This report suggests that robotic repair of giant para-esophageal hernia has a lower recurrence rate than standard laparoscopic methods, but complications and mortality are similar to standard laparoscopic approaches.
Keywords: Paraesophageal, Giant, Robotic, Surgery, Foregut, Hiatal
Abstract
Background and Objectives:
Giant paraesophageal hernia accounts for 5% of all hiatal hernias, and it is commonly seen in elderly patients with comorbidities. Some series report complication rates up to 28%, recurrence rates between 10% and 25%, and a mortality rate close to 2%. Recently, the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) has shown equivocal benefits when used for elective surgeries, whereas for complex procedures, the benefits appear to be clearer. The purpose of this study is to present our preliminary experience in robotic giant paraesophageal hernia repair.
Methods:
We retrospectively collected data from patients who had a diagnosis of giant paraesophageal hernia and underwent a paraesophageal hernia repair with the da Vinci Surgical System.
Results:
Nineteen patients (12 women [63.1%]) underwent surgery for giant paraesophageal hernia at our center. The mean age was 70.4 ± 13.9 years (range, 40–97 years). The mean American Society of Anesthesiologists score was 2.15. The mean surgical time and hospital length of stay were 184.5 ± 96.2 minutes (range, 96–395 minutes) and 4.3 days (range, 2–22 days), respectively. Nissen fundoplications were performed in 3 cases (15.7%), and 16 patients (84.2%) had mesh placed. Six patients (31.5%) presented with gastric volvulus, and 2 patients had other herniated viscera (colon and duodenum). There were 2 surgery-related complications (10.5%) (1 dysphagia that required dilatation and 1 pleural injury) and 1 conversion to open repair (partial gastric resection). No recurrences or deaths were observed in this series.
Conclusion:
In our experience robotic giant paraesophageal hernia repair is not different from the laparoscopic approach in terms of complications and mortality rate, but it may be associated with lower recurrence rates. However, larger series with longer follow-up are necessary to further substantiate our results.
INTRODUCTION
Gastroesophageal reflux disease is a common chronic disorder prevalent in many countries. It is now the most common upper gastrointestinal disease in the Western population, with 10% to 20% of the population having weekly symptoms,1 and has been associated with many risk factors; among them is the presence of hiatal hernia (HH). HH can be defined as herniation of elements of the abdominal cavity through the esophageal hiatus of the diaphragm and into the mediastinum. More than 90% of HHs are considered “sliding,” or type 1; this type of hernia generally has a benign course because most of the patients will never have symptoms and may not require surgical treatment. An HH can also be known as a paraesophageal hernia (PEH), or type 2, and this represents around 5% of all the cases. In addition, the clinical spectrum and presentation of HH also include type 3 (mixed type) and type 4 (with the presence of other organs into the abdominal cavity) that represent <3% of all the cases.2 PEH sometimes is considered a surgical emergency; its management has become one of the most widely debated and controversial areas in surgery. These patients often bear complicating medical comorbidities, making them potentially poor operative candidates.3 The laparoscopic approach and minimally invasive techniques have emerged as good alternatives for the treatment of this pathology, and a great variety of articles have reported better results than those with the open approach. For example, Andujar et al.4 concluded that laparoscopic repair offers superior visualization, which is crucial for the mediastinal mobilization of the esophagus, and Wiechmann et al.5 mentioned that the laparoscopic approach leads to a shorter hospital length of stay and faster return to full activity. Recently, a novel technology, the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA), has become very popular among the surgical community because of 3-dimensional vision, better motion scaling, intuitive movements, and tremor filtration. However, this technology has shown controversial benefits when used for simple operations, whereas for complex procedures, the benefits appear to be clearer. The complexity observed during PEH repair and the high rate of morbidity still seen after laparoscopic repair could be reasons to test novel platforms, such as the da Vinci Surgical System. In addition, many patients are still offered open PEH repair, and the robotic platform may alleviate this occurrence. The purpose of this study is to report our preliminary experience with the use of the robotic platform during the surgical treatment of giant paraesophageal hernias (GPEHs).
MATERIALS AND METHODS
A retrospective review of our surgical group's patient database and medical records identified 19 patients who underwent a GPEH repair from February 2010 to July 2012. The institutional review board approved the study. Inclusion criteria included patients who underwent a GPEH repair with the use of the da Vinci Surgical System. GPEH was defined by the presence of [me]30% of the stomach in the thoracic cavity.6 During the study period, some laparoscopic cases were performed. The criteria that determined whether the procedure was laparoscopic or robotic were influenced by 3 main factors: (1) preference of the patient, (2) preference of the surgeon, (3) and availability of the robotic system. The patients' characteristics (such as age, sex, body mass index [BMI], or presence of comorbidities) did not influence the selection criteria.
