In an experimental setting, the authors demonstrated a trend toward improved preservation of the immune system after laparoscopic hepatic resection compared with open surgery.
Keywords: Liver resection, Laparoscopy, HLA-DR expression, Immunology, Stimulated cellular secretion, Postoperative immunosuppression
Abstract
Background and Objectives:
Major abdominal procedures are strongly associated with postoperative immunosuppression and subsequent increased patient morbidity. It is believed that laparoscopic surgery causes less depletion of the systemic immune function because of the reduced tissue trauma. Various cytokines and monocytic HLA-DR expression have been successfully implemented to assess postoperative immune function. The aim of our study was to show the difference in immunologic profiles after minimally invasive versus conventional liver resection.
Methods:
Ten animals underwent either laparoscopic or conventional open left lateral liver resection. Flow cytometric characteristics of HLA-DR expression on monocytes and lipopolysaccharide-stimulated cellular secretion of tumor necrosis factor α, interferon γ, interleukin 6, and interleukin 8 were measured and analyzed in ex vivo whole blood samples. Intraoperative and postoperative clinical outcome parameters were also documented and evaluated.
Results:
All animals survived the procedures. Postoperative complications were fever (n = 3), wound infections (n = 2), and biloma (n = 1). Open surgery showed a morbidity rate of 80% compared with 40% after laparoscopic surgery. Laparoscopic liver resection showed no postoperative immunoparalysis. Major histocompatibility complex class II expression in this group was elevated, whereas the open surgery group showed decreased major histocompatibility complex class II expression on postoperative day 1. Postoperative secretion of tumor necrosis factor α, interleukin 6, and interferon γ was lower in the open surgery group. Elevated transaminase levels after laparoscopy might have resulted from an ischemia/reperfusion injury caused by the capnoperitoneum.
Conclusion:
Major immunoparalysis depression was not observed in either group. Laparoscopic surgery shows a tendency to improve immunologic recovery after liver resection.
INTRODUCTION
Laparoscopic liver resection of benign and malignant neoplasms was introduced in the early 1990s and has continuously evolved ever since.1 On the basis of technological advances and ongoing experience, laparoscopic liver surgery is considered a safe and feasible technique when performed by an experienced surgeon. Indications for laparoscopic surgery are currently being expanded from wedge resections to more complex procedures such as hemihepatectomies.2 A recent meta-analysis of nonrandomized comparative studies showed that laparoscopic resections resulted in reduced operative blood loss, speedier recovery, and an oncologic clearance comparable with open surgery.3
The immunologic aspects of minimally invasive surgery have been extensively analyzed and compared with conventional surgery for procedures such as cholecystectomy, appendectomy, and colorectal surgery. It is generally accepted that surgery results in an acute metabolic stress response, which correlates with the intensity of the tissue damage. This stress response is usually accompanied by a transient state of postoperative immunosuppression. As the tissue trauma increases, the postoperative immunoparalysis becomes more apparent, leading to increased susceptibility to infection. In contrast to the large number of publications, data on immunologic differences between laparoscopic and open liver resection are limited.4
Burpee et al.5 showed that laparoscopic liver resection resulted in a diminished stress reaction compared with open surgery in an animal model. They measured significantly decreased plasma levels of interleukin (IL) 6 and tumor necrosis factor α (TNF-α) after laparoscopy, translating into a greater preservation of the immune function.
The aim of our study was to compare the effects of open and laparoscopic surgery on postoperative immunosuppression by analyzing monocytic HLA-DR expression and ex vivo stimulated cytokine secretion. Both tests have been evaluated in different studies to assess postoperative immune function.6,7
METHODS
The experimental procedures were approved by the Berlin office for animal protection in accordance with the German law on animal rights (G 261/07). Ten female domestic pigs were randomly subjected to laparoscopic and conventional open left lateral liver resection. All animals were euthanized after a 7-day follow-up period.
Operative Procedure
The pigs had a median weight of 45 kg (range, 41–55 kg) and mean age of 6 months (range, 5–8 months). All animals were premedicated with 10 mL of 10% ketamine, 6 mL of 2% xylazine (Rompun Bayer AG, Leverkusen, Germany), and 3 mL of azaperone (Stresnil Janssen Pharmaceutical, Beerse, Belgium). After induction with propofol, 2 to 7 mg/kg, anesthesia was maintained with isoflurane (0.8%–1.1%) and fentanyl (1–3 μg/kg per hour). The ventilator was set to a tidal volume of 15 to 20 mL/kg at a rate of 12 to 16 breaths per minute. The bladder was catheterized to improve intraoperative visibility and to allow urine monitoring. During the operation, all animals received approximately 1500 mL of electrolyte infusion.
