Abstract
The observation of the locomotor and exploratory behaviors of rodents in an open field is one of the most fundamental methods used in the field of behavioral pharmacology. A variety of behaviors can be recorded automatically and can readily generate a multivariate pattern of pharmacological effects. Nevertheless, the optimal ways to characterize observed behaviors and concomitant drug effects are still under development. The aim of this study was to extract meaningful behavioral factors that could explain variations in the observed variables from mouse exploration. Behavioral data were recorded from male C57BL/6J mice (n = 268) using the Behavioral Pattern Monitor (BPM). The BPM data were subjected to the exploratory factor analysis. The factor analysis extracted four factors: activity, sequential organization, diversive exploration, and inspective exploration. The activity factor and the two types of exploration factors correlated positively with one another, while the sequential organization factor negatively correlated with the remaining factors. The extracted factor structure constitutes a behavioral model of mouse exploration. This model will provide a platform on which one can assess the effects of psychoactive drugs and genetic manipulations on mouse exploratory behavior. Further studies are currently underway to examine the factor structure of similar multivariate data sets from humans tested in a human BPM.
Keywords: Dimension, Exploration, Factor analysis, Locomotion, Open field
1. Introduction
Observing spontaneous activity in rodents is one of the fundamental and most frequently used methods in behavioral neuroscience. This method has been used in various neurobehavioral studies of rodents, including characterizing motor phenotypes, analyzing fear/anxiety, and assessing experimental manipulations. Researchers typically analyze animal behavior through the simultaneous observation of several variables. In these behavioral studies, however, the simultaneous measurement of the same behavior with different activity-monitoring devices could, in fact, measure different aspects of behavior. For example, activity counts measured by an Animex activity meter and a photocell cage, two standard activity-monitoring devices, have been shown not to be correlated with each other [1]. These discrepant findings may have been a consequence of differences in the sensitivities of the monitoring equipment. More importantly, however, animal behavior is not a unitary category. On the contrary, it is composed of several distinct domains of activity, such as overall locomotor activity, exploration, anxiety, and stereotypy; thus, the experimental effects on each of these distinct behaviors must be measured [2, 3]. Therefore, wide-ranging assessment of animal exploratory behavior requires multivariate measurements [4, 5]. The necessity for the simultaneous assessment of different behavioral characteristics is especially important when studying the effects of pharmacological manipulations on behavior, such as the administration of caffeine, nicotine, amphetamine, phencyclidine, 3,4-methylenedioxymethamphetamine (MDMA), and scopolamine. As all of these drugs increase activity despite having differing mechanisms of action, their specific effects on exploration can only be distinguished by the use of a multivariate assessment [6].
A demand for more refined techniques to record drug-induced changes in motor activities has led researchers to design an automated testing chamber that quantifies eight different components of behavior in rats [4]. The chamber is equipped with horizontal and vertical photobeams (the latter for corner positions), and the floor in the box has many small holes for the rodent to poke. With this chamber, the researchers observed that amphetamine increased locomotor activity and the number of times that holes were poked but decreased corner entries and time spent in the corners. In contrast, apomorphine and reserpine reduced all of these variables. After developing another device that consisted of a video-based, automated system, researchers recorded the distance traveled, frequency distribution of speeds, number of entries into an inner field, time spent in an inner field, and number of changes in corner positions [7]. Except for changes in corner positions, methamphetamine increased all measured variables in a dose-dependent manner. Another study using a similar video-based system reported that amphetamine increased the number of entries (from the corner to the edge, etc.), number of rearings made, and distance traveled [8]. These quantities were decreased by low doses (0.2 mg/kg or lower) of apomorphine, whereas a high dose (0.5 mg/kg) did not decrease the number of entries. The average speed and the number of rearings were reduced by all doses of apomorphine tested. In contrast, amphetamine (1–2 mg/kg) did not change the average speed but rather increased the number of rearings (at 2 mg/kg).
Another testing chamber (the Behavioral Pattern Monitor, BPM) included a grid of horizontal beams, a touch plate to detect rearing, floor holes, and wall holes. Thus, it was possible to measure various features of behavioral exploration in rats, including holepoking, rearing, locomotor activity, and locomotor patterns [5, 9]. To assess the spatiotemporal patterns of locomotion in the BPM, Geyer and coworkers developed specific measures, such as spatial d, the spatial and temporal coefficients of variation, and entropy [6, 10, 11]. These measurements quantify the linearity, predictability, and the organization of exploratory behavior. Later, the same laboratory developed a version of the BPM for mice [12] and further investigated the effects of various pharmacological agents, including psychostimulants [5, 10, 13] and hallucinogens [14–18], on locomotor activities in rats and mice. In rats, MDMA, which induces serotonin release, increased the locomotor activity and the spatial coefficient of variation but decreased rearing, holepoking, center entries, and spatial d [6, 10, 19]. In mice, MDMA increased the locomotor activity and the spatial coefficient of variation, decreased spatial d, and produced biphasic dose-dependent effects on entropy [12, 20]. While both amphetamine and apomorphine increased the locomotor activity in rats, amphetamine treatment increased the frequency of holepoking, whereas apomorphine decreased it [4, 21]. Amphetamine also reduced the spatial coefficient of variation in rats [5] but had no effect on spatial d [10]. In contrast, amphetamine administration reduced holepoking and spatial d in mice, while it also led to an increase in activity levels [22].