Three attending surgeons at two institutions performed all the procedures. Two clinical fellows participated in the operations. Information collected included demographics, surgical time, complications, and hospital length of stay. Preoperative evaluation included upper endoscopy and a barium esophagram, and when indicated, manometry and a computed tomography scan were performed. The statistical analysis included quantitative parameters such as age, BMI, operating time, and hospital stay that were presented as mean, with 1 SD and range. The categorical parameters, such as sex, American Society of Anesthesiologists (ASA) score, and complications, were presented as arithmetic values.
Surgical Technique
Under general anesthesia, the patient was placed supine in a reverse 15° Trendelenburg position with both arms extended. The surgical team consisted of a senior attending surgeon, a specialized surgical assistant, an anesthesiologist, a scrub nurse, and an assigned circulating nurse. A minimally invasive fellow was present occupying a position beside the patient as the first assistant and/or as the main surgeon at the console. The anesthesiologist was positioned over the right side of the head of the patient. A 12-mm vertical incision was made above the umbilicus in the midline, and a pneumoperitoneum was created with a Veress needle, by use of an open Hasson technique, or with an optic port device (Endopath Xcel; Ethicon, Somerville, NJ, USA). Subsequently, two 5-mm robotic ports were placed in the right and left upper quadrant over the anterior axillary line, and one 12-mm assistant port was placed on the left side, over the mid-axillary line. A 30° laparoscope was inserted in the 12-mm port, and under direct vision, a Nathanson Hook Liver Retractor (Mediflex Surgical Products, Islandia, NY, USA) was placed in the epigastrium. The robot was brought over the patient's head (Figures 1 and 2). Once the robot instrumentation was inserted and docked, the main surgeon transitioned to the console. The procedure began with the main surgeon dividing the pars flaccida and exposing the right crus; the dissection continued in the hiatal defect, and the stomach was reduced with the da Vinci robotic forceps and with the help of the assistant. A Harmonic scalpel (Intuitive Surgical Inc, Sunnyvale, CA, USA) and monopolar cautery were used in most of the cases. The viability of the stomach was evaluated. In pertinent cases partial stomach resection, omentum resection, or reduction of other organs was performed. The hiatal defect was closed with nonabsorbable suture, and in most of the cases, a biological mesh (Flex HD Acellular Hydrated Dermis; Ethicon) was placed over the closed hiatal defect. The mesh was secured with interrupted stitches to the crus and/or diaphragm and, in some cases, reinforced with fibrin glue (Evicel Fibrin Sealant; Ethicon). If clinically indicated, a 360° wrap stomach Nissen fundoplication was performed (Figures 3–7).
Figure 1.

The robot is placed over the head of the patient.
Figure 2.
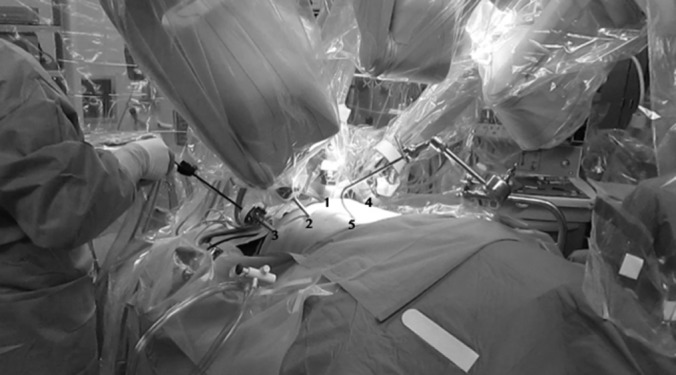
Robot docked: 1, 12-mm robotic camera docked (at umbilicus); 2, 5-mm robotic arm docked (left anterior axillary line); 3, 12-mm laparoscopic assistant trocar (left mid-axillary line); 4, 5-mm robotic arm docked (right anterior axillary line); and 5, Nathanson retractor.
Figure 3.
