Abstract
Mechanistic studies of macromolecular complexes often feature x-ray structures of complexes with bound ligands. The attachment of Adeno-Associated Virus (AAV) to cell surface glycosaminoglycans (GAGs) is an example that has not proven amenable to crystallography, because the binding of GAG analogs disrupts lattice contacts. The interactions of AAV with GAGs are of interest in mediating the cell specificity of AAV-based gene therapy vectors. Previous electron microscopy led to differing conclusions on the exact binding site and the existence of large ligand-induced conformational changes in the virus. Conformational changes are expected during cell entry, but it has remained unclear whether the electron microscopy provided evidence of their induction by GAG-binding. Taking advantage of automated data collection, careful processing and new methods of structure refinement, the structure of AAV-DJ complexed with sucrose octasulfate is determined by electron microscopy difference map analysis to 4.8 Å resolution. At this higher resolution, individual sulfate groups are discernible, providing a stereochemical validation of map interpretation, and highlighting interactions with two surface arginines that have been implicated in genetic studies. Conformational changes induced by the SOS are modest and limited to the loop most directly interacting with the ligand. While the resolution attainable will depend on sample order and other factors, there are an increasing number of macromolecular complexes that can be studied by cryo-electron microscopy at resolutions beyond 5 Å, for which the approaches used here could be used to characterize the binding of inhibitors and other small molecule effectors when crystallography is not tractable.
Introduction
Adeno-Associated Virus (AAV) is a small human single-stranded DNA virus that is enjoying laboratory and clinical successes as a recombinant vector in experimental gene therapies (Carter et al., 2008; Nathwani et al., 2011). The first step in endosomal-mediated viral entry is attachment to one of several extracellular glycans used by different AAVs (Kaludov et al., 2001; Shen et al., 2011), heparan sulfate proteoglycan (HSPG) for type species AAV-2 (Summerford and Samulski, 1998). These interactions have been shown to be important in vivo to the tissue tropism of vectors (Shen et al., 2012), and are of intense interest in efforts to modulate the specificity of cell transduction and limit off-target transgene expression (DiPrimio et al., 2010).
This study focuses on AAV-DJ, a recombinant variant that was selected for resistance to pooled human neutralizing sera and liver-tropism out of a library created by gene shuffling among natural AAV serotypes (Grimm et al., 2008). AAV-DJ is among the first of a growing number of AAV gene delivery vehicles showing varying tissue tropisms in pre-clinical studies and produced through the application of combinatorial technologies (Asokan et al., 2012; Maheshri et al., 2006). AAV-DJ is a chimeric mix of serotypes 2, 8 and 9. AAV-DJ was chosen to check for possible changes in primary receptor binding in the selection of this retargeted recombinant variant. It was also chosen, because the cryo-electron microscopy (cryo-EM) of native AAV-DJ at 4.5 Å resolution (Lerch et al., 2012) suggested that it might be possible to visualize a receptor complex at sufficient resolution to address questions about AAV cell attachment. The cryo-EM of native AAV-DJ virus-like particles (VLPs) had showed a structure that was highly homologous to AAV-2 (Lerch et al., 2012), differing mostly at the binding site of a neutralizing AAV-2 antibody (McCraw et al., 2012), and differing less at the hypothesized site of heparin-binding.
The general area for glycan attachment in AAV-2 was predicted from surface charge seen in the AAV-2 crystal structure (Xie et al., 2002). The region contains five basic amino acids implicated by mutagenesis in heparin-binding and/or cell attachment (Kern et al., 2003). Neither co-crystallization nor soaking yielded high resolution diffraction, so structural studies proceeded through cryo-EM analysis of AAV-2 complexed with heparin fragments (Levy et al., 2009; O'Donnell et al., 2009). In the Levy et al. (2009) study at 18 Å resolution, difference map features at 0.5 to 1.0 σ were interpreted as a bound heparin oligosaccharide, and virus conformational changes that would be induced in making the expected interactions with AAV-2 Arg585 and Arg588. In the 8 Å study of O’Donnell et al. (2009), stronger difference density (6 σ) showed the heparin oligosaccharide in immediate contact with Arg585 and Arg588 and there was no evidence of large-scale conformational change. Nevertheless, the Levy et al. (2009) conclusions are widely cited because they paint an appealing picture of how conformational changes expected at some point during endosomal entry might be triggered (Levy et al., 2009), whereas the O’Donnell et al. (2009) implies that any triggering occurs at some later undetermined post-attachment step. Neither study produced sufficiently detailed maps for recognition of chemical features of the ligand that would have provided decisive internal validation of the map interpretation. This is the goal of the current research. It required not only higher experimental resolution, but use of a different receptor analog. The earlier studies had used chromatographic fractions of heparin whose heterogeneity would limit our ability to identify detailed chemical features of the ligand even if the virus could be imaged at high enough resolution.
