Abstract
Objective
Compare pre and postoperative performance in patients undergoing cochlear implantation (CI) for unilateral severe-to-profound sensorineural hearing loss (single-sided deafness, SSD).
Study design
IRB-approved, prospective
Setting
Tertiary center
Patients
Twenty-nine patients have undergone CI for SSD. SSD was due to Ménière's disease (MD) in 10 subjects; these also suffered from recalcitrant vertigo spells and in these 10 patients along with 2 others the CI was placed simultaneous with a labyrinthectomy.
Intervention(s)
CI with or without labyrinthectomy.
Main outcome measure(s)
CNC word and AzBio sentences in quiet were administered to the implanted ear. A multiple-loudspeaker sound localization test was administered in the bilateral listening condition. All data were collected pre-operatively and 3, 6, 12-months post-operatively with post-operative data available for 19 subjects. Additionally, a tinnitus handicap questionnaire is administered pre- and 12-months post-operatively.
Results
CNC word and AzBio sentence scores showed improvement in the implanted ear. Sound localization appeared to improve in an experience dependent fashion in some patients. Most patients reported diminished tinnitus following cochlear implantation. All patients undergoing labyrinthectomy experienced resolution of vertigo attacks.
Conclusions
CI restores auditory function to the deafened ear. Additionally, the binaural input appears to improve sound localization for most patients. In patients with severe hearing loss and recalcitrant vertigo attacks due to MD, simultaneous labyrinthectomy and CI effectively relieves vertigo attacks and improves auditory function.
Keywords: cochlear implant, unilateral hearing loss, tinnitus, vertigo, endolymphatic hydrops
Introduction
Binaural hearing provides several advantages over monaural hearing including binaural summation, the binaural squelch effect, and the ability to localize sounds (1). Binaural summation occurs when the same stimulus is available to both two ears. Higher order processing of the redundant information received by the two ears provides a 2-6 dB improvement in signal detection threshold and leads to improvement in speech perception in quiet and in noise. The binaural squelch effect represents the ability to combine the noise from the ear with the poorer signal-to-noise (S/N) ratio with the noise from the ear with the more favorable S/N ratio allowing increased speech perception in background noise (2). Finally, the ability to localize sounds depends on the comparison of interaural timing and intensity differences between two independent ears (3).
An additional advantage of hearing with two ears relates to the head shadow effect. This occurs when the head acts as an acoustic barrier, which creates a spectral difference between the two ears and, in turn, a greater S/N ratio at one ear (4). Although two ears are not necessary to benefit from the head shadow, they enable the listener to attend to the ear with a better S/N ratio. Due to the loss of these binaural advantages, patients with severe-to-profound unilateral sensorineural hearing loss (SNHL, single-sided deafness, SSD) experience difficulties in hearing and understanding speech presented to the deaf side, and sound localization.
Standard rehabilitation options for SSD include contralateral routing of sound (CROS) and osseointegrated implants (OI) (5-9). Both help overcome the head shadow effect and restore auditory perception to the side with severe-to-profound SNHL. However, they do not restore binaural hearing and fail to fully rehabilitate the deficits of SSD. In particular, they provide little improvement in sound localization and modest improvement in speech perception in noisy environments (5-10). Electrical stimulation with a cochlear implant (CI) remains the only method to restore auditory perception to a deafened ear. Recent reports suggest that CIs provide improved ability to localize sound and speech perception in some situations with background noise compared to CROS and OI devices (11). Suppression of tinnitus represents another potential advantage of electrical stimulation over CROS and OI devices (12,13). Given the potential advantages and the initial encouraging results we have begun to offer CIs as an off-label indication to patients with SSD.
Some patients with late stage Ménière's disease (MD) experience debilitating vertigo and/or Tumarkin drop attacks that are unresponsive to medical therapy and/or non-ablative surgery (14,15). Many of these patients also suffer severe-to-profound SNHL in the affected ear. Chemical or surgical labyrinthectomy offers a high rate of vertigo control in these patients; however patients still suffer the consequences of SSD (16-18). In an effort to alleviate vertigo spells and rehabilitate the effects of SSD, we have recently offered simultaneous surgical labyrinthectomy and cochlear implantation to patients with MD, poor unilateral hearing and incapacitating vertigo.
Here we provide preliminary speech perception and sound localization outcomes on a cohort of 26 patients that have received a CI as an off-label indication to rehabilitate SSD. This report also includes 12 patients who underwent simultaneous labyrinthectomy and cochlear implantation.