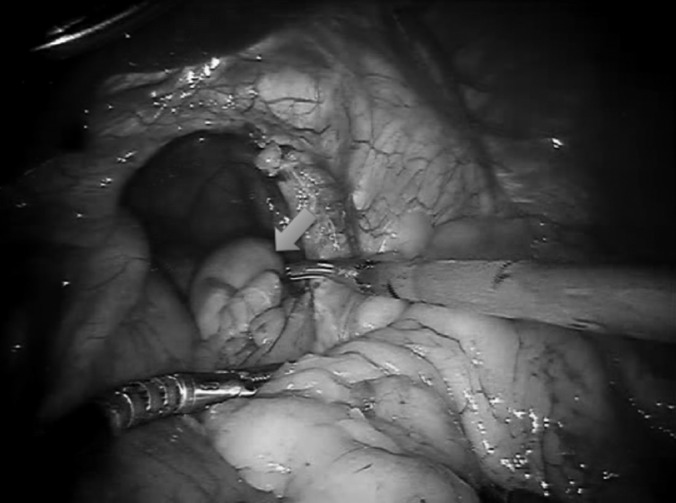
GPEH with entire stomach (blue arrow) in chest.
Figure 4.

Hiatal defect posterior to complete reduction of intrathoracic organs.
Figure 5.
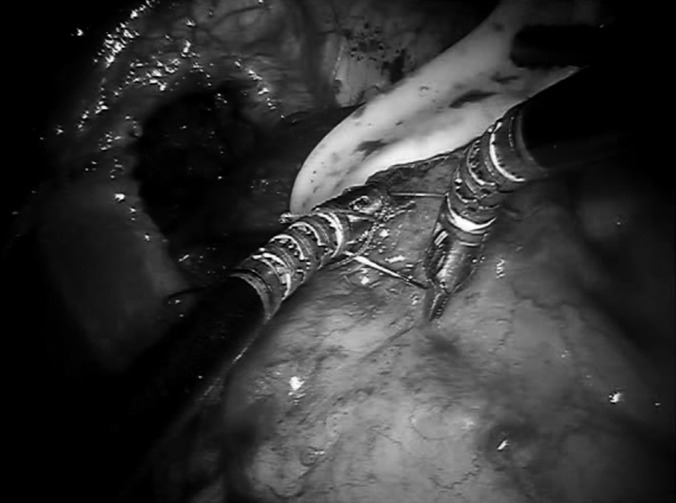
Ergonomic robotic arm during performance of intracorporeal suturing.
Figure 6.
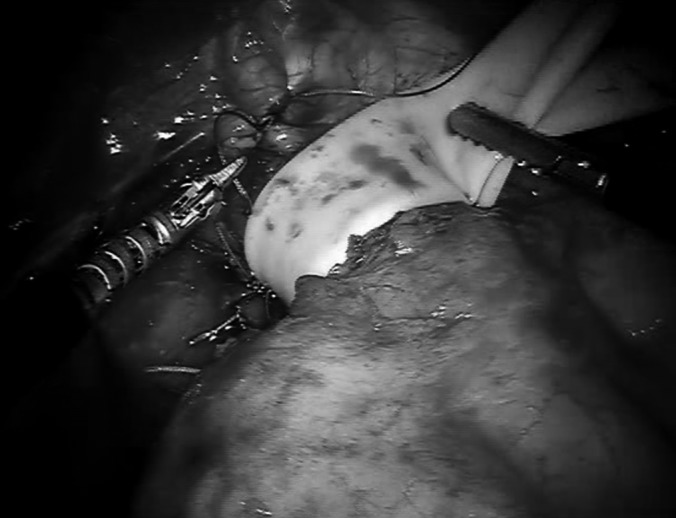
Closed hiatal defect.
Figure 7.
Hiatal defect covered with biological mesh.
RESULTS
The study included 19 patients: 12 women (63.1%) and 7 men (36.9%). The mean age was 70.4 ± 13.9 years (range, 40–97 years), and the mean preoperative BMI was 28.3 ± 6.1 kg/m2 (range, 21.6–44.9 kg/m2). ASA scores of 1, 2, and 3 were reported in 4 patients (21%), 8 patients (42.1%), and 7 patients (36.9%), respectively (mean ASA score, 2.15). Fifteen patients (79%) had at least one chronic comorbidity, and 10 (52.6%) had a history of abdominal surgery. Total comorbidities and the main symptoms of presentation are summarized in Table 1. In 3 patients (15.7%), a 360° fundoplication was performed as part of the PEH repair, and in 16 cases (84.2%), a mesh was used to cover the hiatal defect. Fibrin glue (Evicel Fibrin Sealant) was used in 9 cases (47.4%). Five concurrent procedures (31.2%) were performed during the HH repair. Two patients underwent a robotic cholecystectomy, 1 patient required open repair of a large umbilical hernia, 1 patient underwent a gastrostomy tube placement, and 1 patient required partial gastric resection because of lack of viability of the gastric wall. There were 6 patients (31.5%) with associated gastric volvulus. One patient had a herniated colon, and 1 had colon and duodenum in the hiatal defect. The mean surgical time was 184.5 ± 96.2 minutes (range, 76–395 minutes). The mean hospital length of stay was 4.3 days (range, 2–22 days). There were 2 surgery-related complications (10.5%) and 1 conversion (5.2%) (a detailed explanation of the complications and conversion is given in the next paragraph). No recurrence (with a mean follow-up period of 15.6 ± 9.6 months) or death was seen in this series.