To date, researchers have measured an increasing number of variables that are purported to represent behavioral activities. However, there is little consistency in the sets of variables between studies. For example, to analyze the exploratory behaviors of rodents, some researchers have measured the amount of rearing, while others have measured the amount of holepoking. However, as described above, experimental manipulations can differentially alter these behaviors. There is also a possibility that some observed variables represent more than one aspect of behavior. Rearing, for example, depends not only on exploration per se but also on the amount of locomotor activity. Moreover, rearing may represent both inspective and diversive exploration of an environment, while holepoking is more likely to reflect inspective, investigatory exploration. There should be underlying factors that could explain variations in behaviors, such as locomotor activity, investigation, and exploration. Analyses of animal behaviors with these behavioral factors should be universal; ideally, the results would be independent of any arbitrary selection of variables in individual experiments. Therefore, for a more meaningful characterization of behaviors, the assessment of behaviors should be made on behavioral factors rather than on individual variables. Specifically, understanding the factors contributing to mouse exploration will provide important information on the cross-species translatability of the BPM. This understanding is particularly important given the advent of a human version of the BPM, which is being used to characterize exploratory behavior in psychiatric populations [22–24].
Several principal component analysis attempts have been made thus far to extract independent components from rodent behavioral data. The oldest attempt, to the best of our knowledge, on rat behavior in an open field test extracted three components, which the authors labeled “exploration”, “fear”, and “shifted activity” [25]. Another principal component analysis on behavioral data taken from 137 drug-naive rats during 60-minute sessions of the open field test with the BPM extracted three independent components: “the amount of activity” (i.e., total transitions and photobeam interruptions), “sequential response organization” (i.e., spatial d and entropy), and “exploratory activity” (i.e., the amount of holepoking and rearing) [26]. Later, the same laboratory performed principal component analysis on the behavior of mice (C57 strain, n = 61 and 129 strain, n = 33) in an open field [27]. The measured variables were area transitions, center time, spatial d, and entropy, with no rearing or holepoking variables. The principal component analysis extracted two components: “the amount of locomotor activity” (i.e., area transition and center time) and “the spatial organization of locomotion” (i.e., spatial d and entropy). This factorial structure is similar to that identified in the rat study [26], although it remains difficult to compare across species because of the lack of rearing and holepoking data. Another principal component analysis extracted three components (activity, exploration, and irritation) from 18 observed variables of mouse behavior in an open-field test without a hole board [28]. The exploration component was associated with the spatial component of locomotion. However, given that the test chamber in this study did not have a hole board, the extraction of the exploration component seems incomplete. Despite the importance of factorial analysis on behavioral data, there is no model that has acquired universal characteristics of rat/mouse behaviors. The aim of this study was to construct such a model with a small number of latent variables or factors characterizing mouse behavior in an open field.
2. Materials and methods
2.1. Animals
The subjects were male C57BL/6J mice (n = 268). The mice were obtained from Jackson Laboratories (Bar Harbor, ME) and were allowed to acclimate for approximately 1 week after arrival. They were housed at a vivarium at the University of California San Diego (UCSD), an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-approved animal facility that meets Federal and State requirements for the care and treatment of laboratory animals. All mice were housed 4 per cage in a climate-controlled room with a reversed light cycle (lights on at 20:00, off at 8:00). All testing occurred between 9:00 and 18:00. Food and water were provided ad libitum, except during behavioral testing. Animal testing was conducted according to the Principles of Laboratory Animal Care NIH guidelines and was approved by the UCSD Animal Care Committee. All efforts were made to minimize animal suffering and to reduce the total number of animals used.