Materials and Methods
Subjects
The Institutional Review Board of the University of Iowa approved the study protocol. From 2012- 2013, 29 patients with good to normal hearing in one ear and profound hearing loss in the opposite ear have received a CI at our institution. Of those patients, pre-operative data are available from 25 subjects and post-operative data are available from 19 subjects who have had at least three months of CI experience. Table 1 presents the demographic data for the patients implanted at the University of Iowa. Sixteen of the patients received Nucleus devices and 13 patients received Advanced Bionics devices. The mean age at implantation was 55.2±1.69 (mean±standard error, SE) years (range 31 years to 75 years) and the mean duration of severe to profound hearing loss in the implanted ear was 3.5±0.76 years. Females comprised 52% (15/29) of the patients. The mean pre-operative pure-tone average (PTA) calculated at 0.5, 1, 2, 4 kHz was 76.6±2.71 (SE) for the implanted ear and 22.1±2.31 for the better hearing ear. One patient had tried a CROS hearing aid prior to implantation (patient C1) as part of a separate study and one patient utilized an OI for 5 years prior to implantation (patient 28). All patients were counseled heavily either with a CI audiologist, physician, and/or hearing aid audiologist on their rehabilitative options prior to making the decision of a cochlear implant.
Table 1.
Subject demographics. Pre-operative pure-tone average (PTA) calculated at 0.5, 1, 2, 4 kHz. NU=Nucleus device; AB=Advanced Bionics device;ISSNHL=idiopathic sudden sensorineural hearing loss; IAC=Internal auditory canal; N/A=have not had post-CI testing; UNK=no data available.
| Subject ID | Age at Implant | Duration of Deafness (years) | Etiology | Left PTA | Right PTA | PreOp CI Ear CNC Score | Most Recent Post-Op CNC Score | CI Ear | CI Type |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | 2 | ISSNHL | 84 | 15 | 0 | 64 | Left | NU CI512 |
| 2 | 50 | 2 | Ménière's/ Labyrinthectomy | NR | 16 | 0 | 48 | Left | NU CI512 |
| 3 | 55 | 1 | Ménière's/ Labyrinthectomy | 21 | 86 | 0 | 50 | Right | NU Freedom |
| 4 | 57 | 2 | Ménière's/ Labyrinthectomy | 70 | 28 | UNK | 71 | Left | NU Freedom |
| 5 | 58 | 3.9 | ISSNHL | 64 | 13 | UNK | 39 | Left | AB 90K |
| 7 | 48 | 0.6 | ISSNHL | 13 | 71 | 3 | 15 | Right | AB 90K |
| 8 | 52 | 3 | poorly compensated vestibulopathy/Labyrinthectomy | NR | 15 | 0 | 0 | Left | NU CI422 |
| 9 | 51 | 15 | ISSNHL | NR | 63 | 0 | 36 | Left | AB 90K |
| 10 | 52 | 17 | Post-surgical | 34 | 96 | 0 | 1 | Right | AB 90K |
| 11 | 55 | 0.6 | ISSNHL | 9 | 68 | 16 | 15 | Right | AB 90K |
| 12 | 49 | 1.3 | Ménière's/ Labyrinthectomy | 44 | 75 | 3 | 28 | Right | AB 90K |
| 13 | 64 | 2.8 | ISSNHL | 6 | NR | 0 | UNK | Right | AB 90K |
| 14 | 75 | 0.9 | ISSNHL | 31 | 95 | 0 | 9 | Right | AB 90K |
| 15 | 56 | 1 | Facial nerve schwannoma | 31 | 85 | 29 | 3 | Right | NU CI422 |
| 16 | 61 | 1.6 | Ménière's/ Labyrinthectomy | 65 | 30 | 42 | 26 | Left | NU CI422 |
| 17 | 62 | 4.7 | ISSNHL | 83 | 31 | 11 | 4 | Left | AB 90K |
| 18 | 64 | 3 | ISSNHL | 75 | 10 | 3 | N/A | Left | NU CI422 |
| 19 | 53 | 0.5 | ISSNHL | 81 | 15 | 0 | 23 | Right | AB 90K |
| 20 | 58 | 3.4 | Ménière's/ Labyrinthectomy | 78 | 33 | 6 | 18 | Left | NU CI422 |
| 21 | 63 | 10 | Head Trauma | 90 | 19 | 7 | 58 | Left | NU CI422 |
| 22 | 63 | 8 | Ménière's/ Labyrinthectomy | 16 | 56 | 0 | 18 | Right | NU CI422 |
| 23 | 64 | 0.3 | ISSNHL | 28 | NR | 0 | N/A | Right | AB 90K |
| 26 | 63 | 0.8 | Ménière's/ Labyrinthectomy | 14 | 55 | 59 | N/A | Right | NU CI422 |
| 27 | 58 | 1 | ISSNHL | 93 | 33 | 0 | N/A | Left | AB 90K |
| 28 | 36 | 5 | ISSNHL | 98 | 15 | 0 | N/A | Left | NU CI422 |
| C1 | 59 | .8 | ISSNHL/Facial nerve paralysis | NR | 14 | 0 | N/A | Left | NU Freedom |
| NC1 | 42 | 4.3 | Ménière's/ Labyrinthectomy | 23 | 65 | UNK | UNK | Right | NU CI422 |
| NC2 | 45 | 1.6 | Ménière's/ Labyrinthectomy | 69 | 11 | UNK | UNK | Left | NU CI422 |
| NC3 | 31 | 4 | IAC lipoma/Translabyrinthine approach | 9 | 60 | UNK | UNK | Right | AB 90K |
Idiopathic sudden sensorineural hearing loss (ISSNHL) represented the most common etiology for the hearing loss (14/29, 48.2%). One patient with ISSNHL presented with poly cranial neuropathy including facial paralysis, vertigo, and profound SNHL. Another patient with ISSNHL had a vestibular schwannoma in the contralateral, better hearing ear.