Table 1.
Clinical Presentation and Previous Comorbidities
| Patient | Main Clinical Presentation | Comorbidities |
|---|---|---|
| 1 | Acute hematemesis, epigastric burning, nausea, vomiting | Aortic stenosis, pacemaker placement |
| 2 | Chest discomfort | CADa, COPDa, hyperlipidemia, HTNa, obesity, previous, RAa, MIa, previous CABGa |
| 3 | Episodes of intermittent bowel obstruction | Healthy |
| 4 | Aspiration pneumonia, severe reflux, shortness of breath | Aortic stenosis, COPD, obesity, CHFa, previous PEa |
| 5 | Reflux | Healthy |
| 6 | Heartburn, reflux | Asthma, morbid obesity |
| 7 | Abdominal pain, shortness of breath | Healthy |
| 8 | Dyspepsia | HTN |
| 9 | Dysphagia, early satiety, odynophagia | Obesity |
| 10 | Odynophagia, reflux | Asthma, cerebral ataxia, colonic malformation, hypothyroidism, obesity, previous PE, pulmonary HTN |
| 11 | Abdominal pain, upper GIa bleeding, vomiting | HTN, mitral valve prolapsed |
| 12 | Nausea, vomiting | Healthy |
| 13 | Asymptomatic patient | CHF, prostate cancer, previous cardiac catheterization |
| 14 | Abdominal pain | HTN, dementia |
| 15 | Abdominal pain | HTN, hypothyroidism, obesity, previous mastectomy for breast cancer |
| 16 | Shortness of breath, upper GI bleeding, dysphagia | HTN, depression, obesity, RA |
| 17 | Abdominal distention, discomfort | HTN, hyperlipidemia, RA, placement of knee and hip prosthesis |
| 18 | Severe reflux | Deep venous thrombosis, vertigo |
| 19 | Reflux, mild intermittent abdominal pain | Prostate cancer |
CABG = coronary artery bypass graft, CAD = coronary artery disease, CHF = congestive heart failure, COPD = chronic obstructive pulmonary disease, GI = gastrointestinal, HTN = hypertension, MI = myocardial infarction, PE = pulmonary embolism, RA = rheumatoid arthritis.
The first complication was seen in a 69-year-old healthy woman who presented with shortness of breath to the emergency department. PEH was diagnosed, and the patient was taken to the operating room. During the procedure, a GPEH was found and the stomach was strongly adhered to both the right and left crus. During the dissection, a pleural injury was noticed. Reduction was completed, the pleural injury was repaired with No. 3-0 Vicryl (Ethicon), and the hernia was closed with nonabsorbable suture. No pleural tube was used. A biological mesh was placed and a Nissen fundoplication performed. The total procedure was completed in 258 minutes, and the patient was discharged 3 days later.
Another complication was seen in a 40-year-old man who had a history of PEH repair 8 years ago. His main complaints were dysphagia and occasional vomiting. The procedure was completed in 288 minutes because of adhesions from a previous HH surgery. The patient was discharged 3 days later. However, he was readmitted 2 days after discharge complaining of dysphagia; this was treated successfully with repetitive endoscopic pneumatic dilatations.
The conversion occurred in a 97-year-old man with a history of aortic stenosis and cardiac pacemaker placement. He arrived to the emergency department with acute hematemesis and epigastric burning. During the procedure, a complete organoaxial rotation of the stomach was found with questionable viability of the stomach wall. The robot was undocked, and an open partial gastric resection was completed. The hiatal defect was closed with PDS (Ethicon), and a biological mesh was used. This operation was completed in 395 minutes, and the patient was discharged 5 days later with no major complications. Details of every patient in this series are summarized in Table 2.
Table 2.