2.2. Mouse BPM
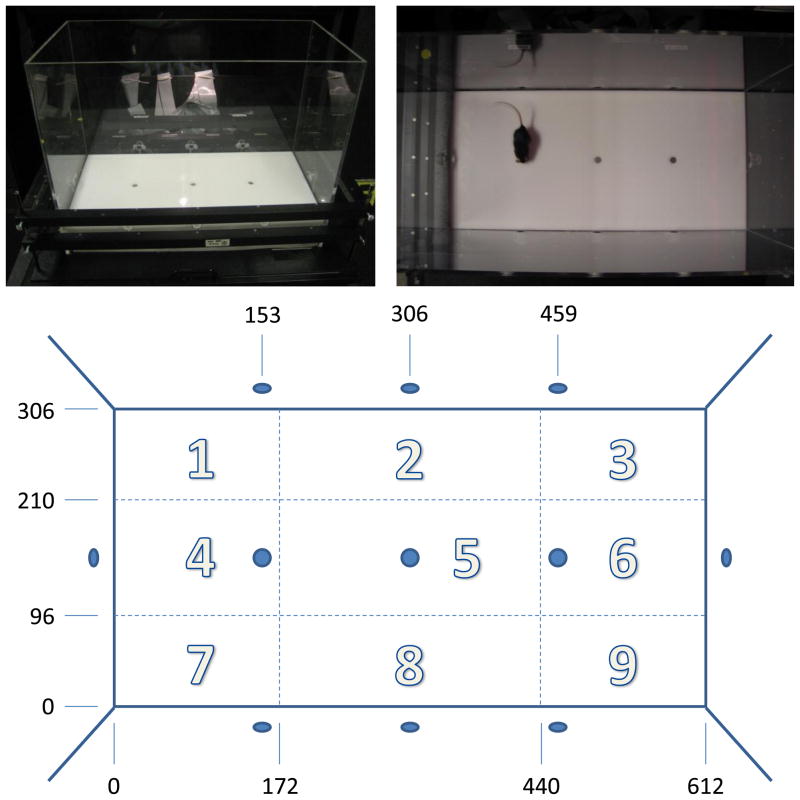
Spontaneous behavioral data were recorded using the BPM (San Diego Instruments, San Diego, CA), as described previously [12, 29, 30]. The mouse BPM chamber is a clear Plexiglas box, which has a Plexiglas hole board floor equipped with three floor holes and eight wall holes located 1.9 cm from the floor (Fig. 1). Each hole (1.2 cm diameter) was equipped with an infrared photobeam to detect holepoking behavior. The chamber was illuminated by a single light source above the arena (producing 350 lux in the center and 92 lux in the four corners). Mice were tested during the dark phase of their light cycle. During testing, a white noise generator produced background noise at 65 dB. At the start of each test session, a mouse was placed in the bottom left-handed corner of the chamber, facing the corner, and the test session started immediately. The location of the mouse was obtained from a grid of 12 × 24 infrared photobeams located 1 cm above the floor. Rearing behavior was detected by an array of 16 infrared photobeams placed 2.5 cm above the floor and aligned with the long axis of the chamber. The measurement of transitions, center time, and spatial coefficient of variation were based on the nine divided regions of the chambers. The status of the photobeams was sampled every 0.1 s. The session lasted 60 min. Raw data were transformed into the location of the animal (in x-y coordinates), whether holepoking or rearing occurred (events), and the duration of each event (time). The chambers were cleaned thoroughly between testing sessions.
Fig. 1.
The mouse BPM chamber (upper left panel). The chamber is a clear Plexiglas box with a Plexiglas hole board floor that is equipped with three floor holes and eight wall holes (upper right panel). Each hole (1.2 cm diameter) is equipped with an infrared photobeam so that holepoking behavior could be detected. The location of the mouse was obtained from a grid of 12 × 24 infrared photobeams located 1 cm above the floor. The arena was divided into nine horizontal regions (lower panel), on which the measurement of transitions, center time, and spatial coefficient of variation are based. The small figures [mm] indicate the boundaries of the nine regions and the positions of the holes.
2.3. Analysis
The BPM data that were transformed into nine variables are described in Table 1. Among the nine variables, the variables that were obtained directly from the measurement were as follows: counts, transitions, center time, %vrear, and %vpoke. As holepokes and rearings depend on the amount of activity, these two variables were normalized with respect to the amount of activity required to increase the independence of these measures from each other. Only the initial holepoke or rearing event in a series of responses not interrupted by another behavior was counted. Spatial d was calculated using a scaling hypothesis and resembled a fractal dimension [10]. The calculation of entropy was based on the dynamical system theory [31]. The spatial coefficient of variation was calculated from the distribution of transitions between the divided regions, while the temporal coefficient of variation was calculated from the distribution of time spent in the divided regions [5]. For more detailed descriptions and definitions of the variables, please refer to other studies [6, 10, 11, 31]. These variables had different variations within the test, and all of the variations are not considered to provide information equally. On the contrary, there would exist a correlation structure between the observed variables. For example, some variables may have very weak correlations with the other variables; they may have a unique piece of information on the behavior of mice. However, there may be variables that correlate strongly with one another. In this case, they might share common information on mouse behavior. Therefore, by interrelating a set of observed variables, mouse behavior could possibly be described with a smaller number of “latent variables”. Exploratory factor analysis is a statistical method that is used to extract such variables, called “factors”, by analyzing the pattern of relationships among the observed variables [32]. We performed exploratory factor analysis on the observed behavioral data. The estimation of factor loadings was made with the maximum likelihood estimation method. We ran the entire analysis under the R statistical computing environment (The R Project for Statistical Computing, http://www.r-project.org/). All of the observed variables were standardized before the analyses. In the factor analysis, we employed promax rotation, an oblique factor rotation, due to the fact that we expected the extracted factors to be correlated with one another.
Table 1.
