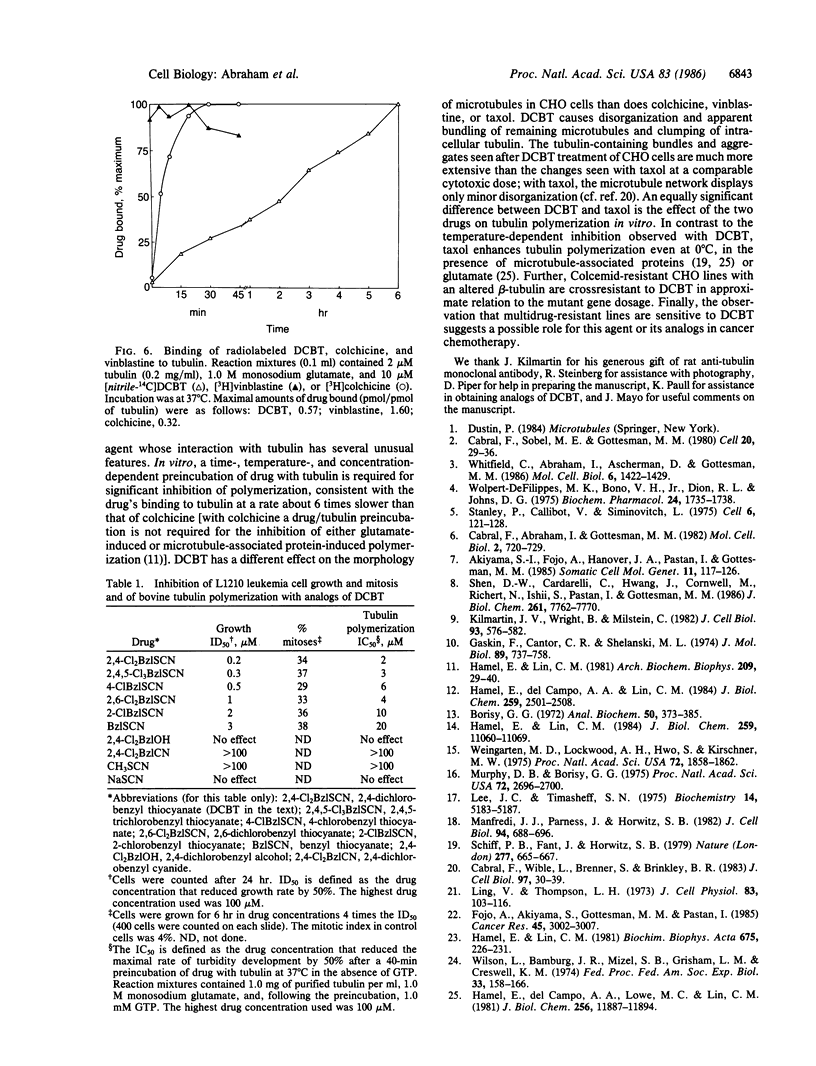
A compound of simple structure, 2,4-dichlorobenzyl thiocyanate (DCBT), is an antimitotic agent with a number of unusual properties. The drug causes an extreme reorganization of microtubules in cells in culture. Most normal microtubules disappear, and remaining tubulin-containing structures appear to be bundled or aggregated. DCBT irreversibly inhibits in vitro polymerization of purified tubulin, but only after a prolonged preincubation of the protein with the drug. Binding of radiolabeled DCBT to tubulin similarly requires a long incubation time, with the reaction not being complete even after 6 hr at 37 degrees C. A specific interaction with tubulin is also shown by the crossresistance to DCBT of Colcemid-resistant cells with an altered beta-tubulin. A human KB carcinoma cell line and a Chinese hamster ovary cell line selected for crossresistance to multiple chemotherapeutic agents, including most antimitotic drugs, are sensitive to DCBT. Initial structure-function studies have demonstrated weak antimitotic and antitubulin activity with the parent compound benzyl thiocyanate. Chlorination at either position 2 or position 4 of the phenyl ring produces compounds of intermediate activity (4-chlorobenzyl thiocyanate is more active than 2-chlorobenzyl thiocyanate). The thiocyanate moiety appears to be essential for activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S., Fojo A., Hanover J. A., Pastan I., Gottesman M. M. Isolation and genetic characterization of human KB cell lines resistant to multiple drugs. Somat Cell Mol Genet. 1985 Mar;11(2):117–126. doi: 10.1007/BF01534700. [DOI] [PubMed] [Google Scholar]
- Borisy G. G. A rapid method for quantitative determination of microtubule protein using DEAE-cellulose filters. Anal Biochem. 1972 Dec;50(2):373–385. doi: 10.1016/0003-2697(72)90046-2. [DOI] [PubMed] [Google Scholar]
- Cabral F., Abraham I., Gottesman M. M. Revertants of a Chinese hamster ovary cell mutant with an altered beta-tubulin: evidence that the altered tubulin confers both colcemid resistance and temperature sensitivity on the cell. Mol Cell Biol. 1982 Jun;2(6):720–729. doi: 10.1128/mcb.2.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral F., Sobel M. E., Gottesman M. M. CHO mutants resistant to colchicine, colcemid or griseofulvin have an altered beta-tubulin. Cell. 1980 May;20(1):29–36. doi: 10.1016/0092-8674(80)90231-7. [DOI] [PubMed] [Google Scholar]
- Cabral F., Wible L., Brenner S., Brinkley B. R. Taxol-requiring mutant of Chinese hamster ovary cells with impaired mitotic spindle assembly. J Cell Biol. 1983 Jul;97(1):30–39. doi: 10.1083/jcb.97.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojo A., Akiyama S., Gottesman M. M., Pastan I. Reduced drug accumulation in multiply drug-resistant human KB carcinoma cell lines. Cancer Res. 1985 Jul;45(7):3002–3007. [PubMed] [Google Scholar]
- Gaskin F., Cantor C. R., Shelanski M. L. Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol. 1974 Nov 15;89(4):737–755. doi: 10.1016/0022-2836(74)90048-5. [DOI] [PubMed] [Google Scholar]
- Hamel E., Lin C. M. Glutamate-induced polymerization of tubulin: characteristics of the reaction and application to the large-scale purification of tubulin. Arch Biochem Biophys. 1981 Jun;209(1):29–40. doi: 10.1016/0003-9861(81)90253-8. [DOI] [PubMed] [Google Scholar]
