Abstract
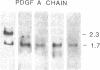
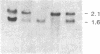
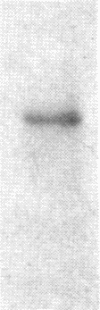
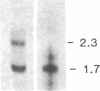
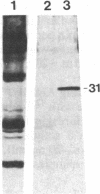
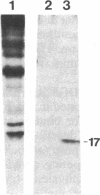
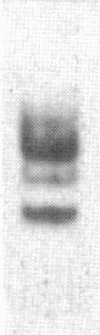
It is shown here that the myogenic cell line L6J1, primary skeletal myoblasts, and primary adult arterial smooth muscle cells express the gene for the A chain but not the gene for the B chain (c-sis) of platelet-derived growth factor (PDGF). It is further demonstrated that conditioned media from L6J1 cultures contain material that (i) competes with 125I-labeled PDGF for binding to human fibroblasts, (ii) is specifically precipitated by antibodies against PDGF, and (iii) has a relative molecular mass comparable to that of PDGF and, after reduction, its constituent subunit chains. The secretion of PDGF-receptor-competing activity was at a maximum in exponentially growing cultures but remained at a high level also after the cells had become confluent, stopped dividing, and fused to form multinucleate myotubes. Similarly, it was previously demonstrated that adult rat arterial smooth muscle cells in primary culture produce a mitogenic protein with immunological and structural properties similar to PDGF. In accordance with these findings, it was recently shown that secretion of PDGF-like mitogens by a number of human tumor cell lines correlates with expression of the gene for the A chain rather than the B chain of PDGF. The results suggest that production of homodimers of PDGF A chains may stimulate proliferation of skeletal myoblasts and arterial smooth muscle cells in an autocrine or paracrine manner. This could fulfill important functions during myogenesis in the embryo as well as in tissue repair and atherogenesis in the adult.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T. B., Gajdusek C. M., Schwartz S. M., McDougall J. K., Benditt E. P. Expression of the sis gene by endothelial cells in culture and in vivo. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6772–6774. doi: 10.1073/pnas.81.21.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C., Heldin C. H., Nister M., Ek B., Wasteson A., Westermark B. Synthesis of a PDGF-like growth factor in human glioma and sarcoma cells suggests the expression of the cellular homologue to the transforming protein of simian sarcoma virus. Biochem Biophys Res Commun. 1983 Nov 30;117(1):176–182. doi: 10.1016/0006-291x(83)91557-7. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B., Lind P., Urdea M. S., Eddy R., Shows T. B., Philpott K., Mellor A. L. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature. 1986 Apr 24;320(6064):695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Westermark B., Ek B., Heldin C. H. Coexpression of a PDGF-like growth factor and PDGF receptors in a human osteosarcoma cell line: implications for autocrine receptor activation. Cell. 1984 Dec;39(3 Pt 2):447–457. doi: 10.1016/0092-8674(84)90452-5. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Vogel A., Ross R. Production of platelet-derived growth factor-like molecules and reduced expression of platelet-derived growth factor receptors accompany transformation by a wide spectrum of agents. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2396–2400. doi: 10.1073/pnas.81.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Chiu I. M., Reddy E. P., Givol D., Robbins K. C., Tronick S. R., Aaronson S. A. Nucleotide sequence analysis identifies the human c-sis proto-oncogene as a structural gene for platelet-derived growth factor. Cell. 1984 May;37(1):123–129. doi: 10.1016/0092-8674(84)90307-6. [DOI] [PubMed] [Google Scholar]
- Clarke M. F., Westin E., Schmidt D., Josephs S. F., Ratner L., Wong-Staal F., Gallo R. C., Reitz M. S., Jr Transformation of NIH 3T3 cells by a human c-sis cDNA clone. 1984 Mar 29-Apr 4Nature. 308(5958):464–467. doi: 10.1038/308464a0. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Huang S. S., Stroobant P., Waterfield M. D. Expression of a platelet-derived growth factor-like protein in simian sarcoma virus transformed cells. Science. 1983 Sep 30;221(4618):1348–1350. doi: 10.1126/science.6310754. [DOI] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Edmonds M., Caramela M. G. The isolation and characterization of adenosine monophosphate-rich polynucleotides synthesized by Ehrlich ascites cells. J Biol Chem. 1969 Mar 10;244(5):1314–1324. [PubMed] [Google Scholar]
- Eva A., Robbins K. C., Andersen P. R., Srinivasan A., Tronick S. R., Reddy E. P., Ellmore N. W., Galen A. T., Lautenberger J. A., Papas T. S. Cellular genes analogous to retroviral onc genes are transcribed in human tumour cells. Nature. 1982 Jan 14;295(5845):116–119. doi: 10.1038/295116a0. [DOI] [PubMed] [Google Scholar]
- Garrett J. S., Coughlin S. R., Niman H. L., Tremble P. M., Giels G. M., Williams L. T. Blockade of autocrine stimulation in simian sarcoma virus-transformed cells reverses down-regulation of platelet-derived growth factor receptors. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7466–7470. doi: 10.1073/pnas.81.23.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goustin A. S., Betsholtz C., Pfeifer-Ohlsson S., Persson H., Rydnert J., Bywater M., Holmgren G., Heldin C. H., Westermark B., Ohlsson R. Coexpression of the sis and myc proto-oncogenes in developing human placenta suggests autocrine control of trophoblast growth. Cell. 1985 May;41(1):301–312. doi: 10.1016/0092-8674(85)90083-2. [DOI] [PubMed] [Google Scholar]