Observed variables extracted from the mouse BPM data.
| Symbols | Variables | Description |
|---|---|---|
| cnts | counts | The number of times the mouse changes direction or activity type (e.g., when moving forward, it changes direction or completes a rear and then a holepoke). |
| trans | transitions | The BPM chamber is divided into nine different regions (Fig. 1) – a transition is when the mouse moves from one region to another. |
| CT | center time | The amount of time [sec] the mouse is in the center region (Region 5). |
| h | entropy | Entropy quantifies the variety of different locomotor patterns (h = 0 for a repetitive locomotion). Higher entropy indicates larger variability in locomotion, hence lower predictability of next movement. |
| d | spatial d | Spatial d, or dimensionality, quantifies the geometrical structure of the movement of the animal through space, showing whether the movement is in long, one-dimensional lines (d=1.0), in a meandering path (d=~1.5), or in more two-dimensional, localized movements (d=2.0). |
| sCV | spatial CV | Spatial coefficient of variation in the transition from one region to another. A more preferential exhibition of a subset of transitions results in a higher value of sCV. |
| tCV | temporal CV | Temporal coefficient of variation. A measure of the predictability of time spent in regions. Higher values of tCV indicate higher predictability. |
| %vrear | %varied rears normalized | %vrear = (vrearn/cnts)*100, where vrearn = total rears - rrears, and rrears is the number of repeated rears at the same location. |
| %vpoke | %varied pokes normalized | %vpokn = (vpokn/cnts)*100, where vpokn = total pokes - rpokes, and rpokes is the number of repeated pokes in the same hole without an intervening transition or rearing. Thus, varied pokes reflects bouts of holepoking behavior. |
3. Results
3.1. Correlations
The means and standard deviations of the observed variables are given in Table 2. The Pearson’s correlations between the observed variables are shown in Table 3. The counts and transitions had high correlations with each other, and entropy had high correlations with the counts and transitions. However, the temporal coefficient of variation had negative correlations with counts and transitions. The spatial d and the spatial coefficient of variation highly correlated with each other. While %vrear had modest correlations with entropy and transitions, %vpoke had rather weak correlations with all of the remaining variables. There was no correlation between %vrear and %vpoke.
Table 2.
Means and standard deviations (SDs) of the observed variables.
| Mean | SD | |
|---|---|---|
| cnts | 13675 | 3099 |
| trans | 1170 | 362 |
| CT | 6.89 | 3.33 |
| h | 0.193 | 0.024 |
| d | 1.36 | 0.075 |
| sCV | 0.977 | 0.119 |
| tCV | 1.35 | 0.647 |
| %vpoke | 0.239 | 0.097 |
| %vrear | 0.264 | 0.137 |
Table 3.
Pearson’s correlations between the observed variables. The correlations with absolute values greater than 0.500 are in bold.
