Patients with median lobe enlargement of the prostate have different natural history of lower urinary tract symptoms following robotic prostatectomy as compared with patients without this finding.
Keywords: Lower urinary tract symptoms, Urinary incontinence, Radical prostatectomy, Benign prostatic hyperplasia
Abstract
Background:
We report on the natural history of lower urinary tract symptoms (LUTS) and urinary continence in patients with median lobe enlargement (MLE) after robotic radical prostatectomy (RP).
Methods:
Patients treated with RP from October 2008 to March 2012 completed American Urological Association symptom index (AUAI) and continence assessments at the preoperative visit and each postoperative visit. Two cohorts were established based on the presence or absence of a median lobe intraoperatively.
Results:
A total of 698 validated questionnaires were completed by 175 patients with a median of 4 AUAI scores per patient. The 36 patients (21%) with MLE required a longer time to achieve urinary continence (P = .05, log-rank test), although ultimately, no difference was seen in long-term continence probability between the two cohorts (P = .63). On multivariate analysis, the presence of a median lobe reduced the odds of early continence recovery (P = .02). By use of a generalized estimating equation, the cohort-average AUAI scores after RP are presented. Patients with MLE had faster improvement in LUTS after surgery, whereas those without MLE had temporary worsening in LUTS before improvement.
Conclusion:
Patients with MLE have a different natural history of LUTS and continence after RP as compared with patients without this finding. Therefore, radiographic or cystoscopic evaluation for the presence of a median lobe before RP may improve patient counseling about urinary outcomes.
INTRODUCTION
Prostate cancer is the most common noncutaneous malignancy in American men, with a median age at diagnosis of 68 years.1,2 In this age group, many men are simultaneously afflicted with lower urinary tract symptoms (LUTS) due to prostatic hyperplasia. Median lobe enlargement (MLE) is an anatomic form of hyperplasia in which prostate tissue protrudes intravesically.3 MLE has been associated with a higher risk of urinary retention and failure of nonsurgical therapies for LUTS.4
Though primarily a cancer surgery, radical prostatectomy (RP) has the added benefit of improving LUTS in most patients.5 However, RP's effect in patients with MLE has not been well elucidated. We hypothesized that this subset of patients might have a different natural history of urinary symptoms after surgery compared with men without MLE. To date, most studies of RP in patients with MLE have focused largely on surgical technique and certain intraoperative parameters.6,7
The purpose of this investigation was to study the evolution of LUTS after RP in men with MLE. Our goal was to determine what differences such patients might expect in terms of urinary symptom improvement and continence recovery compared with those without this finding. We believed that such data might be clinically useful and improve patient counseling before RP.
METHODS
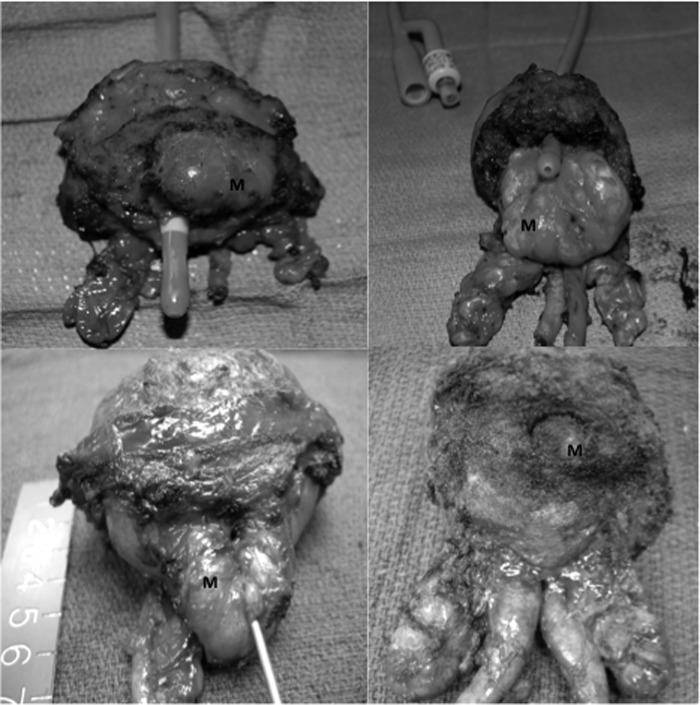
After approval by the institutional Human Research Review Committee, we retrospectively reviewed the charts of all patients who had undergone robot-assisted RP at a single institution from October 2008 through March 2012. As part of routine care, patients had completed questionnaires at the preoperative visit and every postoperative visit, including (1) American Urological Association symptom index (AUAI) and (2) continence assessment. Typically, follow-up visits were at 1, 3, 6, 9, and 12 months postoperatively, although not every patient adhered to this schedule. Two cohorts of patients were established based on the presence (MLE) or absence (N-MLE) of a median lobe, defined as any degree of intravesical prostatic protrusion visualized intraoperatively. MLE was identified and recorded on the operative report prospectively. Examples of the wide variation in the degree of MLE encountered are shown in Figure 1. Urinary continence was defined as the use of 0 to 1 pads per day. Patients were considered disease free if the most recent prostate-specific antigen level was <0.1 ng/mL, without adjuvant or salvage therapies.