Ménière's disease was the second most common etiology, accounting for the unilateral hearing loss in 10 patients (38.5%). All patients with MD had stage IV disease based on the modified system of the AAOHNS Committee on Hearing and Equilibrium (19). All 10 patients with MD underwent simultaneous labyrinthectomy for management of intractable vertigo. In another patient a labyrinthectomy was performed for management persistent dizziness following 3 doses of intratympanic gentamicin at another institution; however the patient did not fulfill diagnostic criteria for definite MD and was not including in the MD cohort. One other patient underwent simultaneous labyrinthectomy and CI during a translabyrinthine approach to an internal auditory canal lipoma with pre-existing profound unilateral HL and intractable vertigo. In these 12 cases the primary indication for surgical intervention was control of dizziness; cochlear implantation to improve hearing was performed simultaneously. One case each of unilateral HL resulted from middle ear surgery, head trauma, and an ipsilateral facial nerve schwannoma involving the internal auditory canal.
Speech perception
Speech perception was measured in quiet using recorded Consonant-Nucleus-Consonant monosyllabic words (CNC) (20) and AzBio sentences (21). CNC scoring was based on percent-correct performance at both the word and the phoneme levels and the AzBio sentences were scored by dividing the total number of words correctly identified by the total number of words possible. Two lists of CNC words and AzBio sentences were presented to each subject. All speech perception lists were randomized between subjects and no subject received two of the same lists during any test session.
Pre-operatively, the CNC words and AzBio sentences were presented in the sound field, in a 10′× 9.3′× 6.5′ sound-treated booth at 60 dB A. In order to isolate the poorer ear, masking, using speech-shaped noise, was presented to the better ear via an insert ear phone. If hearing loss allowed, a hearing aid was verified and fit in the poorer ear. Post-operatively, direct electrical connection to the cochlear implant sound processor was used to present CNC words allowing us to test the ear with the CI in isolation.
Sound localization
A localization test was administered using everyday sounds presented at 60 dB A from (22) an array of eight loudspeakers spanning a horizontal arc of 108°. Loudspeaker one and loudspeaker eight were placed 54° to the left and to the right of the straight-ahead (0°) position. Each of the 16 different sound items (23) were repeated six times randomly from one of the eight loudspeakers for a total of 96 presentations for the entire test. The subject was told to identify the loudspeaker number from which the sound originated, but not to identify the sound itself. Subjects were not given feedback as to a right or wrong answer. Localization performance was determined by calculating the average Root Mean Square (RMS)-error in degrees. All presentations of the sounds were used to calculate the Average RMS-error in degrees.
Results
All patients except one (patient 2) wear their devices throughout the waking day and all report satisfaction with their devices. Patient 2 wears his device daily, but intermittently.
Speech perception outcomes
Figures 1 and 2 present preoperative and 3, 6, 12, and 24-month postoperative speech perception scores in the implanted ear (CNC words and AzBio sentences, respectively) for the patients for which the data were available. For both tests, the data indicate a high degree of individual variability, as is commonly seen in regards to CI performance. Thirteen subjects have experienced some level of benefit (most recent post minus pre score) on word scores (average benefit 32% ± 5.7 SE; range 9% to 64%) and 14 subjects have experienced some level of benefit on sentence scores (average benefit 39% ± 7.7 SE; range 4% to 92%). Average scores for the cohort demonstrate improved hearing in the implanted ear following implantation and continued improvement with experience. Overall, the average improvement on CNC word scores for the 19 subjects with pre- and most recent post-operative data was 28% ± 5.1 (mean ± SE). This improvement ranged from 64% for one subject to a decrement of 26%. Overall, the average improvement on CNC word scores for the 19 subjects with pre- and post-operative data was 28% ± 5.1 (mean ± SE). This improvement ranged from 64% for one subject to a decrement of 26%. The differences in preoperative CNC word scores compared to most recent postoperative scores was statistically different (p<.05, Wilcoxon Signed Ranks test). On AzBio sentences, average improvement was 40% ± 6.6 with a range from 92% improvement for one subject to a decrement of 57% for another subject. The differences in preoperative AzBio word scores compared to most recent postoperative scores was statistically different (p<.05, Wilcoxon Signed Ranks test).