| cnts | trans | CT | h | d | sCV | tCV | %vrear | %vpoke | |
|---|---|---|---|---|---|---|---|---|---|
| cnts | 1 | 0.942 | 0.336 | 0.705 | −0.286 | −0.456 | −0.721 | 0.360 | −0.017 |
| trans | 0.942 | 1 | 0.342 | 0.815 | −0.168 | −0.423 | −0.595 | 0.411 | −0.070 |
| CT | 0.336 | 0.342 | 1 | 0.404 | −0.330 | −0.571 | −0.407 | 0.347 | 0.174 |
| h | 0.705 | 0.815 | 0.404 | 1 | −0.231 | −0.567 | −0.465 | 0.483 | 0.024 |
| d | −0.286 | −0.168 | −0.330 | −0.231 | 1 | 0.628 | 0.410 | −0.209 | −0.169 |
| sCV | −0.456 | −0.423 | −0.571 | −0.567 | 0.628 | 1 | 0.495 | −0.380 | −0.149 |
| tCV | −0.721 | −0.595 | −0.407 | −0.465 | 0.410 | 0.495 | 1 | −0.266 | −0.229 |
| %vrear | 0.360 | 0.411 | 0.347 | 0.483 | −0.209 | −0.380 | −0.266 | 1 | 0.075 |
| %vpoke | −0.017 | −0.070 | 0.174 | 0.024 | −0.169 | −0.149 | −0.229 | 0.075 | 1 |
3.2. Four-factor solution
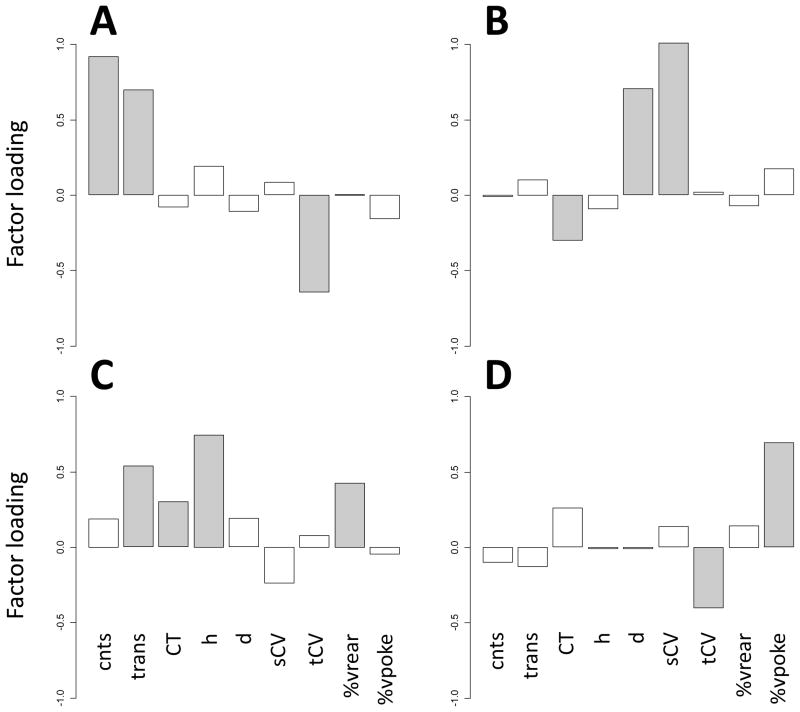
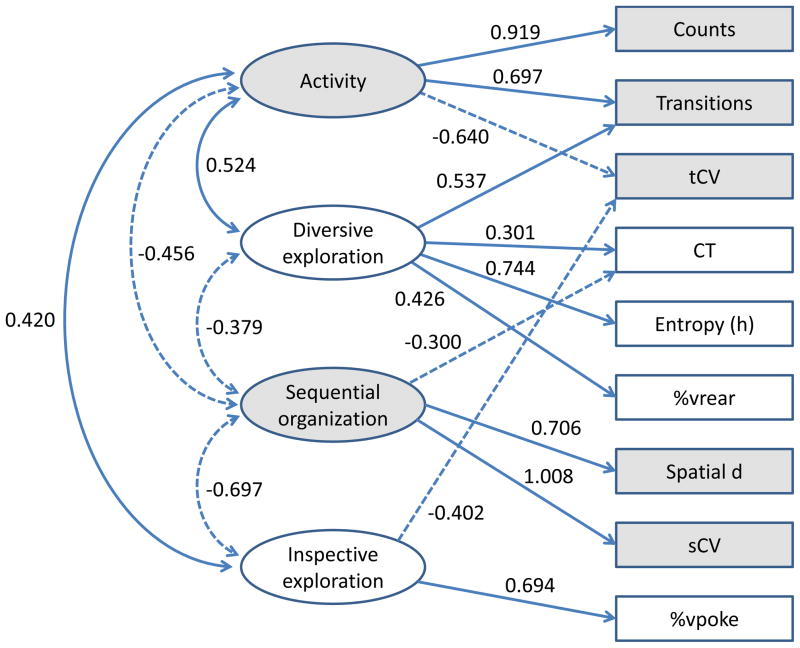
The factor analysis extracted four factors. The loadings of the observed variables on the extracted factors are shown in Table 4 and Fig. 2. The counts and transitions had high factor loadings on the first factor. This factor was labeled “activity”. Spatial d and spatial coefficient of variation had high factor loadings on the second factor, which was labeled “sequential organization”. The third factor was labeled “diversive exploration” due to the fact that the entropy, transitions, and %vrear had high factor loadings on this factor. This factor describes diversive behavior for stimulus/sensation-seeking. The fourth factor increases %vpoke and decreases the temporal coefficient of variation. This factor was considered to describe the inspection of particular sources that could supply precise information that the animal misses [3]; it was labeled “inspective exploration”. A path diagram of the four-factor model is shown in Fig. 3. The extracted factors were correlated with one another, and the Pearson’s correlations between the factors are given in Table 5.
Table 4.
Loadings of the observed variables on the factors extracted by the exploratory factor analysis with promax rotation (n = 268). The factor loadings whose magnitudes were greater than or equal to 0.300 are indicated in bold type.
| Factor | ||||
|---|---|---|---|---|
| Activity | Sequential organization | Diversive exploration | Inspective exploration | |
| cnts | 0.919 | −0.007 | 0.188 | −0.097 |
| trans | 0.697 | 0.102 | 0.537 | −0.128 |
| CT | −0.077 | −0.300 | 0.301 | 0.260 |
| h | 0.193 | −0.089 | 0.744 | −0.008 |
| d | −0.105 | 0.706 | 0.191 | −0.009 |
| sCV | 0.085 | 1.008 | −0.237 | 0.138 |
| tCV | −0.640 | 0.020 | 0.078 | −0.402 |
| %vrear | 0.004 | −0.069 | 0.426 | 0.143 |
| %vpoke | −0.154 | 0.174 | −0.043 | 0.694 |
Fig. 2.
Factor loading profiles of the observed variables. A: The activity factor. B: The sequential organization factor. C: The diversive exploration factor. D: The inspective exploration factor. The gray bars indicate factor loadings whose magnitudes were greater than 0.300.
Fig. 3.