Details of 19 Patients
| Case | Sex | Age, yr | ASA Score | Previous Abdominal Surgeries | BMI, kg/m2 | Fundoplication | Mesh | Fibrin Glue | Concomitant Procedures | Volvulus | % Stomach Into Chest | Other Intrathoracic Organs | Surgical Time, min | Length of Stay, d | Conversion | Complication |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ma | 97 | 3 | 23 | Yes | Yes | Yes | >30 | 395 | 5 | Yes | |||||
| 2 | M | 79 | 3 | 30 | Yes | Yes | >30 | Yes | 133 | 3 | ||||||
| 3 | M | 85 | 1 | 23 | Yes | Yes | Yes | 100 | 167 | 5 | ||||||
| 4 | Fa | 72 | 3 | Yes | 34 | Yes | Yes | 90 | 370 | 22 | ||||||
| 5 | M | 59 | 1 | 25 | Yes | >30 | 120 | 2 | ||||||||
| 6 | F | 60 | 2 | 44 | Yes | Yes | Yes | >30 | — | 4 | ||||||
| 7 | F | 69 | 1 | 25 | Yes | Yes | 90 | 258 | 3 | Yes | ||||||
| 8 | M | 67 | 2 | 23 | Yes | Yes | Yes | >30 | — | 4 | ||||||
| 9 | M | 40 | 2 | Yes | 35 | Yes | 100 | 288 | 3 | Yes | ||||||
| 10 | F | 79 | 3 | Yes | 33 | Yes | Yes | >30 | 303 | 3 | ||||||
| 11 | F | 59 | 3 | Yes | 23 | Yes | >30 | 81 | 2 | |||||||
| 12 | F | 50 | 1 | Yes | 25 | Yes | Yes | 100 | 149 | 2 | ||||||
| 13 | M | 89 | 3 | 21 | Yes | Yes | Yes | >30 | 86 | 7 | ||||||
| 14 | F | 80 | 2 | Yes | 24 | Yes | Yes | 100 | 76 | 2 | ||||||
| 15 | F | 80 | 2 | Yes | 32 | Yes | Yes | Yes | 100 | 160 | 8 | |||||
| 16 | F | 73 | 2 | Yes | 34 | Yes | 100 | 143 | 2 | |||||||
| 17 | F | 71 | 2 | Yes | 26 | Yes | Yes | 90 | Yes | 195 | 2 | |||||
| 18 | F | 57 | 3 | 27 | Yes | Yes | Yes | >50 | 95 | 2 | ||||||
| 19 | F | 73 | 2 | Yes | 21 | >30 | 154 | 2 |
F = female, M = male.
DISCUSSION
PEH repair continues to be a challenge to surgeons and physicians. Recently, the role of nonoperative management in asymptomatic patients with PEH was revisited, and the authors reported a successful “watchful-waiting” approach in these cases.7 However, currently, most surgeons recommend surgical repair of PEH regardless of symptomatology8 because this entity is associated with a high incidence of life-threatening complications.9 When surgery is indicated, the best approach is laparoscopic because incision-related complications are fewer and recovery time and hospital length of stay are shorter when compared with open access.10,11 A large multicenter study (2069 laparoscopic PEH repairs vs 657 open repairs) reported that for elective procedures, the laparoscopic approach was associated with a shorter hospital stay, less requirement for intense care unit admission, a lower rate of overall complications, fewer 30-day readmissions, and a lower cost.12 Schauer et al.13 showed similar results when laparoscopic repair was compared with open repair. However, even with the clear improvements in outcomes with laparoscopic surgery, complication and mortality rates continue to be high in patients after PEH repair. For example, in a series of 100 GPEH repairs, Luketich et al.14 reported more than 20 complications, and another experienced center reported a 2% mortality rate among 94 laparoscopic PEH repairs.15 In addition, recurrence is one of the major concerns when a PEH is treated because the probability of recurrence is high. Some authors report lower recurrence rates with laparoscopic surgery,16 others show similar results when compared with open surgery,17 and still others even favor the open approach.18 To prevent the recurrence of this entity, several studies have been performed and different surgical authorities have reported their experience adding some technical details. For example, Ponsky et al.19 recommend an anterior gastropexy, and Oelschlager et al.20 in prospective randomized study favored the use of a biological prosthesis to prevent recurrence. In addition, there are surgeons who still offer patients open repair as the only option. With an understanding of the benefits of minimally invasive surgery, this seems counterintuitive. This occurs mainly because of the surgeon's lack of laparoscopic expertise.