Figure 1.
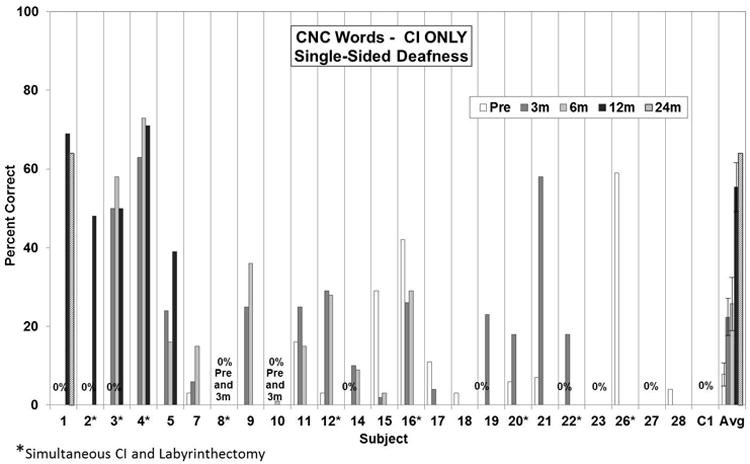
Pre- and post-operative CNC word scores in the implanted ear. Individual preoperative and 3, 6, and 12-month postoperative scores. The bars at the far right present the group mean. Error bars present standard error. The differences in preoperative scores compared to 6 and 12-month postoperative scores were statistically significant (p<0.05, Kruskall-Wallis ANOVA with post-hoc Dunn's method) whereas the preoperative scores were not statistically different than the 3-month postoperative scores.
Figure 2.
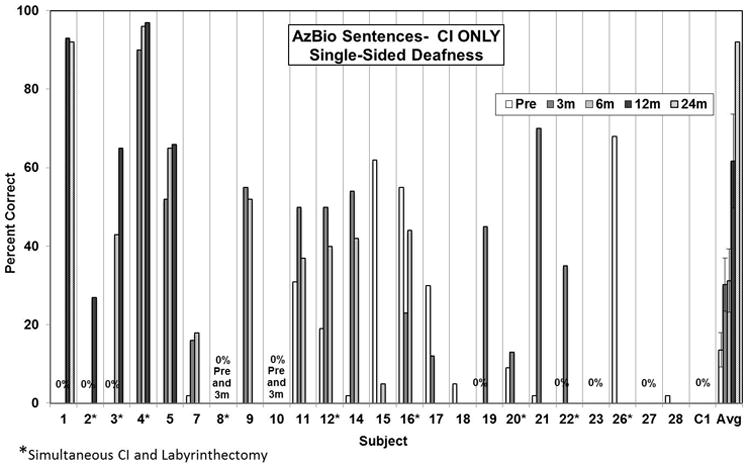
Pre- and post-operative AzBio sentence scores in the implanted ear. Individual preoperative and 3, 6, and 12-month postoperative scores. The bars at the far right present the group mean. Error bars present standard error. The differences in preoperative scores compared to 3 and 6-month postoperative scores were statistically significant (p=0.020 and p=0.018, respectively, one-way ANOVA with post-hoc Holm-Sidak method) whereas the preoperative scores were not statistically different than the 12-month postoperative scores.
Sound localization
Figure 3 presents preoperative and 3, 6, and 12-month postoperative individual and group average sound localization results. Performance on this task improved post-operatively in most, but not all, individuals. Further, as demonstrated by the average data for the group, performance improved from 3 to 12-months postoperatively suggesting that sound localization is experience dependent.
Figure 3.
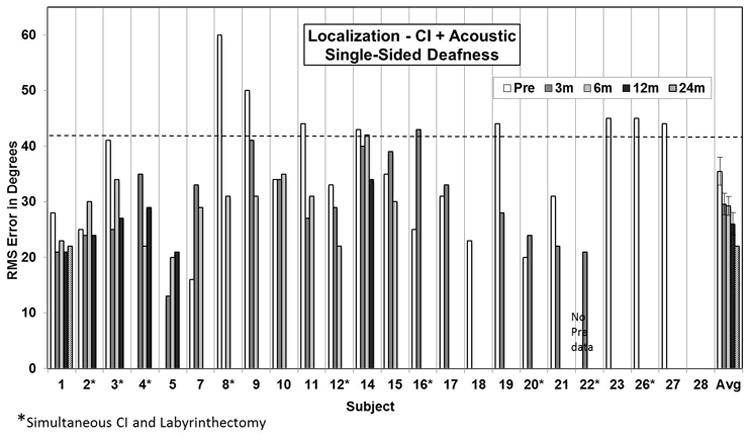
Pre- and post-operative sound localization results in the bilateral hearing condition. Individual preoperative and 3, 6, and 12-month postoperative root mean square (RMS) error in degrees scores are presented. The bars at the far right present the group mean. Error bars present standard error.