Path diagram of the four-factor model with promax (oblique) factor rotation. The diagram shows the paths with factor loadings whose magnitudes were greater than or equal to 0.300. The bidirectional arrows show correlations between the factors.
Table 5.
Pearson’s correlations between the extracted factors.
| Factor | Activity | Sequential organization | Diversive exploration | Inspective exploration |
|---|---|---|---|---|
| Activity | 1 | −0.456 | 0.524 | 0.420 |
| Sequential organization | −0.456 | 1 | −0.379 | −0.697 |
| Diversive exploration | 0.524 | −0.379 | 1 | 0.253 |
| Inspective exploration | 0.420 | −0.697 | 0.253 | 1 |
4. Discussion
4.1. Factor loadings
The activity factor has a positive influence on both the counts and transitions, but it has a negative influence on the temporal coefficient of variation. The former influences are intelligible, and the latter negative influence would be due to the fact that high activity decreased the predictability of time spent in regions and then decreased the temporal coefficient of variation. The sequential organization factor increases spatial dimension and the spatial coefficient of variation due to patterned locomotion. The reason why this factor decreases center time would be that mice tended to exhibit such patterned locomotion in the periphery of the field. The spatial coefficient of variation is increased when the animal preferentially repeats certain transitions, hence reflecting the predictability of movement [5]. This increase would also increase spatial d; however, diversive exploration would preferentially decrease repeated transitions. Therefore, the spatial coefficient of variation had a positive loading on the sequential organization factor but a negative loading on the diversive exploration factor. The diversive exploration factor increases transitions, center time, entropy, and %vrear, whereas the inspective exploration factor increases %vpoke but decreases the temporal coefficient of variation. This marked difference in loading profiles between the diversive and inspective exploration factors supports the notion that exploration is subdivided into diversive and inspective (or specific) exploration [3].
4.2. Differences between the current mouse model and previous rat models
The previous model describing rat exploratory behavior had three components: the amount of activity, exploratory activity, and sequential response organization [26]. This three-component model, which was extracted from six observed variables, is similar to the current model for mice. On the other hand, there exist differences between these two models. In the rat study, spatial d and entropy were positively correlated and loaded onto the sequential response organization component [26]. This means that locomotion with higher spatial d tends to have higher entropy. Interestingly, mouse behavior seems to be contrary to the result in rats. In this study, spatial d and entropy were negatively correlated and loaded onto distinct factors. This difference between the rat and mouse models might be due to the difference in the size of the animals relative to the arena dimension. Moreover, the spatial coefficient of variation, along with spatial d, loads onto the sequential organization factor in our mouse model. However, rat model did not have the variable of the spatial coefficient of variation; therefore, the correspondence between the sequential organization factor in our model and the sequential response organization component in the previous model for rats is incomplete.
4.3. Rearing vs. holepoking
In a previous model for rat behavior, the number of rearings and holepoking loaded onto the exploratory activity component [26]. In contrast, in our models, rearing and holepoking load onto separate exploratory factors. This finding is consistent with pharmacological evidence that GBR 12909 and modafinil differentially affect rearing and holepoking in C57BL/6J and 129 strain mice, respectively [13, 30]. Additional pharmacological evidence that differentiates rearing and holepoking in C57BL/6J mice comes from the finding that psilocin, a hallucinogen, reduces holepoking by activating the 5-HT1A receptor, while reducing rearing through a different receptor mechanism [18]. Although psilocin administration affected both rearing and holepoking in mice, the fact that distinct receptor mechanisms were responsible for the reductions of rearing and holepoking supports the differentiation of these variables [15].
4.4. Four-factor model
The extraction of the factors of activity and sequential organization is consistent with both the previous rat [26] and mouse [27] models. Increasing the number of observed variables in the rat model disclosed exploration as an additional dimension of rodent behavior. This analysis demonstrated that exploration is further subdivided into diversive and inspective exploration. Interestingly, holepoking correlated significantly with risk-taking behavior in dopamine transporter knockdown mice [33], which are a mouse model of bipolar mania [34], while rearing did not. Thus, the inspective exploration factor in mice may provide information on specific behaviors related to frontal functioning and environmental manipulation. These findings are consistent with the dissociation of the inspective exploration factor from the diversive exploration factor in mice, resulting in a fourth dimension of mouse behavior. Therefore, the model extracted in this study has four behavioral dimensions, each of which corresponds to the factors extracted. This four-dimensional structure is based on the C57BL/6J mouse data. Although this structure seems general, it remains to be determined whether other mouse strains have different associations of variables with these factors. This model can be used for future model-based studies of the effects of drugs, genetic manipulation, and polymorphisms. Under varying conditions, one would be able to characterize behavioral changes in rodents in a systematic or consistent manner through the use of this model. This approach would be useful for several purposes, including drug development and the testing of animal models of psychiatric disorders. The results of these evaluations will be generalizable and comparable between studies. It is likely that this model-based approach is also applicable to human studies [2, 22]. Studies are currently underway to examine the factor structure of similar multivariate datasets from humans tested in a human BPM.