- Graves D. T., Owen A. J., Barth R. K., Tempst P., Winoto A., Fors L., Hood L. E., Antoniades H. N. Detection of c-sis transcripts and synthesis of PDGF-like proteins by human osteosarcoma cells. Science. 1984 Nov 23;226(4677):972–974. doi: 10.1126/science.6209798. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Johnsson A., Wennergren S., Wernstedt C., Betsholtz C., Westermark B. A human osteosarcoma cell line secretes a growth factor structurally related to a homodimer of PDGF A-chains. Nature. 1986 Feb 6;319(6053):511–514. doi: 10.1038/319511a0. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Platelet-derived growth factor. Mol Cell Endocrinol. 1985 Mar;39(3):169–187. doi: 10.1016/0303-7207(85)90061-9. [DOI] [PubMed] [Google Scholar]
- Johnsson A., Betsholtz C., Heldin C. H., Westermark B. Antibodies against platelet-derived growth factor inhibit acute transformation by simian sarcoma virus. Nature. 1985 Oct 3;317(6036):438–440. doi: 10.1038/317438a0. [DOI] [PubMed] [Google Scholar]
- Johnsson A., Betsholtz C., von der Helm K., Heldin C. H., Westermark B. Platelet-derived growth factor agonist activity of a secreted form of the v-sis oncogene product. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1721–1725. doi: 10.1073/pnas.82.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Wasteson A., Westermark B., Deuel T. F., Huang J. S., Seeburg P. H., Gray A., Ullrich A., Scrace G. The c-sis gene encodes a precursor of the B chain of platelet-derived growth factor. EMBO J. 1984 May;3(5):921–928. doi: 10.1002/j.1460-2075.1984.tb01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs S. F., Guo C., Ratner L., Wong-Staal F. Human-proto-oncogene nucleotide sequences corresponding to the transforming region of simian sarcoma virus. Science. 1984 Feb 3;223(4635):487–491. doi: 10.1126/science.6318322. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Martinet Y., Bitterman P. B., Mornex J. F., Grotendorst G. R., Martin G. R., Crystal R. G. Activated human monocytes express the c-sis proto-oncogene and release a mediator showing PDGF-like activity. Nature. 1986 Jan 9;319(6049):158–160. doi: 10.1038/319158a0. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Nilsson J., Sjölund M., Palmberg L., Thyberg J., Heldin C. H. Arterial smooth muscle cells in primary culture produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4418–4422. doi: 10.1073/pnas.82.13.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistér M., Heldin C. H., Wasteson A., Westermark B. A glioma-derived analog to platelet-derived growth factor: demonstration of receptor competing activity and immunological crossreactivity. Proc Natl Acad Sci U S A. 1984 Feb;81(3):926–930. doi: 10.1073/pnas.81.3.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris J. S., Cornett L. E., Hardin J. W., Kohler P. O., MacLeod S. L., Srivastava A., Syms A. J., Smith R. G. Autocrine regulation of growth: II. Glucocorticoids inhibit transcription of c-sis oncogene-specific RNA transcripts. Biochem Biophys Res Commun. 1984 Jul 18;122(1):124–128. doi: 10.1016/0006-291x(84)90448-0. [DOI] [PubMed] [Google Scholar]
- Owen A. J., Pantazis P., Antoniades H. N. Simian sarcoma virus--transformed cells secrete a mitogen identical to platelet-derived growth factor. Science. 1984 Jul 6;225(4657):54–56. doi: 10.1126/science.6328659. [DOI] [PubMed] [Google Scholar]
- Pantazis P., Pelicci P. G., Dalla-Favera R., Antoniades H. N. Synthesis and secretion of proteins resembling platelet-derived growth factor by human glioblastoma and fibrosarcoma cells in culture. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2404–2408. doi: 10.1073/pnas.82.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Seifert R. A., Schwartz S. M., Bowen-Pope D. F. Developmentally regulated production of platelet-derived growth factor-like molecules. Nature. 1984 Oct 18;311(5987):669–671. doi: 10.1038/311669a0. [DOI] [PubMed] [Google Scholar]
- Sejersen T., Sümegi J., Ringertz N. R. Density-dependent arrest of DNA replication is accompanied by decreased levels of c-myc mRNA in myogenic but not in differentiation-defective myoblasts. J Cell Physiol. 1985 Dec;125(3):465–470. doi: 10.1002/jcp.1041250315. [DOI] [PubMed] [Google Scholar]
- Shimokado K., Raines E. W., Madtes D. K., Barrett T. B., Benditt E. P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Cline M. J. Expression of cellular oncogenes during embryonic and fetal development of the mouse. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7141–7145. doi: 10.1073/pnas.81.22.7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyberg J., Palmberg L., Nilsson J., Ksiazek T., Sjölund M. Phenotype modulation in primary cultures of arterial smooth muscle cells. On the role of platelet-derived growth factor. Differentiation. 1983;25(2):156–167. doi: 10.1111/j.1432-0436.1984.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zoelen E. J., van de Ven W. J., Franssen H. J., van Oostwaard T. M., van der Saag P. T., Heldin C. H., de Laat S. W. Neuroblastoma cells express c-sis and produce a transforming growth factor antigenically related to the platelet-derived growth factor. Mol Cell Biol. 1985 Sep;5(9):2289–2297. doi: 10.1128/mcb.5.9.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]