Vertigo
All patients with MD undergoing labyrinthectomy reported complete resolution of their vertigo attacks at their latest follow-up. Four patients with less than 6 months of follow-up from the labyrinthectomy noted mild dysequilibrium as they undergo vestibular compensation. Most patients do not yet have 18-24-month follow-up data so it is not possible to classify them according to the AAOHNS Committee on Hearing and Equilibrium guidelines, yet assuming continued control they would all be regarded as class A (19). The other two patients with disabling dizziness that underwent labyrinthectomy likewise experienced a resolution of the dizziness.
Tinnitus
In addition to improved speech perception in the implanted ear, most patients reported subjective improvement in tinnitus. We are actively collecting prospective pre and post-implantation tinnitus questionnaires to quantify changes in tinnitus perception over time; however at this time we do not have sufficient 12-month follow-up data to report.
Discussion
SSD significantly impacts auditory perception and performance, which in turn impacts function in educational, social and employment settings (24-26). For patients who seek rehabilitation, CROS and OIs systems remain the standard management options with proven efficacy (5-9). Cochlear implantation has recently emerged as another off-label option for the rehabilitation of SSD (11,27,28). Here we report our preliminary results with cochlear implantation in a series of patients with SSD and good hearing in the contralateral ear.
Speech perception
Recent reports have demonstrated improved speech perception for patients with SSD following cochlear implantation in the implanted ear (11,27,28). They also demonstrated that cochlear implantation enhanced speech perception in noise, particularly when the noise is presented to the implant side (11). Our results are largely consistent with this experience, with the majority of patients demonstrating significant improvement in speech perception in the deafened ear following CI. Three subjects demonstrated decreased CNC word scores 3-6 months post-operatively compared to the preoperative data. We do not yet have longer-term data on these two subjects and will continue to monitor their progress over the next 24 months. In one case (patient 15), poorer performance may be related to device issues. This patient required deactivation of 8 electrodes and demonstrated rising impedances. We are continuing to monitor the integrity and function of this device. Another patient (patient 16), underwent a simultaneous labyrinthectomy so the preoperative hearing does not reflect the actual hearing status of the ear without a CI. For this individual along with the other patients undergoing simultaneous labyrinthectomy, we are beginning to test these individuals postoperatively with or without the use of the CI to better compare the benefit of CI for speech perception with their current hearing status. Most patients in this study do not yet have >6 months experience with their CI so we have not been able to obtain speech perception in noise testing in patients with significant CI experience. Thus, outcomes for speech perception in noise testing for our patients will require further follow-up.
Our study was limited to adult patients with acquired hearing loss. SSD occurs in children and likely impacts development and performance (29). Whether or not rehabilitation of children with acquired or congenital SSD with CIs is beneficial remains to be shown, however early case reports are encouraging (30,31).
Sound localization
The ability to localize sounds depends on binaural input. It is not clear how well the brain can integrate acoustic signals from one ear with input from a CI in the other ear to localize sounds. The data presented here confirm that patients with SSD perform poorly on sound localization tasks. We only have 12-month postoperative localization data available for six subjects, limiting the conclusions that can be drawn on the ability of a CI to improve sound localization in patients with SSD. Also, some patients demonstrated decreased sound localization ability following CI in early post-operative testing. Nevertheless, the data from the entire cohort viewed collectively appear to support the notion that rehabilitation of SSD with a CI allows for improved sound localization and that this ability improves with time. Apparently with experience the brain is able to integrate acoustic signals from one ear with those provided by a CI in the opposite ear to help localize the source of a sound. For those patients undergoing simultaneous labyrinthectomy that had some residual hearing prior to labyrinthectomy, preoperative sound localization measures likely overestimate the patients' function without a CI. As with speech perception measures, we are beginning to compare sound localization in these individuals postoperatively with or without the use of the CI to better determine the benefit of CI in sound localization for these subjects. Additional follow-up data in a larger cohort of patients will be necessary to confirm these preliminary findings. Nevertheless, an improved ability to localize sounds represents one likely advantage of a CI over CROS or OI devices for rehabilitation of SSD.