Figure 1.
Variations in median lobes (M). A, Eccentric right-sided anterior median lobe. B, Wide posterior median lobe extending intravesically for >4 cm. C, Posterior median lobe protruding 2 cm intravesically. D, Small posterior median lobe.
Surgical Technique
Robotic-assisted RP was performed with a reduced-port technique by use of 4 or 5 trocars for most cases. For proper exposure of the posterior bladder neck, a No. 0 Vicryl tie (Ethicon, Somerville, New Jersey, USA) was tied loosely through the eye of an 18-F council tip catheter at the beginning of the surgery. After division of the anterior bladder neck, the catheter balloon was deflated and the suture was exteriorized in the suprapubic region by use of a port closure device to provide countertraction and exposure. In cases of MLE, the bladder mucosa was divided high on the median lobe and the mucosa was carefully dissected away from the hyperplastic tissue, leaving all prostatic tissue en bloc with the specimen. In some cases, upward traction was placed on the median lobe with suture or a robotic cobra grasper. Single-layer vesico-urethral anastomosis was performed by use of poliglecaprone sutures tied together in a classic Van Velthoven manner. No bladder neck reconstruction was performed. When the bladder neck and urethra caliber were obviously discrepant, we compensated by increasing the distance between each suture pass in the bladder.
Statistical Analysis
Continuous parameters of the two cohorts were compared by use of the Mann-Whitney U test. Dichotomous variables were compared by use of the Fisher exact test. The Kaplan-Meier method and the log-rank test were used to compare the time interval to urinary continence recovery between the two cohorts. Logistic regression analysis was performed to determine which patient and tumor factors were associated with early return of continence (≤3 months postoperatively). The natural history of LUTS after RP was delineated graphically with generalized estimating equation (GEE) models derived from AUAI scores obtained at each visit. Because of variation in patient follow-up, each graphed time point represents the midpoint of a time interval of follow-up. For GEE analysis, we excluded patients in whom bladder neck contractures developed and those without a minimum of one preoperative and one postoperative AUAI score. GEE was used in lieu of standard regression analysis to account for the correlation among repeated AUAI scores in each patient, which were not independent observations. Statistical analysis was performed by use of STATA version 11.2 for Windows (StataCorp, College Station, Texas, USA). P < .05 and odds ratios whose 95% confidence interval excluded 1 were considered significant.
RESULTS
A total of 698 validated questionnaires were completed by 175 patients, over a median follow-up period of 25 months (range, 4–45 months). At least 1 preoperative and 1 postoperative AUAI score was available in 160 patients (91%), with a median of 4 AUAI scores per patient (range, 1–8 scores). MLE was noted in 36 patients (21%). At last follow-up, 31 MLE patients (86%) and 127 N-MLE patients (91%) were disease free. The MLE cohort was older (median age, 64 years vs 59 years; P < .001), was more frequently receiving medical therapy for LUTS (36% vs 6%, P < .0001), had a larger RP specimen weight (median, 62 g vs 47 g; P < .001), and had a higher frequency of disease with a Gleason score of 6 (67% vs 46%, P = .04) compared with those without MLE. Other demographic and tumor characteristics are presented in Table 1.