5. Conclusions
The factor analysis extracted four behavioral factors – activity, sequential organization, diversive exploration, and inspective exploration – that constitute a four-dimensional behavioral model in mice. This model has an advantage in the evaluation of drug effects on mouse behaviors because of the universal nature of the extracted factors. This model will also be useful for characterizing the effects of genetic manipulations on exploratory behaviors and in the animal models of psychiatric and neural diseases.
Acknowledgments
We thank Richard Sharp for his assistance. The original animal experiments were supported by the following NIH awards: MH071916 (MAG), DA002925 (MAG), and DA025412 (ALH). They were also supported by the Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center. This analytical study was supported by the Human Informatics Research Center at Sophia University (ST).
Footnotes
Authors’ contributions
ST performed the analysis. JWY, ALH, VLM, and MAG performed the original animal experiments. All authors read and approved the final manuscript.
Conflict of Interest
JWY reports receiving funding from Cerca Insights, but it had no bearing on this manuscript. MAG has received consulting compensation from Abbott, Addex, Cerca, Merck, Omeros, Takeda, and Teva and holds an equity interest in San Diego Instruments. ST, ALH, and VLM have no conflicts of interest.
References
- 1.Ljungberg T. Reliability of two activity boxes commonly used to assess drug induced behavioural changes. Pharmacology Biochemistry and Behavior. 1978;8:191–5. doi: 10.1016/0091-3057(78)90336-2. [DOI] [PubMed] [Google Scholar]
- 2.Henry BL, Minassian A, Young JW, Paulus MP, Geyer MA, Perry W. Cross-species assessments of motor and exploratory behavior related to bipolar disorder. Neuroscience & Biobehavioral Reviews. 2010;34:1296–306. doi: 10.1016/j.neubiorev.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berlyne DE. Curiosity and exploration. Science. 1966;153:25–33. doi: 10.1126/science.153.3731.25. [DOI] [PubMed] [Google Scholar]
- 4.Ljungberg T, Ungerstedt U. A method for simultaneous recording of eight behavioral parameters related to monoamine neurotransmission. Pharmacology Biochemistry and Behavior. 1978;8:483–9. doi: 10.1016/0091-3057(78)90088-6. [DOI] [PubMed] [Google Scholar]
- 5.Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: Pharmacological and behavioral analyses. Pharmacology Biochemistry and Behavior. 1986;25:277–88. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- 6.Geyer MA, Paulus MP. Multivariate and nonlinear approaches to characterizing drug effects on the locomotor and investigatory behavior of rats. NIDA Res Monogr. 1992;124:203–35. [PubMed] [Google Scholar]
- 7.Tanger HJ, Vanwersch RAP, Wolthuis OL. Automated TV-based system for open field studies: Effects of methamphetamine. Pharmacology Biochemistry and Behavior. 1978;9:555–7. doi: 10.1016/0091-3057(78)90058-8. [DOI] [PubMed] [Google Scholar]
- 8.Nickolson VJ. Detailed analysis of the effects of apomorphine and d-amphetamine on spontaneous locomotor behaviour of rats as measured in a TV-based, automated open-field system. European Journal of Pharmacology. 1981;72:45–56. doi: 10.1016/0014-2999(81)90295-8. [DOI] [PubMed] [Google Scholar]
- 9.Flicker C, Geyer MA. Behavior during hippocampal microinfusions. I. Norepinephrine and diversive exploration. Brain Research Reviews. 1982;4:79–103. doi: 10.1016/0165-0173(82)90006-6. [DOI] [PubMed] [Google Scholar]
- 10.Paulus MP, Geyer MA. A temporal and spatial scaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology (Berl) 1991;104(1):6–16. doi: 10.1007/BF02244547. [DOI] [PubMed] [Google Scholar]
- 11.Paulus MP, Geyer MA. A scaling approach to find order parameters quantifying the effects of dopaminergic agents on unconditioned motor activity in rats. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1991;15:903–19. doi: 10.1016/0278-5846(91)90018-v. [DOI] [PubMed] [Google Scholar]
- 12.Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential Contributions of Dopamine D1, D2, and D3 Receptors to MDMA-Induced Effects on Locomotor Behavior Patterns in Mice. Neuropsychopharmacology. 2006;31:2349–58. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- 13.Young J, Goey A, Minassian A, Perry W, Paulus M, Geyer M. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology. 2010;208:443–54. doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebs-Thomson K, Paulus MP, Geyer MA. Effects of hallucinogens on locomotor and investigatory activity and patterns: influence of 5-HT2A and 5-HT2C receptors. Neuropsychopharmacology. 1998;18(5):339–51. doi: 10.1016/S0893-133X(97)00164-4. [DOI] [PubMed] [Google Scholar]