Tinnitus suppression
Several studies suggest that cochlear implantation reduces tinnitus severity. CIs were initially considered in patients with SSD and severe tinnitus as a potential treatment to reduce tinnitus (28,32). Although we do not yet have 12-month post-operative tinnitus survey results to publish, most patients reported tinnitus suppression following activation of the CI. Tinnitus suppression by electrical stimulation may be limited to the duration of the stimulation while in other patients the suppression may persist beyond the period of electrical stimulation (12,13,33)
Simultaneous labyrinthectomy and cochlear implantation in MD
Chemical and surgical labyrinthectomy represent highly effective methods to reduce or eliminate vertigo attacks in patients with MD (16-18). Surgical labyrinthectomy results in profound deafness and is typically reserved those patients with pre-existing severe-to-profound hearing loss in the affected ear. The outcomes reported here mirror those reported by others confirming that surgical labyrinthectomy relieves vertigo spells in nearly all patients with intractable MD. Patients require a period of recuperation following chemical or surgical labyrinthectomy to allow for vestibular compensation. Nevertheless, most patients welcome the relief from unpredictable, severe vertigo spells.
Cochlear implantation is the only available therapy to restore auditory perception to an ear deafened by MD. Assuming the encouraging preliminary hearing results seen in our patients hold up over time, simultaneous labyrinthectomy and cochlear implantation not only provides relief from vertigo attacks, but also provides improved hearing for these patients. Depending on the long-term hearing outcomes following cochlear implantation, it may be reasonable to extend the indication of labyrinthectomy to patients with intractable vertigo and some residual hearing. Another advantage of cochlear implantation with or without labyrinthectomy in ears deafened by MD relates to the fact that over time some patients will develop the disease in the contralateral ear placing the patient at risk for bilateral deafness (34-38). Indeed many adults receiving a cochlear implant for bilateral deafness suffer from MD (39). Having a functioning CI in the initially deafened ear will allow the patient to continue hearing in the event that the hearing in the contralateral eventually deteriorates.
We prefer to perform simultaneous labyrinthectomy and CI in patients with severe-to-profound SNHL and intractable vertigo attacks for several reasons. These include reduced risks associated with another surgical procedure and anesthetic. Also, surgical labyrinthectomy may lead to soft tissue scarring or even ossification in the cochlea which could prohibit later implantation (40), although this is not a uniform response (41,42). Finally, immediate implantation reduces the time of deafness and performance with a CI is correlated with duration of deafness (43). One disadvantage of simultaneous labyrinthectomy and CI is that patients with residual hearing preoperatively will have not experienced the full consequences of SSD and may not fully appreciate the benefit of the CI for rehabilitation of the new deficit.
Conclusions
Patients with SSD experience significant problems with sound localization and speech perception in background noise leading many to seek rehabilitation options. CROS and OI devices provide significant benefit, yet fail to address deficits that require binaural auditory processing. The data reported here, coupled with recent reports, raise the possibility that restoration of binaural auditory perception via cochlear implantation offers advantages not provided by CROS or OI devices. The extent to which the advantages of CI outweigh those provided by CROS or OI technologies requires further comparative studies.
Acknowledgments
Support: NIDCD P50 DC00242
Footnotes
Conflicts: Bruce J. Gantz has served as a consultant for Advanced Bionics, Corp. and Cochlear Corp on new electrode designs.
References
- 1.Kamal SM, Robinson AD, Diaz RC. Cochlear implantation in single-sided deafness for enhancement of sound localization and speech perception. Curr Opin Otolaryngol Head Neck Surg. 2012;20:393–7. doi: 10.1097/MOO.0b013e328357a613. [DOI] [PubMed] [Google Scholar]
- 2.Dirks DD, Wilson RH. The effect of spatially separated sound sources on speech intelligibility. J Speech Hear Res. 1969;12:5–38. doi: 10.1044/jshr.1201.05. [DOI] [PubMed] [Google Scholar]
- 3.Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiol Rev. 2010;90:983–1012. doi: 10.1152/physrev.00026.2009. [DOI] [PubMed] [Google Scholar]