The da Vinci technology attempts to provide the same benefits of laparoscopic surgery but with enhancement in technical proficiency for some surgeons. Currently, such robotic technology is used for a great variety of procedures (gynecologic, thoracic, urologic, gastrointestinal) because this platform allows 6 df, combined with the 3-dimensional high-definition image and steady operating arms. It also allows the surgeon to operate in an extremely precise and meticulous manner and provides varying levels of magnification (12× to 40×) depending on its proximity to the target tissue. However, the robot is still inaccessible to some health systems because of the associated high cost; this may be the reason many surgeons restrict its use only to technically difficult cases (such as bariatric revision surgery or cardiac procedures) and in cases with limited space to work (such as radical prostatectomy and low anterior colonic resections) because in these operations the da Vinci system has obtained its best results and its cost has been justified. In our facility we have access to many robotic systems; therefore implementation of robotic technology was without difficulty. We believe that using the robot for those “difficult” cases, such as those presented in this series, would be of most benefit. As previously mentioned, the PEH constitutes a technical challenge in which careful dissection must be conducted and advanced surgical maneuvers are frequently performed. The robotic experience in this group of patients and surgery type is restricted only to few series. Braumann et al.21 included 14 robotic PEH repairs, and Draaisma et al.22 presented their experience with 40 consecutive robotic cases. Finally, one other experience was reported recently: in a comparative study Gehrig et al.23 reported a series of 12 robotic PEH repairs and compared such cases with their laparoscopic and open experience. They mentioned that the robotic approach was superior to the open approach but similar to the laparoscopic approach.
Our report represents one of the few publications evaluating the use of the da Vinci Surgical System during GPEH repair. The preoperative comorbidities presented in our series are similar to those reported in the literature.24,25 This entity is seen frequently in elderly patients with comorbidities. In our report the mean age was 70.5 years and 79% of the patients had a history of at least one chronic disease. In addition, the great majority of our patients were diagnosed because of the presence of symptoms, and some presented with life-threatening complications (Table 1). We observed the occurrence of postoperative complications in two patients (10.5%) and no deaths during the mean follow-up period (15.6 ± 9.6 months). This is comparable with the complication rate with the laparoscopic approach that is reported in the literature (between 6% and >20%).26,27 In addition, our series reported a mean surgical time of 184.5 minutes; this is similar to that reported by Gehrig et al.23 in their robotic experience (172 minutes). Moreover, our surgical time compared with laparoscopic surgery was similar to some large series. For example, Andujar et al.4 reported 160 minutes and Horgan et al.28 reported 210 minutes. No difference was noted in hospital length of stay when we compared our experience with other reports in the literature. Our study shows that the robotic approach has outcomes similar to the laparoscopic approach in terms of complication rate, total surgical time, and hospital length of stay. In our series, there were no recurrences after the robotic procedure after a mean follow-up period of 15.6 months. The use of robotic surgery could decrease the risk of recurrence because most of the laparoscopic series report recurrences rates between 2% and 12%.5,15,16 It is important to note that the follow-up period is short, just over a year, and patients may report to other surgeons with our recurrences. Larger numbers of patients with longer follow-up will alleviate this limitation. Nevertheless, the reason for this finding may be related to dissection of the sac because it is well known that extensive dissection and excision of the sac decrease the risk of recurrence.29
Our study has some limitations. First, the number of patients is small compared with other major laparoscopic series.19 Second, technical variations (presence and type of mesh, fibrin sealant, fundoplication, and so on) represent an important variable for the results. Finally, the follow-up period in our study is limited. The robotic technique used in this series presents new technical challenges (such as docking and undocking of the robot and changing of robotic instruments), but with experience, efficiencies were improved. When a robotic PEH is performed, we recommend following the same surgical principles described in laparoscopic cases (careful dissection of the left and right crus, transection of the short gastric vessels if a fundoplication is to be performed, removal of the sac, and complete mobilization of the esophagus before closure of the hiatal defect). As mentioned before, a mesh, a fibrin sealant, or Nissen fundoplication was used during the repair in some cases in this series; however, the issues surrounding these methods are still controversial, and their discussion goes beyond the scope of this article.
We believe that the principal limitation of the robotic technology is the cost; to that end, the Society of American Gastrointestinal and Endoscopic Surgeons agrees that the most significant limitation of the surgical robotics is the economic implications.30 Many institutions do not have robotic systems available, and when they exist, they are used for procedures in which a clear benefit has been shown (e.g., prostatectomy and hysterectomy). Our health system has many robotic systems, and our adoption of the technology in these more complex cases in the arena of general surgery is to determine whether there exists a benefit similar to that seen in urology and gynecology. Because of the cost, some surgeons restrict the use of robotic systems to “complex” cases in which a clear justification is provided. Although this may be correct, we believe that to acquire experience with the robotic platform, it is necessary to begin initially with simple cases in which the complete surgical team can gain experience and comfort with the system. In addition, the da Vinci technology requires collaboration of a surgical assistant, scrub nurse, and circulating nurse. Our practice and recommendation are to use the robotic platform frequently and consistently in simple cases with the same operative team so that experience is gained for those complex procedures.