Table 1.
Cohort Characteristics
| MLE (n = 36) | No MLE (n = 139) | P Value | |
|---|---|---|---|
| Median age (y) | 64 (55–77) | 59 (42–74) | <.001 |
| Median BMIa (kg/m2) | 27.3 (21.7–37.9) | 27.2 (19.4–37.6) | .62 |
| Median ASAa score | 2 (2–3) | 2 (1–4) | .94 |
| Median PSAa (ng/mL) | 6.0 (1.7–13.2) | 5.4 (1.1–37.2) | .40 |
| No. receiving α-blocker and/or 5-ALPHA REDUCTASE INHIBITOR | 13 (36%) | 8 (6%) | <.0001 |
| No. with Gleason score of 6 | 24 (67%) | 64 (46%) | .04 |
| Median prostate weight (g) | 62 (27–125) | 47 (20–103) | <.001 |
| Median surgery time (min) | 256 (206–377) | 249 (185–358) | .37 |
| No. with bladder neck contracture | 0 | 4 (3%) | .58 |
| No. with stage pT3x | 3 (8%) | 30 (22%) | .09 |
| No. with positive surgical margin | 3 (8%) | 25 (18%) | .20 |
ASA = American Society of Anesthesiologists; BMI = body mass index; PSA = prostate-specific antigen.
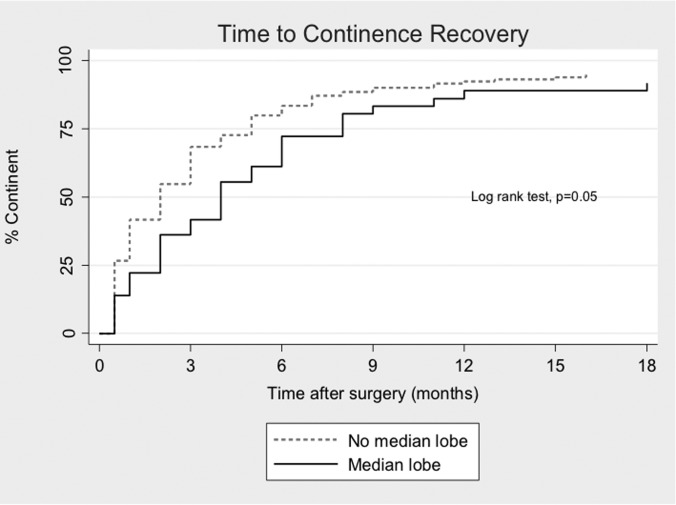
After RP, 34 of 36 MLE patients (94%) and 134 of 139 N-MLE patients (96%) regained continence (P = .63). However, as shown in Figure 2, patients with MLE required a longer time to achieve urinary recovery compared with those without MLE (P = .05). The median time to continence was 4 months in the MLE cohort and 2 months in the N-MLE cohort (P = .02). Age >65 years and the presence of MLE were associated with reduced odds of early continence recovery on univariate analysis, but only the presence of MLE continued to have a significant independent influence on multivariate analysis (Table 2).
Figure 2.
Recovery of urinary continence in patients with and without MLE by use of Kaplan-Meier method.
Table 2.