All pigs were placed in the supine position and received a left lateral liver lobe resection with a 1064-nm Nd:YAG (neodymium:yttrium-aluminum-garnet) laser (Dornier MedTech, Wessling, Germany). The laser was operated with a flexible bare fiber of 4 m in length and 600 μm in diameter. Tissue dissection was performed with direct contact of the bare fiber to the liver (contact mode) and coagulation of small vessels and parenchymal bleeding with laser application from a 0.5- to 1-cm distance (noncontact mode). Laser application for both modes was in continuous wave mode with a fixed energy level at 40 W. Bleeding from larger vessels with a diameter >2 mm was controlled through temporary compression and clip application. A clear operative field was maintained by intermittent saline solution irrigation and suction.
Conventional Open Surgery
Access to the abdominal cavity was achieved by a midline incision ranging from the xiphoid to approximately 10 cm below the umbilicus. The abdominal wall was drawn back with a metal retractor, and the left liver lobe was manually exposed. The resection was performed as previously described.8 Wound closure was achieved with two layers of single sutures for skin and fascia.
Laparoscopy
A capnoperitoneum of 15 mm Hg was established after insertion of a Veress needle. The 10-mm camera access port was introduced blindly 2 cm above the umbilicus. A 30° laparoscope (MGB, Berlin, Germany) connected to a high-resolution camera (WOM, Berlin, Germany) allowed endoscopic visualization. Two additional 10-mm ports were inserted in a semicircular formation under videoscopic guidance. The animals were positioned in a reverse Trendelenburg angle to facilitate free access to the left liver lobe. Laser dissection demanded intermittent smoke evacuation with the suction and irrigation for clear visibility. Larger vessels were either clipped (Ethicon, Norderstedt, Germany) or occluded with an endoscopic bipolar forceps (Wolf, Knittlingen, Germany). The resected specimen was retrieved through an extended midline incision of the camera port.
Postoperative Course
Immediately after the operation, all animals received water and food and returned to their cage. During the postoperative course, blood samples were obtained and clinical parameters were assessed immediately after surgery and on postoperative day (POD) 1, POD 3, and POD 7. A standardized antibiotic regimen of perioperative intravenous ampicillin on POD 1 and intramuscular tetracycline on POD 1 and POD 3 was administered. On POD 7, all animals were euthanized with a lethal dose of thiopental, pancuronium bromide, and potassium chloride. Macroscopic autopsy findings were obtained and documented.
Laboratory Analysis
Cytokine secretion after lipopolysaccharide stimulation.
In this experimental setting, TNF-α, interferon (IFN) γ, IL-6, and IL-8 secretion was measured after ex vivo stimulation of whole blood samples with the lipopolysaccharide (LPS) endotoxin. The blood was retrieved from the femoral vein and immediately processed. Fifty microliters of blood was taken from each sample, diluted with 200 μL of RPMI Medium (RPMI 1640; Biochrom KG, Berlin, Germany), and incubated for 24 hours (37°C, 5% carbon dioxide) with 5000-ng/mL LPS endotoxin (Escherichia coli 0127:B8) (Sigma-Aldrich, Deisenhofen, Germany). Cell-free supernatants were harvested after centrifugation at 3000g at 4°C for 10 minutes. All samples were stored in 2-mL pyrogen-free polypropylene screw-cap tubes (Sarstedt, Nümbrecht-Rommelsdorf, Germany) at –85°C until final analysis. Measurements were conducted with commercially available enzyme-linked immunosorbent assay sets (Quantikine Porcine IFN-γ Immunoassay, DuoSet ELISA Porcine CXL8/IL-8, DuoSet ELISA Porcine IL-6, and DuoSet ELISA Porcine TNF-α; R&D Systems, Wiesbaden, Germany).
HLA-DR measurement on monocytes.