- Hamel E., Lin C. M. Guanosine 5'-O-(3-thiotriphosphate), a potent nucleotide inhibitor of microtubule assembly. J Biol Chem. 1984 Sep 10;259(17):11060–11069. [PubMed] [Google Scholar]
- Hamel E., Lin C. M. Stabilization of the colchicine-binding activity of tubulin by organic acids. Biochim Biophys Acta. 1981 Jul;675(2):226–231. doi: 10.1016/0304-4165(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Hamel E., del Campo A. A., Lin C. M. Stability of tubulin polymers formed with dideoxyguanosine nucleotides in the presence and absence of microtubule-associated proteins. J Biol Chem. 1984 Feb 25;259(4):2501–2508. [PubMed] [Google Scholar]
- Hamel E., del Campo A. A., Lowe M. C., Lin C. M. Interactions of taxol, microtubule-associated proteins, and guanine nucleotides in tubulin polymerization. J Biol Chem. 1981 Nov 25;256(22):11887–11894. [PubMed] [Google Scholar]
- Kilmartin J. V., Wright B., Milstein C. Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J Cell Biol. 1982 Jun;93(3):576–582. doi: 10.1083/jcb.93.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. The reconstitution of microtubules from purified calf brain tubulin. Biochemistry. 1975 Nov 18;14(23):5183–5187. doi: 10.1021/bi00694a025. [DOI] [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Manfredi J. J., Parness J., Horwitz S. B. Taxol binds to cellular microtubules. J Cell Biol. 1982 Sep;94(3):688–696. doi: 10.1083/jcb.94.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Borisy G. G. Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2696–2700. doi: 10.1073/pnas.72.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff P. B., Fant J., Horwitz S. B. Promotion of microtubule assembly in vitro by taxol. Nature. 1979 Feb 22;277(5698):665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Shen D. W., Cardarelli C., Hwang J., Cornwell M., Richert N., Ishii S., Pastan I., Gottesman M. M. Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. J Biol Chem. 1986 Jun 15;261(17):7762–7770. [PubMed] [Google Scholar]
- Stanley P., Caillibot V., Siminovitch L. Selection and characterization of eight phenotypically distinct lines of lectin-resistant Chinese hamster ovary cell. Cell. 1975 Oct;6(2):121–128. doi: 10.1016/0092-8674(75)90002-1. [DOI] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C., Abraham I., Ascherman D., Gottesman M. M. Transfer and amplification of a mutant beta-tubulin gene results in colcemid dependence: use of the transformant to demonstrate regulation of beta-tubulin subunit levels by protein degradation. Mol Cell Biol. 1986 May;6(5):1422–1429. doi: 10.1128/mcb.6.5.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L., Bamburg J. R., Mizel S. B., Grisham L. M., Creswell K. M. Interaction of drugs with microtubule proteins. Fed Proc. 1974 Feb;33(2):158–166. [PubMed] [Google Scholar]
- Wolpert-DeFilippes M. K., Bono V. H., Jr, Dion R. L., Johns D. G. Initial studies on maytansine-induced metaphase arrest in L1210 murine leukemia cells. Biochem Pharmacol. 1975 Sep 15;24(18):1735–1738. doi: 10.1016/0006-2952(75)90017-9. [DOI] [PubMed] [Google Scholar]