- 4.Shaw EA. Transformation of sound pressure level from the free field to the eardrum in the horizontal plane. J Acoust Soc Am. 1974;56:1848–61. doi: 10.1121/1.1903522. [DOI] [PubMed] [Google Scholar]
- 5.Nicolas S, Mohamed A, Yoann P, et al. Long-term benefit and sound localization in patients with single-sided deafness rehabilitated with an osseointegrated bone-conduction device. Otol Neurotol. 2013;34:111–4. doi: 10.1097/MAO.0b013e31827a2020. [DOI] [PubMed] [Google Scholar]
- 6.Desmet JB, Wouters K, De Bodt M, et al. Comparison of 2 implantable bone conduction devices in patients with single-sided deafness using a daily alternating method. Otol Neurotol. 2012;33:1018–26. doi: 10.1097/MAO.0b013e31825e79ba. [DOI] [PubMed] [Google Scholar]
- 7.Hol MK, Kunst SJ, Snik AF, et al. Pilot study on the effectiveness of the conventional CROS, the transcranial CROS and the BAHA transcranial CROS in adults with unilateral inner ear deafness. European archives of oto-rhino-laryngology. 2010;267:889–96. doi: 10.1007/s00405-009-1147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin LM, Bowditch S, Anderson MJ, et al. Amplification in the rehabilitation of unilateral deafness: speech in noise and directional hearing effects with bone-anchored hearing and contralateral routing of signal amplification. Otol Neurotol. 2006;27:172–82. doi: 10.1097/01.mao.0000196421.30275.73. [DOI] [PubMed] [Google Scholar]
- 9.Bishop CE, Eby TL. The current status of audiologic rehabilitation for profound unilateral sensorineural hearing loss. Laryngoscope. 2010;120:552–6. doi: 10.1002/lary.20735. [DOI] [PubMed] [Google Scholar]
- 10.Linstrom CJ, Silverman CA, Yu GP. Efficacy of the bone-anchored hearing aid for single-sided deafness. Laryngoscope. 2009;119:713–20. doi: 10.1002/lary.20164. [DOI] [PubMed] [Google Scholar]
- 11.Arndt S, Aschendorff A, Laszig R, et al. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol. 2011;32:39–47. doi: 10.1097/MAO.0b013e3181fcf271. [DOI] [PubMed] [Google Scholar]
- 12.Pan T, Tyler RS, Ji H, et al. Changes in the tinnitus handicap questionnaire after cochlear implantation. Am J Audiol. 2009;18:144–51. doi: 10.1044/1059-0889(2009/07-0042). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amoodi HA, Mick PT, Shipp DB, et al. The effects of unilateral cochlear implantation on the tinnitus handicap inventory and the influence on quality of life. Laryngoscope. 2011;121:1536–40. doi: 10.1002/lary.21851. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Fernandez N, Montes-Jovellar L, Cervera-Paz J, et al. Auditory and vestibular assessment of patients with Meniere's disease who suffer Tumarkin attacks. Audiol Neurootol. 2010;15:399–406. doi: 10.1159/000310899. [DOI] [PubMed] [Google Scholar]
- 15.Kentala E, Havia M, Pyykko I. Short-lasting drop attacks in Meniere's disease. Otolaryngology--head and neck surgery. 2001;124:526–30. doi: 10.1067/mhn.2001.115169. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen KD, Minor LB, Della Santina CC, et al. Vestibular function and vertigo control after intratympanic gentamicin for Meniere's disease. Audiol Neurootol. 2009;14:361–72. doi: 10.1159/000241893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diaz RC, LaRouere MJ, Bojrab DI, et al. Quality-of-life assessment of Meniere's disease patients after surgical labyrinthectomy. Otol Neurotol. 2007;28:74–86. doi: 10.1097/01.mao.0000233815.71671.6c. [DOI] [PubMed] [Google Scholar]
- 18.Teufert KB, Doherty J. Endolymphatic sac shunt, labyrinthectomy, and vestibular nerve section in Meniere's disease. Otolaryngologic clinics of North America. 2010;43:1091–111. doi: 10.1016/j.otc.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere's disease. American Academy of Otolaryngology-Head and Neck Foundation, Inc. Otolaryngology--head and neck surgery. 1995;113:181–5. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- 20.Tillman TW, Carhart R. An expanded test for speech discrimination utilizing CNC monosyllabic words. Northwestern University Auditory Test 6 SAM-TR-66-55. Tech Rep SAM-TR. 1966:1–12. doi: 10.21236/ad0639638. [DOI] [PubMed] [Google Scholar]
- 21.Spahr AJ, Dorman MF, Litvak LM, et al. Development and validation of the AzBio sentence lists. Ear and hearing. 2012;33:112–7. doi: 10.1097/AUD.0b013e31822c2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn CC, Tyler RS, Witt SA. Benefit of wearing a hearing aid on the unimplanted ear in adult users of a cochlear implant. J Speech Lang Hear Res. 2005;48:668–80. doi: 10.1044/1092-4388(2005/046). [DOI] [PubMed] [Google Scholar]