For the flow cytometric analysis of HLA-DR expression, 50 μL of whole blood in a Vacutainer tube (Becton Dickinson Biosciences, San Jose, CA, USA) containing EDTA was stained with 30 μL of monoclonal phycoerythrin-conjugated anti–HLA-DR and PerCP-Cy5.5–conjugated anti-CD14 antibodies (Quantibrite; Becton Dickinson Biosciences) in the dark at room temperature for 30 minutes. Erythrocyte lysis was achieved with 0.5 mL of lysing solution (Becton Dickinson Biosciences) for another 30 minutes at room temperature. Finally, the cells were washed with 1 mL of fluorescence activated cell sorting (FACS) buffer and analyzed on a FACSCalibur cytometer (Becton Dickinson Biosciences) to assess HLA-DR surface expression.
Statistical Analysis
Biometric analysis was performed with SPSS software (SPSS, Chicago, IL, USA). Regarding the small study group, nonparametric tests (Mann-Whitney U test) were applied to evaluate the data. Statistically significant differences were defined as P < .05.
RESULTS
Operation and Postoperative Course
Ten animals were randomly subjected to either laparoscopic or open left lateral liver resection. No major adverse events occurred during any of the surgeries. The mean dissection time was 43.6 minutes (± 9.2) for open surgery and 64.2 minutes (± 4.6) for laparoscopy. The mean blood loss was 337 mL (± 178) for conventional open surgery and 182 mL (± 93.2) for laparoscopy. The detailed results of the surgical procedures have been previously published.8 Adverse events during the postoperative course included transient fever in 3 animals (2 in the open surgery group and 1 in the laparoscopy group), 1 wound infection in each group, and 1 bilioma after open surgery. This resulted in a morbidity rate of 80% for open surgery compared with 40% after laparoscopy. Necropsy findings showed significantly increased formation of adhesions in the open surgery group.
Biochemical Parameters
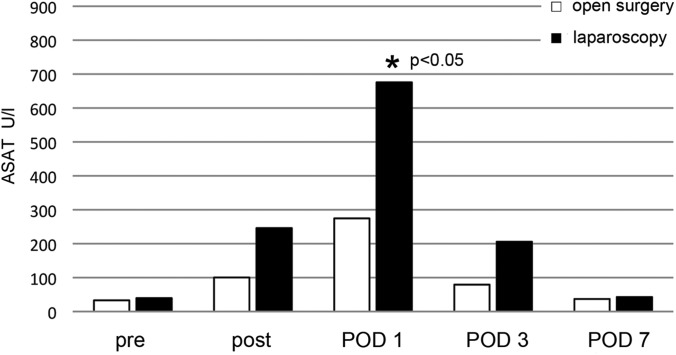
Postoperatively, transaminase levels were increased in both groups (Figure 1). On POD 1, laparoscopy showed a significant elevation of Aspartat-Aminotransferase (ASAT) glutamic oxaloacetic transaminase (GOT), with 675 U/L, compared with 274 U/L after open surgery. On POD 3, ASAT levels remained elevated and returned to baseline values on POD 7 in both groups. Other biochemical parameters and blood cell counts showed no significant differences.
Figure 1.
ASAT results for open surgery and laparoscopy before surgery (pre), immediately after surgery (post), and during the postoperative course (reference at 37°C, <52 U/L). Asterisk, P < .05.