Predictors of Early Continence Recovery After RP
| Characteristic | Univariate |
Multivariate |
||
|---|---|---|---|---|
| ORa (95% CIa) | P Value | Adjusted OR (95% CI) | P Value | |
| Patient age (y) | ||||
| <50 | 1.0 (reference) | — | 1.0 (reference) | — |
| 51–64 | 0.30 (0.08–1.09) | .07 | 0.38 (0.10–1.52) | .17 |
| ≥65 | 0.24 (0.06–0.93) | .04 | 0.37 (0.09–1.59) | .18 |
| BMIa (kg/m2) | ||||
| <30 | 1.0 (reference) | — | 1.0 (reference) | — |
| ≥30 | 0.80 (0.39–1.63) | .53 | 0.89 (0.40–1.98) | .78 |
| PSAa (ng/mL) | ||||
| <10 | 1.0 (reference) | — | 1.0 (reference) | — |
| ≥10 | 0.92 (0.40–2.12) | .85 | 1.03 (0.36–2.90) | .96 |
| Gleason score | ||||
| 6 | 1.0 (reference) | — | 1.0 (reference) | — |
| ≥7 | 0.81 (0.44–1.49) | .50 | 0.61 (0.28–1.36) | .23 |
| Surgeon experience (cases) | ||||
| ≤50 | 1.0 (reference) | — | 1.0 (reference) | — |
| 51–100 | 1.65 (0.74–3.71) | .22 | 1.69 (0.60–4.79) | .32 |
| 101–150 | 1.51 (0.68–3.38) | .31 | 1.27 (0.44–3.66) | .66 |
| 151–175 | 1.81 (0.66–4.96) | .25 | 1.21 (0.36–4.15) | .76 |
| MLE | ||||
| No | 1.0 (reference) | — | 1.0 (reference) | — |
| Yes | 0.34 (0.16–0.73) | .005 | 0.32 (0.13–0.81) | .02 |
| Bladder neck sparing | ||||
| No | 1.0 (reference) | — | 1.0 (reference) | — |
| Yes | 1.60 (0.86–2.97) | .13 | 1.53 (0.74–3.20) | .25 |
| Posterior reconstruction | ||||
| No | 1.0 (reference) | — | 1.0 (reference) | — |
| Yes | 0.67 (0.23–1.93) | .46 | 0.76 (0.18–3.18) | .71 |
| Potency nerve sparing | ||||
| None | 1.0 (reference) | — | 1.0 (reference) | — |
| Unilateral | 1.07 (0.42–2.70) | .89 | 0.93 (0.31–2.75) | .90 |
| Bilateral | 1.55 (0.71–3.35) | .27 | 1.20 (0.43–3.35) | .73 |
| Prostate weight (g) | ||||
| <50 | 1.14 (0.61–2.13) | .69 | 0.69 (0.31–1.55) | .38 |
| 50–79.9 | 1.0 (reference) | — | 1.0 (reference) | — |
| ≥80 | 0.80 (0.20–3.21) | .75 | 1.20 (0.22–6.55) | .84 |
| Pathologic stage | ||||
| pT2x | 1.0 (reference) | — | 1.0 (reference) | — |
| pT3x | 1.26 (0.57–2.81) | .56 | 1.51 (0.58–3.91) | .40 |
BMI = body mass index; CI = confidence interval; OR, odds ratio; PSA = prostate-specific antigen.
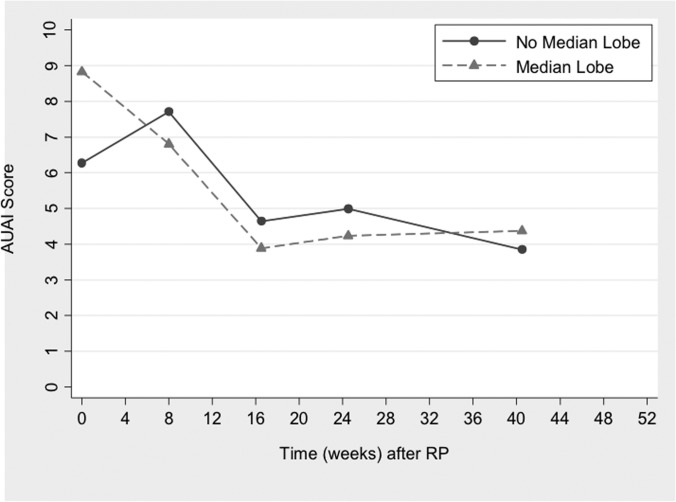
The natural history of urinary symptoms after RP is presented graphically in Figure 3, excluding patients without sufficient follow-up and those in whom bladder neck contractures developed. Patients with MLE had faster improvement in LUTS and reached a nadir AUAI score at 4.5 months. Interesting, those without MLE had a temporary worsening of LUTS in the initial months after surgery. At 11 months postoperatively, the AUAI score was similar regardless of cohort, with 32 of 34 MLE patients (94%) and 100 of 122 N-MLE patients (82%) achieving equal or lower AUAI scores compared with before surgery (P = .11). However, those with MLE had a greater reduction in AUAI scores (4.9 points vs 2.42 points) compared with those without MLE.