- 15.Halberstadt AL, Geyer MA. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61:364–81. doi: 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams LM, Geyer MA. LSD-induced alterations of locomotor patterns and exploration in rats. Psychopharmacology (Berl) 1982;77:179–85. doi: 10.1007/BF00431945. [DOI] [PubMed] [Google Scholar]
- 17.Adams LM, Geyer MA. A proposed animal model for hallucinogens based on LSD’s effects on patterns of exploration in rats. Behavioral Neuroscience. 1985;99:881–900. doi: 10.1037//0735-7044.99.5.881. [DOI] [PubMed] [Google Scholar]
- 18.Halberstadt AL, Koedood L, Powell SB, Geyer MA. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. Journal of Psychopharmacology. 2011;25:1548–61. doi: 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold LH, Koob GF, Geyer MA. Stimulant and hallucinogenic behavioral profiles of 3,4-methylenedioxymethamphetamine and N-ethyl-3,4-methylenedioxyamphetamine in rats. Journal of Pharmacology and Experimental Therapeutics. 1988;247:547–55. [PubMed] [Google Scholar]
- 20.Powell SB, Lehmann-Masten VD, Paulus MP, Gainetdinov RR, Caron MG, Geyer MA. MDMA “ecstasy” alters hyperactive and perseverative behaviors in dopamine transporter knockout mice. Psychopharmacology (Berl) 2004;173(3–4):310–7. doi: 10.1007/s00213-003-1765-7. [DOI] [PubMed] [Google Scholar]
- 21.Geyer MA, Light RK, Rose GJ, Petersen LR, Horwitt DD, Adams LM, et al. A characteristic effect of hallucinogens on investigatory responding in rats. Psychopharmacology (Berl) 1979;65:35–40. doi: 10.1007/BF00491975. [DOI] [PubMed] [Google Scholar]
- 22.Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, et al. A Reverse-Translational Study of Dysfunctional Exploration in Psychiatric Disorders: From Mice to Men. Archives of General Psychiatry. 2009;66:1072–80. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young JW, Minassian A, Paulus MP, Geyer MA, Perry W. A reverse-translational approach to bipolar disorder: Rodent and human studies in the Behavioral Pattern Monitor. Neuroscience & Biobehavioral Reviews. 2007;31:882–96. doi: 10.1016/j.neubiorev.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minassian A, Henry BL, Geyer MA, Paulus MP, Young JW, Perry W. The quantitative assessment of motor activity in mania and schizophrenia. Journal of Affective Disorders. 2010;120:200–6. doi: 10.1016/j.jad.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markel AL, Galaktionov YuK, Efimov VM. Factor analysis of rat behavior in an open field test. Neuroscience and Behavioral Physiology. 1989;19(4):279–86. doi: 10.1007/BF01236015. [DOI] [PubMed] [Google Scholar]
- 26.Paulus MP, Geyer MA. Three independent factors characterize spontaneous rat motor activity. Behavioural Brain Research. 1993;53(1–2):11–20. doi: 10.1016/s0166-4328(05)80262-1. [DOI] [PubMed] [Google Scholar]
- 27.Paulus MP, Dulawa SC, Ralph RJ, Geyer MA. Behavioral organization is independent of locomotor activity in 129 and C57 mouse strains. Brain Research. 1999;835:27–36. doi: 10.1016/s0006-8993(99)01137-3. [DOI] [PubMed] [Google Scholar]
- 28.Jähkel M, Rilke O, Koch R, Oehler J. Open field locomotion and neurotransmission in mice evaluated by principal component factor analysis-effects of housing condition, individual activity disposition and psychotropic drugs. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2000;24:61–84. doi: 10.1016/s0278-5846(99)00081-0. [DOI] [PubMed] [Google Scholar]
- 29.Halberstadt AL, van der Heijden I, Ruderman MA, Risbrough VB, Gingrich JA, Geyer MA, et al. 5-HT2A and 5-HT2C Receptors Exert Opposing Effects on Locomotor Activity in Mice. Neuropsychopharmacology. 2009;34:1958–67. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young JW, Kooistra K, Geyer MA. Dopamine Receptor Mediation of the Exploratory/Hyperactivity Effects of Modafinil. Neuropsychopharmacology. 2011;36:1385–96. doi: 10.1038/npp.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulus MP, Geyer MA, Gold LH, Mandell AJ. Application of entropy measures derived from the ergodic theory of dynamical systems to rat locomotor behavior. Proceedings of the National Academy of Sciences. 1990;87:723–7. doi: 10.1073/pnas.87.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Gruijter DNM, van der Kamp LJT. Statistical Test Theory for the Behavioral Science. Boca Raton: Chapman & Hall/CRC; 2008. [Google Scholar]
- 33.Young JW, van Enkhuizen J, Winstanley CA, Geyer MA. Increased risk-taking behavior in dopamine transporter knockdown mice: further support for a mouse model of mania. Journal of Psychopharmacology. 2011;25:934–43. doi: 10.1177/0269881111400646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young JW, Henry BL, Geyer MA. Predictive animal models of mania: hits, misses and future directions. British Journal of Pharmacology. 2011;164:1263–84. doi: 10.1111/j.1476-5381.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]