Cytokine Secretion After Ex Vivo Stimulation of Whole Blood
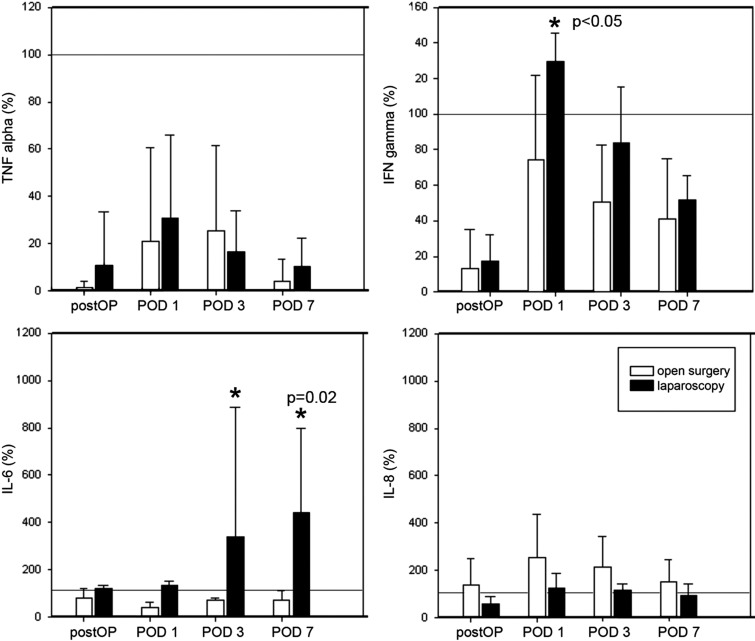
The preoperative measurements were used as baseline values (100%). TNF-α showed a significant drop in both groups immediately after the operation, with a recovery on POD 1 and another relapse on POD 3 to POD 7 (Figure 2). No statistically significant differences between open and laparoscopic surgery were observed. IFN-γ levels showed an 80% decrease after surgery and an elevation on POD 1 above baseline in the laparoscopy group (0.3-fold). By analogy with the TNF-α curve, IFN-γ values decreased on POD 3 and POD 7 in both groups. IFN-γ values were higher on all days in the laparoscopy group (P > .05). IL-6 levels decreased below the preoperative level in the open surgery group, with a minimum value on POD 1 and a slow increase on POD 3 and POD 7. After laparoscopy, a 0.2-fold increase was seen in postoperative IL-6 measurements, with a continuous rise during the complete course and a maximum 4.3-fold increase on POD 7 (P = .02). IL-8 showed a persistent increase after open surgery, with a maximum value on POD 1 (2.3-fold) and a return to baseline levels on POD 3 and POD 7. Laparoscopy presented an initial decrease (0.2-fold) and a light increase on POD 1 and POD 3, remaining below open surgical values throughout the follow-up period.
Figure 2.
Cytokine expression after whole blood induction with LPS endotoxin and 24 hours' incubation. The preoperative measurements were used as baseline values (100%). a, TNF-α showed a significant drop in both groups immediately after the operation (postOP), with a recovery on POD 1 and another relapse on POD 3, continuing on POD 7. b, IFN-γ levels showed an 80% decrease after surgery and an elevation on POD 1 above baseline in the laparoscopy group. Laparoscopy yielded higher IFN-γ values on all days (P > .05). c, IL-6 levels fell below the preoperative level in the open surgery group, with a minimum value on POD 1 and a slow increase on POD 3 and POD 7. After laparoscopy, a maximum 4.3-fold rise on POD 7 was visible (P = .02). d, IL-8 showed a persistent increase after open surgery, with a maximum value on POD 1 (2.3-fold) and a return toward baseline levels on POD 3 and POD 7. Laparoscopy presented an initial decrease (0.2-fold) and a light rise on POD 1 and POD 3, remaining constantly below open surgical values.
HLA-DR Expression on Monocytes
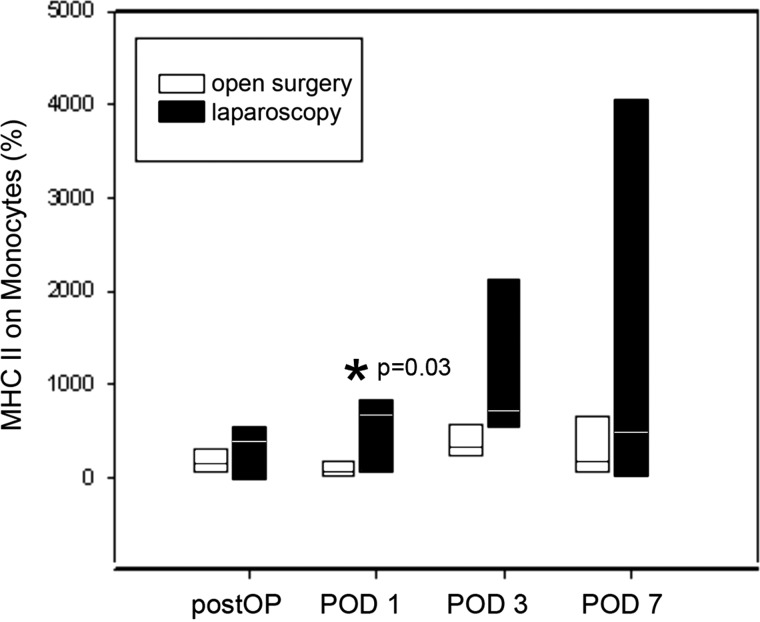
The preoperative median fluorescent intensity value represents the baseline value for the postoperative measurements (Figure 3). Immediate postoperative major histocompatibility complex class II density was increased in the laparoscopy group and remained elevated throughout POD 1 and POD 3 (maximum of 5.9-fold). After open surgery, a 0.35-fold decrease on POD 1 was observed, followed by an increase above baseline levels on POD 3. Both groups showed a return to baseline levels on POD 7. Differences on POD 1 were statistically significant (P = .03).