Sucrose octasulfate (SOS) is a commonly used glycosaminoglycan (GAG) analog that is chemically homogeneous. With 55 non-hydrogen atoms, a 1nm dimension and a mass at 1% of the target, the small size of the ligand would present challenges in resolution and sensitivity, which would be compounded by partial occupancy and perhaps by flexibility in ligand binding. Structure-function studies of complexes with small ligands (substrates, inhibitors, effectors and other ligands) have a storied history in protein crystallography (Johnson and Phillips, 1965; Sigler et al., 1966; Stryer et al., 1964). Early in this history, it was recognized that difference map analysis provided a sensitive means of imaging molecular interactions (Henderson and Moffat, 1971). Difference imaging has been used with EM to localize subunits in large complexes (Stewart et al., 1993), but small ligands have largely remained in the exclusive purview of x-ray crystallography due to the aforementioned challenges. AAV would provide a case study, exploring the current capacity of EM in the analysis of small ligand binding to macromolecular complexes in cases where ligand binding is incompatible with well-ordered crystal lattices.
Materials and Methods
Virus-like particles (VLPs) of AAV-DJ were expressed in insect cells from a baculovirus construct as previously described (Lerch et al., 2012). Empty capsids were purified by three rounds of CsCl density gradient ultracentrifugation, as before, followed by additional chromatography using a heparin affinity column. Following elution with an NaCl gradient, AAV-DJ was dialyzed into 10 mM Tris buffer pH 6.8 with 125 mM NaCl and 1 mM MgCl2. SOS (Sigma-Aldrich) was added in 400-fold molar excess relative to capsid subunit at 3 mg/mL and incubated for 45 min at room temperature. Immediately before flash-freezing the AAV-DJ was diluted to 1.5 mg/mL.
Sample aliquots of 3 µL were placed on 400 mesh carbon-coated grids (C-flat) that had been glow discharged for 5s on a Gatan Model 950. Samples were flash-frozen by plunging into liquid ethane from an environment of 100% humidity and 4°C, using a Vitrobot Mark IV (FEI Inc.), blotting both sides of the grid for 2.5s. Data were collected on an FEI Titan Krios at 120 keV using an electron dose of 15 e¯/Å2. Nominal defocus on the microscope was randomly chosen between −0.8 and −2.0 µm. The resulting images had a mean estimated defocus of −1.58 µm with a standard deviation of 0.49 µm. Images were recorded at a magnification of 120,000× on a 4k × 4k pixel Gatan Ultrascan CCD camera, which resulted in a pixel size of 0.65 Å at the specimen level. 8155 high magnification images were automatically acquired over the course of 4 days using Leginon (Suloway et al., 2005), and 5207 of the images were selected for further processing.
Particles were processed semi-automatically using Appion (Lander et al., 2009) for picking, contrast transfer function (CTF) estimation, and stack making. Particles were selected automatically using template matching. CTFs were estimated with the ACE (Automated CTF Estimation) software package (Mallick et al., 2005), rejecting images with confidence value < 0.7, and flipping phases for individual particles according to their ACE-estimated defocus. These analyses resulted in 94,123 particles that were used for alignment and classification. FREALIGN (Grigorieff, 2007) was used to refine Euler angles and generate the reconstruction, starting from Euler angles estimated by EMAN using an initial model generated directly from the data using the starticos sub-program. Euler angle refinement in FREALIGN converged in 12 iterations, and 70,725 particles contributed to the final EM map. Resolution was estimated from the EM data at 4.8 Å by FSC0.143 (FSC0.5 = 5.6 Å) (Böttcher et al., 1997; Grigorieff, 2000; Harauz and van Heel, 1986; Rosenthal and Henderson, 2003; Sousa and Grigorieff, 2007). Signal attenuation was corrected using EM-Bfactor (Fernandez et al., 2008) using a cut-off of 4.8 Å.