Figure 3.
Natural history of LUTS after RP with cohort-average changes in AUAI score by use of GEE.
DISCUSSION
This study adds to the current body of knowledge regarding RP by highlighting the differences that patients with MLE can expect in terms of LUTS and urinary continence recovery. We found faster improvement in LUTS in patients with MLE, whereas those without this finding actually had initial worsening in LUTS. In this study MLE independently reduced the odds of early return of urinary continence. For these reasons, presurgery identification of MLE, with ultrasonography, endorectal magnetic resonance imaging, or cystoscopy, may provide information that improves patient counseling regarding urinary recovery. Indeed, a population-based study reported MLE to be present in about 40% of the study cohort, suggesting that our findings may be applicable to a large number of patients.8
An underreported aspect of RP is the temporary worsening of LUTS it can cause in some men. Many large RP series have either focused entirely on continence or reported only long-term American Urological Association symptom scores rather than the month-by-month variation during the recovery period, thereby missing this important finding.5,9 In our study we noted worsening of LUTS in the initial months after RP in patients without MLE but were unable to characterize this further. Similarly, Namiki et al.10 reported increased irritative voiding symptoms (nocturia and frequency) in some men after RP. Wang et al.11 also noted some worsening in urinary symptoms 3 months after RP in patients with mild LUTS preoperatively. Hence another “take-home” message from our study is the importance of counseling patients about this temporary worsening in urinary symptoms after RP in patients without MLE.
A strength of this study is the use of the GEE to track the evolution of LUTS after RP. This methodology is robust because it accounts for repeated measures in the same individual. We noted faster improvement in LUTS after RP in men with MLE. Other authors have noted a correlation between the preoperative severity of LUTS and the speed at which urinary symptoms improve after surgery, but they have not specifically addressed patients with MLE.11,12 Henderson et al.13 showed improved flow rates and IPSS scores in patients after RP, although their methodology treated these scores as independent measures. Another study of patients undergoing robotic RP graphically showed how LUTS change after RP but reported median International Prostate Symptom Score score at set time points and did not account for repeated measures from the same individual.14
In men with bladder outlet obstruction from benign disease, MLE portends a 3 times greater chance of needing medications to treat LUTS.8 Other studies have shown that significant intravesical prostatic protrusion puts men at higher risk of clinical progression, yields less success with oral therapy, and puts men at higher risk of acute urinary retention.14,15 In context with our study, men with MLE and prostate cancer may be ideal candidates for RP in that both the cancer and urinary problems can be treated with one procedure. It is noteworthy that MLE patients had more favorable tumor characteristics than those without this finding, consistent with what has been reported for patients with large prostate gland size.16 Although the reasons for this are unclear, patients with benign prostatic hyperplasia can have higher prostate-specific antigen values, introducing lead-time bias.
Several caveats of this study deserve mention. First, the MLE patients represented a heterogeneous group because the degree of intravesical prostatic protrusion was not measured. Second, we had a moderately sized study population, but this was offset by a high number of data points related to LUTS due to close follow-up after RP. Third, although LUTS were measured with a validated questionnaire, incontinence was not. All data were still patient reported, not physician reported, thereby reducing bias. Fourth, MLE was related to gland size and age, with only a few patients having isolated MLE. Therefore, we could not statistically determine MLE's independent influence on urinary symptoms. Lastly, although we adjusted for intraoperative factors that could have influenced urinary outcomes (bladder neck sparing, posterior reconstruction), nuances of RP technique among surgeons could result in alternate findings.6
CONCLUSIONS
Patients with MLE have a different natural history of LUTS and urinary continence after RP as compared with patients without this finding. After surgery, such patients have (1) a longer time to achieve continence and (2) faster improvement in LUTS compared with patients without MLE. These differences may warrant radiographic or cystoscopic evaluation for the presence of a median lobe before RP to improve patient counseling about urinary outcomes.