Figure 3.
HLA-DR expression on monocytes after laparoscopic and open surgery. The preoperative measurements were used as baseline values. Immediately postoperatively (postOP), major histocompatibility complex class II (MHC II) density was increased in the laparoscopy group, and it remained elevated on POD 1 and POD 3 (maximum of 5.9-fold). After open surgery, a 0.35-fold decrease on POD 1 was observed, followed by an elevation above baseline level on POD 3. Both groups showed a drop in major histocompatibility complex class II density on POD 7. Differences on POD 1 were statistically significant (P = .03 [asterisk]).
DISCUSSION
Laparoscopic resection of the liver has been successfully established at centers of expertise in hepatobiliary and minimally invasive surgery. Today, laparoscopic liver surgery is considered to be a safe and feasible technique for the resection of benign and malignant liver lesions.9 Several studies, including a meta-analysis, have proven the beneficial effects of laparoscopic liver resection compared with conventional surgery, with decreased postoperative pain, reduced rates of wound infection, and an accelerated return to normal activity without compromising the oncologic outcome.10,11
Although the immunologic aspects of laparoscopy have been thoroughly analyzed, data on laparoscopic liver resection remain limited (Table 1). It is generally accepted that surgical trauma results in a corresponding acute metabolic stress syndrome depending on the intensity of the initial damage. This postoperative metabolic stress response is characterized by the release of stress hormones, neuropeptides, proinflammatory cytokines (eg, TNF-α, IL-1, and IL-6), and acute phase proteins.5 The initial excessive inflammatory response is usually accompanied by a transient state of immunosuppression with a paralysis of cell-mediated immunity. These effects have been said to be responsible for an increased susceptibility to surgical infections and sepsis.6
Table 1.
Published Data on Immunologic Changes Comparing Laparoscopic and Open Liver Resection
| Study | Year | Operation | Animal | Parameters | Results | Other |
|---|---|---|---|---|---|---|
| Burpee et al5 | 2002 | Laparoscopic vs open liver resection | Pig (N = 14) | TNF-α; CRP; IL-6; cortisol | Open surgery group: IL-6 increaseda; TNF-α increaseda; CRP and cortisol increased and decreased (no difference) | Adhesions decreased after laparoscopy |
| Shimada et al4 | 2002 | Laparoscopic liver section vs open liver resection plus steroids vs open liver resection with no steroids | Human (N = 62) | CRP; IL-6; blood loss | Open surgery group: IL-6 decreaseda after steroid application | |
| Laparoscopic group: blood loss decreased; length of hospital stay decreased | ||||||
| Schmidt et al22 | 2010 | Capnoperitoneum vs no capnoperitoneum | Rat (N = 60) | IL-6; KI 67; liver enzymes | Laparoscopic group: liver regeneration impaired; IL-6 increased |
Parameters showed statistically significant differences (P < .05).
Various studies have shown that the extent of postoperative immune depletion is less apparent after laparoscopy compared with similar open surgical procedures.12,13 In a porcine model, Burpee et al.5 showed a significantly higher expression of TNF-α and IL-6 after open hepatic resection. In the same study, the response to delayed-type hypersensitivity was greater after laparoscopic liver resection. These results suggest a better preservation of immune response after laparoscopic liver surgery.
The primary focus of our experimental setting was the assessment of postoperative immune function through measurement of cytokine release by peripheral blood mononuclear cells and fluorescence activated cell sorting (FACS) analysis of the expression of the class II major histocompatibility molecule HLA-DR on monocytes. Postoperative expression of the HLA-DR antigen on monocytes is essential for antigen presentation and initiation of the specific immune response to infection.14,15 Several studies suggest a greater decrease or slower recovery of HLA-DR expression after laparotomy compared with laparoscopy.13,16,17 However, other authors have found a similar expression of HLA-DR after laparoscopic and conventional surgery.18,19 In our study the HLA-DR expression was increased in the laparoscopic group, though with a lack of significance. This finding may be interpreted as a trend toward better preservation of the nonspecific immune system after laparoscopic liver resection compared with the open procedure.