- 23.Kramer SE. Assessment of hearing disability and handicap: A multidimensional approach. Amersfoort, The Netherlands: Print Partner Ipskamp; 1998. [Google Scholar]
- 24.Chiossoine-Kerdel JA, Baguley DM, Stoddart RL, et al. An investigation of the audiologic handicap associated with unilateral sudden sensorineural hearing loss. Am J Otol. 2000;21:645–51. [PubMed] [Google Scholar]
- 25.Lieu JE, Tye-Murray N, Fu Q. Longitudinal study of children with unilateral hearing loss. Laryngoscope. 2012;122:2088–95. doi: 10.1002/lary.23454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieu JE, Tye-Murray N, Karzon RK, et al. Unilateral hearing loss is associated with worse speech-language scores in children. Pediatrics. 2010;125:e1348–55. doi: 10.1542/peds.2009-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Firszt JB, Holden LK, Reeder RM, et al. Auditory abilities after cochlear implantation in adults with unilateral deafness: a pilot study. Otol Neurotol. 2012;33:1339–46. doi: 10.1097/MAO.0b013e318268d52d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vermeire K, Van de Heyning P. Binaural hearing after cochlear implantation in subjects with unilateral sensorineural deafness and tinnitus. Audiol Neurootol. 2009;14:163–71. doi: 10.1159/000171478. [DOI] [PubMed] [Google Scholar]
- 29.Kral A, Hubka P, Heid S, et al. Single-sided deafness leads to unilateral aural preference within an early sensitive period. Brain. 2013;136:180–93. doi: 10.1093/brain/aws305. [DOI] [PubMed] [Google Scholar]
- 30.Plontke SK, Heider C, Koesling S, et al. Cochlear implantation in a child with posttraumatic single-sided deafness. European archives of oto-rhino-laryngology. 2013 doi: 10.1007/s00405-013-2350-2. [DOI] [PubMed] [Google Scholar]
- 31.Hassepass F, Aschendorff A, Wesarg T, et al. Unilateral deafness in children: audiologic and subjective assessment of hearing ability after cochlear implantation. Otol Neurotol. 2013;34:53–60. doi: 10.1097/MAO.0b013e31827850f0. [DOI] [PubMed] [Google Scholar]
- 32.Arts RA, George EL, Stokroos RJ, et al. Review: cochlear implants as a treatment of tinnitus in single-sided deafness. Curr Opin Otolaryngol Head Neck Surg. 2012;20:398–403. doi: 10.1097/MOO.0b013e3283577b66. [DOI] [PubMed] [Google Scholar]
- 33.Rubinstein JT, Tyler RS, Johnson A, et al. Electrical suppression of tinnitus with high-rate pulse trains. Otol Neurotol. 2003;24:478–85. doi: 10.1097/00129492-200305000-00021. [DOI] [PubMed] [Google Scholar]
- 34.Clemmens C, Ruckenstein M. Characteristics of patients with unilateral and bilateral Meniere's disease. Otol Neurotol. 2012;33:1266–9. doi: 10.1097/MAO.0b013e31826426b9. [DOI] [PubMed] [Google Scholar]
- 35.Belinchon A, Perez-Garrigues H, Tenias JM. Evolution of symptoms in Meniere's disease. Audiol Neurootol. 2012;17:126–32. doi: 10.1159/000331945. [DOI] [PubMed] [Google Scholar]
- 36.Sumi T, Watanabe I, Tsunoda A, et al. Longitudinal study of 29 patients with Meniere's disease with follow-up of 10 years or more (In commemoration of Professor Emeritus Isamu Watanabe) Acta oto-laryngologica. 2012;132:10–5. doi: 10.3109/00016489.2011.627570. [DOI] [PubMed] [Google Scholar]
- 37.Huppert D, Strupp M, Brandt T. Long-term course of Meniere's disease revisited. Acta oto-laryngologica. 2010;130:644–51. doi: 10.3109/00016480903382808. [DOI] [PubMed] [Google Scholar]
- 38.Nabi S, Parnes LS. Bilateral Meniere's disease. Curr Opin Otolaryngol Head Neck Surg. 2009;17:356–62. doi: 10.1097/MOO.0b013e3283304cb3. [DOI] [PubMed] [Google Scholar]
- 39.Lustig LR, Arts HA, Brackmann DE, et al. Hearing rehabilitation using the BAHA bone-anchored hearing aid: results in 40 patients. Otol Neurotol. 2001;22:328–34. doi: 10.1097/00129492-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Swartz JD, Mandell DM, Faerber EN, et al. Labyrinthine ossification: etiologies and CT findings. Radiology. 1985;157:395–8. doi: 10.1148/radiology.157.2.3931172. [DOI] [PubMed] [Google Scholar]
- 41.Osborn HA, Yeung R, Lin VY. Delayed cochlear implantation after surgical labyrinthectomy. The Journal of laryngology and otology. 2012;126:63–5. doi: 10.1017/S0022215111002374. [DOI] [PubMed] [Google Scholar]
- 42.Chen DA, Linthicum FH, Jr, Rizer FM. Cochlear histopathology in the labyrinthectomized ear: implications for cochlear implantation. Laryngoscope. 1988;98:1170–2. doi: 10.1288/00005537-198811000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Rubinstein JT, Parkinson WS, Tyler RS, et al. Residual speech recognition and cochlear implant performance: effects of implantation criteria. Am J Otol. 1999;20:445–52. [PubMed] [Google Scholar]