Contributor Information
Satyan K. Shah, Department of Surgery, University of New Mexico School of Medicine, Albuquerque, NM, USA..
Trisha Fleet, Department of Surgery, University of New Mexico School of Medicine, Albuquerque, NM, USA..
Betty Skipper, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque, NM, USA..
References:
- 1. American Cancer Society: Cancer Facts and Figures 2008. Available at: http://www.cancer.org/acs/groups/content/@nho/ documents/document/2008cafffinalsecuredpdf.pdf Accessed July 28, 2012
- 2. SEER Cancer Statistics Review (1975–2007). Available at: http://www.seer.cancer.gov/csr/1975_2007/index.html Accessed July 28, 2012
- 3. Roehrborn CG. Pathology of benign prostatic hyperplasia. Int J Impot Res. 2008;20(Suppl 3):S11–S18 [DOI] [PubMed] [Google Scholar]
- 4. Tan YH, Foo KT. Intravesical prostatic protrusion predicts the outcome of a trial without catheter following acute urine retention. J Urol. 2003;170:2339–2341 [DOI] [PubMed] [Google Scholar]
- 5. Slova D, Lepor H. The short-term and long-term effects of radical prostatectomy on lower urinary tract symptoms. J Urol. 2007;178:2397–2400 [DOI] [PubMed] [Google Scholar]
- 6. Coelho RF, Chauhan S, Guglielmetti GB, et al. Does the presence of median lobe affect outcomes of robot-assisted laparoscopic radical prostatectomy? J Endourol. 2012;26:264–270 [DOI] [PubMed] [Google Scholar]
- 7. Huang AC, Kowalczyk KJ, Hevelone ND, et al. The impact of prostate size, median lobe, and prior benign prostatic hyperplasia intervention on robot-assisted laparoscopic prostatectomy: technique and outcomes. Eur Urol. 2011;59:595–603 [DOI] [PubMed] [Google Scholar]
- 8. Lieber MM, Jacobson DJ, McGree ME, St Sauver JL, Girman CJ, Jacobsen SJ. Intravesical prostatic protrusion in men in Olmsted County, MN. J Urol. 2009;182:2819–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037–1063 [DOI] [PubMed] [Google Scholar]
- 10. Namiki S, Saito S, Ishidoya S, et al. Adverse effect of radical prostatectomy on nocturia and voiding frequency symptoms. Urology. 2005;66:147–151 [DOI] [PubMed] [Google Scholar]
- 11. Wang L, Chung SF, Yip SK, Lau WK, Cheng CW, Sim HG. The natural history of voiding function after robot-assisted laparoscopic radical prostatectomy. Urol Oncol. 2011;29:177–182 [DOI] [PubMed] [Google Scholar]
- 12. Schwartz EJ, Lepor H. Radical retropubic prostatectomy reduces symptom scores and improves quality of life in men with moderate and severe lower urinary tract symptoms. J Urol. 1999;161:1185–1188 [PubMed] [Google Scholar]
- 13. Henderson A, Laing RW, Langley SE. Improvement in urinary symptoms after radical prostatectomy: a prospective evaluation of flow rates and symptom scores. BJU Int. 2004;93:180–181 [DOI] [PubMed] [Google Scholar]
- 14. Lee LS, Sim HG, Lim KB, Wang D, Foo KT. Intravesical prostatic protrusion predicts clinical progression of benign prostatic enlargement in patients receiving medical treatment. Int J Urol. 2010;17:69–74 [DOI] [PubMed] [Google Scholar]
- 15. Mariappan P, Brown DJ, McNeill AS. Intravesical prostatic protrusion is better than prostate volume in predicting the outcome of trial without catheter in white men presenting with acute urinary retention: a prospective clinical study. J Urol. 2007;178:573–577 [DOI] [PubMed] [Google Scholar]
- 16. Link BA, Nelson R, Josephson DY, et al. The impact of prostate gland weight in laparoscopic radical prostatectomy. J Urol. 2008;180:928–932 [DOI] [PubMed] [Google Scholar]