We can conclude that paraesophageal herniation is a potentially devastating condition of the gastroesophageal hiatus commonly manifesting in patients of advanced age with other significant medical problems. When compared with the literature, the robotic platform appears to have the same benefits as those of the laparoscopic approach in terms of complication rate, total surgical time, and hospital length of stay; in addition, the robotic platform may be associated with a lower recurrence rate than the laparoscopic approach. Because our sample size is small and the follow-up period is relatively short, our findings represent preliminary results. We are cautiously optimistic and recommend that additional studies with larger numbers of cases and randomized designs be developed to support our observations.
Contributor Information
Rupa Seetharamaiah, Department of General and Bariatric Surgery, Baptist Health South Florida, Miami, FL, USA..
Rey Jesús Romero, Department of General and Bariatric Surgery, Baptist Health South Florida, Miami, FL, USA..
Radomir Kosanovic, Department of General and Bariatric Surgery, Baptist Health South Florida, Miami, FL, USA..
Michelle Gallas, Center for Research & Grants, Baptist Health South Florida, Miami, FL, USA..
Juan-Carlos Verdeja, Department of General and Bariatric Surgery, Baptist Health South Florida, Miami, FL, USA..
Jorge Rabaza, Department of General and Bariatric Surgery, Baptist Health South Florida, Miami, FL, USA..
Anthony Michael Gonzalez, Department of General and Bariatric Surgery, Baptist Health South Florida, Miami, FL, USA..
References:
- 1. Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54(5):710–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kahrilas PJ, Kim HC, Pandolfino JE. Approaches to the diagnosis and grading of hiatal hernia. Best Pract Res Clin Gastroenterol. 2008;22(4):601–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis SS., Jr Current controversies in paraesophageal hernia repair. Surg Clin North Am. 2008;88(5):959–978 [DOI] [PubMed] [Google Scholar]
- 4. Andujar JJ, Papasavas PK, Birdas T, et al. Laparoscopic repair of large paraesophageal hernia is associated with a low incidence of recurrence and reoperation. Surg Endosc. 2004;18(3):444–447 [DOI] [PubMed] [Google Scholar]
- 5. Wiechmann RJ, Ferguson MK, Naunheim KS, et al. Laparoscopic management of giant paraesophageal herniation. Ann Thorac Surg. 2001;71(4):1080–1086 [DOI] [PubMed] [Google Scholar]
- 6. Stavropoulos G, Flessas II, Mariolis-Sapsakos T, et al. Laparoscopic repair of giant paraesophageal hernia with synthetic mesh: 45 consecutive cases. Am Surg. 2012;78(4):432–435 [PubMed] [Google Scholar]
- 7. Stylopoulos N, Gazelle GS, Rattner DW. Paraesophageal hernias: operation or observation? Ann Surg. 2002;236(4):492–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kehdy F. Current management paraesophageal hernia. Am Surg. 2011;77(12):1565–1573 [PubMed] [Google Scholar]
- 9. Skinner DB, Belsey RH. Surgical management of esophageal reflux and hiatus hernia. Long-term results with 1,030 patients. J Thorac Cardiovasc Surg. 1967;53(1):33–54 [PubMed] [Google Scholar]
- 10. Torres-Villalobos G, Martín-Del Campo LA, Vásquez-Sanchez L, Carranza-Martínez I, Santiago-Andrade R, Santillán-Doherty P. Improvement results in paraesophageal hernia. Cir Cir. 2011;79(4):351–355 [PubMed] [Google Scholar]
- 11. Hussain A, Singhal T, Aravind B, Chandra A, El-Hasani S. Management of acute paraesophageal hernia. Surg Endosc. 2009;23(12):2858–2859 [DOI] [PubMed] [Google Scholar]
- 12. Nguyen NT, Christie C, Masoomi H, Matin T, Laugenour K, Hohmann S. Utilization and outcomes of laparoscopic versus open paraesophageal hernia repair. Am Surg. 2011;77(10):1353–1357 [PubMed] [Google Scholar]