Regarding proinflammatory TNF-α, IFN-γ, and IL-6 release, laparoscopy showed higher levels over the postoperative course (except TNF-α on POD 3). However, except for IL-6 levels on POD 3 and POD 7, the differences were not significant. The higher expression of proinflammatory cytokines may result from the pneumoperitoneum itself. These findings are in accordance with other studies and are the result of ischemia/reperfusion injury after pneumoperitoneum and subsequent desufflation.20–22 The ischemia/reperfusion injury can also explain the higher levels of serum ASAT in the laparoscopic group. The slightly higher values of INF-γ in the laparoscopic group may reflect an advantage for cell-mediated immunity.23 These effects support the thesis of an improved immune preservation after laparoscopy. The initial drop below baseline values of TNF-α and IFN-γ can be interpreted as a general immunosuppression after surgical trauma. Interestingly, proangiogenic and proinflammatory IL-8 showed an opposite course, with elevated levels after open surgery. A possible explanation may be the greater blood loss after open hepatectomy in our study in accordance with Gion et al.,24 who found a positive correlation between postoperative IL-8 levels and estimated blood loss in patients undergoing hepatic resection.
Summary
In an experimental setting, we were able to show a trend toward improved preservation of the immune system after laparoscopic hepatic resection compared with open surgery. The main limitation of this study is certainly the small number of animals (N = 10). Thus further studies investigating the postoperative stress response are necessary to evaluate the potential immunologic advantages of laparoscopic liver surgery.
Contributor Information
Sascha S. Chopra, Department of General, Visceral, and Transplantation Surgery, Charité Campus Virchow Clinic, University Medicine Berlin, Berlin, Germany..
Nadine Haacke, Department of General, Visceral, and Transplantation Surgery, Charité Campus Virchow Clinic, University Medicine Berlin, Berlin, Germany..
Christian Meisel, Institute of Medical Immunology, Charité Campus Mitte, University Medicine Berlin, Berlin, Germany..
Nadine Unterwalder, Institute of Medical Immunology, Charité Campus Mitte, University Medicine Berlin, Berlin, Germany..
Panagiotis Fikatas, Department of General, Visceral, and Transplantation Surgery, Charité Campus Virchow Clinic, University Medicine Berlin, Berlin, Germany..
Sven C. Schmidt, Department of General, Visceral, and Transplantation Surgery, Charité Campus Virchow Clinic, University Medicine Berlin, Berlin, Germany..
References:
- 1. Gagner M, Rogula T, Selzer D. Laparoscopic liver resection: benefits and controversies. Surg Clin North Am. 2004;84(2):451–462 [DOI] [PubMed] [Google Scholar]
- 2. Schon MR. Value of laparoscopic liver resection [in German]. Chirurg. 2010;81(6):516–525 [DOI] [PubMed] [Google Scholar]
- 3. Simillis C, Constantinides VA, Tekkis PP, et al. Laparoscopic versus open hepatic resections for benign and malignant neoplasms—a meta-analysis. Surgery. 2007;141(2):203–211 [DOI] [PubMed] [Google Scholar]
- 4. Shimada M, Harimoto N, Maehara S, et al. Minimally invasive hepatectomy: modulation of systemic reactions to operation or laparoscopic approach? Surgery. 2002;131(1 Suppl):S312–317 [DOI] [PubMed] [Google Scholar]
- 5. Burpee SE, Kurian M, Murakame Y, Benevides S, Gagner M. The metabolic and immune response to laparoscopic versus open liver resection. Surg Endosc. 2002;16(6):899–904 [DOI] [PubMed] [Google Scholar]
- 6. Angele MK, Faist E. Clinical review: immunodepression in the surgical patient and increased susceptibility to infection. Crit Care. 2002;6(4):298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spies CD, Kip M, Lau A, et al. Influence of vaccination and surgery on HLA-DR expression in patients with upper aerodigestive tract cancer. J Int Med Res. 2008;36(2):296–307 [DOI] [PubMed] [Google Scholar]
- 8. Chopra SS, Schmidt SC, Wiltberger G, et al. Laparoscopic radiofrequency ablation of liver tumors: comparison of MR guidance versus conventional laparoscopic ultrasound for needle positioning in a phantom model. Minim Invasive Ther Allied Technol. 2011;20(4):212–217 [DOI] [PubMed] [Google Scholar]
- 9. Baker TB, Jay CL, Ladner DP, et al. Laparoscopy-assisted and open living donor right hepatectomy: a comparative study of outcomes. Surgery. 2009;146(4):817–823; discussion 823–825 [DOI] [PubMed] [Google Scholar]
- 10. Vigano L, Tayar C, Laurent A, Cherqui D. Laparoscopic liver resection: a systematic review. J Hepatobiliary Pancreat Surg. 2009;16(4):410–421 [DOI] [PubMed] [Google Scholar]
- 11. Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250(5):831–841 [DOI] [PubMed] [Google Scholar]
- 12. Vittimberga FJ, Jr, Foley DP, Meyers WC, Callery MP. Laparoscopic surgery and the systemic immune response. Ann Surg. 1998;227(3):326–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ordemann J, Jacobi CA, Schwenk W, Stosslein R, Muller JM. Cellular and humoral inflammatory response after laparoscopic and conventional colorectal resections. Surg Endosc. 2001;15(6):600–608 [DOI] [PubMed] [Google Scholar]
- 14. Cheadle WG, Hershman MJ, Wellhausen SR, Polk HC., Jr HLA-DR antigen expression on peripheral blood monocytes correlates with surgical infection. Am J Surg. 1991;161(6):639–645 [DOI] [PubMed] [Google Scholar]
- 15. Sietses C, Beelen RH, Meijer S, Cuesta MA. Immunological consequences of laparoscopic surgery, speculations on the cause and clinical implications. Langenbecks Arch Surg. 1999;384(3):250–258 [DOI] [PubMed] [Google Scholar]
- 16. Kloosterman T, von Blomberg BM, Borgstein P, Cuesta MA, Scheper RJ, Meijer S. Unimpaired immune functions after laparoscopic cholecystectomy. Surgery. 1994;115(4):424–428 [PubMed] [Google Scholar]
- 17. Veenhof AA, Sietses C, von Blomberg BM, et al. The surgical stress response and postoperative immune function after laparoscopic or conventional total mesorectal excision in rectal cancer: a randomized trial. Int J Colorectal Dis. 2011;26(1):53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dunker MS, Ten Hove T, Bemelman WA, Slors JF, Gouma DJ, Van Deventer SJ. Interleukin-6, C-reactive protein, and expression of human leukocyte antigen-DR on peripheral blood mononuclear cells in patients after laparoscopic vs. conventional bowel resection: a randomized study. Dis Colon Rectum. 2003;46(9):1238–1244 [DOI] [PubMed] [Google Scholar]
- 19. Klava A, Windsor A, Boylston AW, Reynolds JV, Ramsden CW, Guillou PJ. Monocyte activation after open and laparoscopic surgery. Br J Surg. 1997;84(8):1152–1156 [PubMed] [Google Scholar]
- 20. Nickkholgh A, Barro-Bejarano M, Liang R, et al. Signs of reperfusion injury following CO2 pneumoperitoneum: an in vivo microscopy study. Surg Endosc. 2008;22(1):122–128 [DOI] [PubMed] [Google Scholar]
- 21. Eleftheriadis E, Kotzampassi K, Botsios D, Tzartinoglou E, Farmakis H, Dadoukis J. Splanchnic ischemia during laparoscopic cholecystectomy. Surg Endosc. 1996;10(3):324–326 [DOI] [PubMed] [Google Scholar]
- 22. Schmidt SC, Schumacher G, Klage N, Chopra S, Neuhaus P, Neumann U. The impact of carbon dioxide pneumoperitoneum on liver regeneration after liver resection in a rat model. Surg Endosc. 2010;24(1):1–8 [DOI] [PubMed] [Google Scholar]
- 23. Buunen M, Gholghesaei M, Veldkamp R, Meijer DW, Bonjer HJ, Bouvy ND. Stress response to laparoscopic surgery: a review. Surg Endosc. 2004;18(7):1022–1028 [DOI] [PubMed] [Google Scholar]
- 24. Gion T, Taketomi A, Shirabe K, et al. The role of serum interleukin-8 in hepatic resections. Surg Today. 2010;40(6):543–548 [DOI] [PubMed] [Google Scholar]