- 13. Schauer PR, Ikramuddin S, McLaughlin RH, et al. Comparison of laparoscopic versus open repair of paraesophageal hernia. Am J Surg. 1998;176(6):659–665 [DOI] [PubMed] [Google Scholar]
- 14. Luketich JD, Raja S, Fernando HC, et al. Laparoscopic repair of giant paraesophageal hernia: 100 consecutive cases. Ann Surg. 2000;232(4):608–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li J, Rosenthal RJ, Roy M, Szomstein S, Sesto M. Experience of laparoscopic paraesophageal hernia repair at a single institution. Am J Surg. 2012;204(1):60–65 [DOI] [PubMed] [Google Scholar]
- 16. Zehetner J, Demeester SR, Ayazi S, et al. Laparoscopic versus open repair of paraesophageal hernia: the second decade. J Am Coll Surg. 2011;212(5):813–820 [DOI] [PubMed] [Google Scholar]
- 17. Edye MB, Canin-Endres J, Gattorno F, Salky BA. Durability of laparoscopic repair of paraesophageal hernia. Ann Surg. 1998;228(4):528–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rathore MA, Bhatti MI, Andrabi SI, McMurray AH. Laparoscopic repair of paraesophageal hernia requires cautious enthusiasm. Int J Surg. 2008;6(5):404–408 [DOI] [PubMed] [Google Scholar]
- 19. Ponsky J, Rosen M, Fanning A, Malm J. Anterior gastropexy may reduce the recurrence rate after laparoscopic paraesophageal hernia repair. Surg Endosc. 2003;17(7):1036–1041 [DOI] [PubMed] [Google Scholar]
- 20. Oelschlager BK, Pellegrini CA, Hunter JG, et al. Biologic prosthesis to prevent recurrence after laparoscopic paraesophageal hernia repair: long-term follow-up from a multicenter, prospective, randomized trial. J Am Coll Surg. 2011;213(4):461–468 [DOI] [PubMed] [Google Scholar]
- 21. Braumann C, Jacobi CA, Menenakos C, Ismail M, Rueckert JC, Mueller JM. Robotic-assisted laparoscopic and thoracoscopic surgery with the da Vinci system: a 4-year experience in a single institution. Surg Laparosc Endosc Percutan Tech. 2008;18(3):260–266 [DOI] [PubMed] [Google Scholar]
- 22. Draaisma WA, Gooszen HG, Consten EC, Broeders IA. Mid-term results of robot-assisted laparoscopic repair of large hiatal hernia: a symptomatic and radiological prospective cohort study. Surg Technol Int. 2008;17:165–170 [PubMed] [Google Scholar]
- 23. Gehrig T, Mehrabi A, Fischer L, et al. Robotic-assisted paraesophageal hernia repair—a case-control study. Langenbecks Arch Surg. 2013;398(5):691–696 [DOI] [PubMed] [Google Scholar]
- 24. Rosen M, Ponsky J. Laparoscopic repair of giant paraesophageal hernias: an update for internists. Cleve Clin J Med. 2003;70(6):511–514 [DOI] [PubMed] [Google Scholar]
- 25. Khanna A, Finch G. Paraesophageal herniation: a review. Surgeon. 2011;9(2):104–111 [DOI] [PubMed] [Google Scholar]
- 26. Mattar SG, Bowers SP, Galloway KD, Hunter JG, Smith CD. Long-term outcome of laparoscopic repair of paraesophageal hernia. Surg Endosc. 2002;16(5):745–749 [DOI] [PubMed] [Google Scholar]
- 27. Diez-Tarbenilla M, Ruiz-Tovar J, Grajal-Marino R, et al. Paraesophageal hiatal hernia. Open vs. laparoscopic surgery. Rev Esp Enferm Dig. 2009;101(10):706–711 [DOI] [PubMed] [Google Scholar]
- 28. Horgan S, Eubanks TR, Jacobsen G, Omelanczuk P, Pellegrini CA. Repair of paraesophageal hernias. Am J Surg. 1999;177(5):354–358 [DOI] [PubMed] [Google Scholar]
- 29. Oelschlager BK, Petersen RP, Brunt LM, et al. Laparoscopic paraesophageal hernia repair: defining long-term clinical and anatomic outcomes. J Gastrointest Surg. 2012;16(3):453–459 [DOI] [PubMed] [Google Scholar]
- 30. Hanly EJ, Zand J, Bachman SL, Marohn MR, Talamini MA. Value of the SAGES Learning Center in introducing new technology. Surg Endosc. 2005;19(4):477–483 [DOI] [PubMed] [Google Scholar]