A difference map was calculated between the newly acquired reconstruction of the AAVDJ-SOS complex and the previously published native AAV-DJ (Lerch et al., 2012). A magnification correction was first refined for each reconstruction using RSRef (Chapman et al., 2013) by least-squares minimizing the differences between density levels of the reconstruction and those calculated from the crystal structure of homolog AAV-2 that had been superimposed rigidly according to the icosahedral symmetry elements. The relative magnification corrections are remarkably consistent, considering that they were refined independently against different reconstructions, and were small, 1.011 for native and 1.010 for complex. Nevertheless, at the outside of a 275 Å diameter virus a 1.01 correction corresponds to a 1.4 Å positional error, and its application appreciably affects the superimposition of the AAV structure on the SOS difference peak.
After a preliminary atomic refinement (see below), the difference map calculation was improved modestly by first bringing the density levels of each reconstruction to a common scale using the atomic models as a reference. An additive and multiplicative constant were applied to all voxels in each reconstruction that least squares minimized differences from the calculated density within an envelope drawn 4.9 Å around the respective atomic models (Chapman et al., 2013). Following subtraction of the native reconstruction from the complex, the difference map was low-pass filtered to 5.0 Å using EMAN (Ludtke et al., 1999), minimizing possible artifacts arising from the slightly different resolution limits (4.8 and 4.5 Å respectively).
An atomic model for SOS was placed approximately in the difference map using Coot (Emsley et al., 2010). RSRef (Chapman et al., 2013) was used for the atomic refinements described below. RSRef is a real-space method, optimizing the fit of the model to map values throughout the molecular volume, incorporating the microscope envelope function and resolution attenuation in calculation of the density expected from the atomic model. It was used in combination with CNS (Brünger et al., 1998) for stereochemically restrained structure optimization by gradient descent or simulated annealing. It was used in a stand-alone mode for optimization of imaging, occupancy and B-factor parameters (when atom positions were fixed). Throughout, the icosahedral symmetry was applied as a constraint.
The SOS difference map showed the two saccharide rings and eight sulfate peaks, but it was not immediately obvious which ring or sulfate should be assigned to each feature. With many possible conformers and combinations of dihedral angles, the possibilities were explored systematically. Twelve starting structures related to each other by 622 pseudo-symmetry were constructed to sample possible orientations at 60° intervals. Each SOS was fit into the difference density using a simulated annealing protocol that allowed rigid body changes as well as full exploration of alternative glycosidic and sulfate dihedral angles. The needed stereochemical force field terms for SOS were generated through semi-empirical quantum mechanics calculation using eLBOW (Moriarty et al., 2009). The SOS model with highest correlation to the difference density (0.73) was used in modeling of the complex (below).
A close contact with Arg587, and SOS density weaker than protein suggested fractional occupancy of the binding site, and the presence of two side chain rotamers for SOS-bound and unbound Arg587. The occupancy of the bound-form rotamer was constrained to that of the SOS, while the unbound rotamer was exempted from close contact restraints with the SOS. Initial refinement of the protein structure was by torsion angle simulated annealing against the map of the complex. A flat-bottom potential was added to restrain protein backbone dihedrals within the favored areas of a Ramachandran plot (Chapman et al., 2013). In optimizing the agreement between model and map, grid points within 4.9 Å of any atom were used, and these grid points incorporated contributions from atoms up to 12 Å away. Isotropic thermal parameters were retained at values refined for uncomplexed AAV-DJ (Lerch et al., 2012). Combined refinement of protein and ligand atoms positions and ligand occupancy was by gradient descent. Riding counter-ions were added to the SOS sulfates if there was residual density where they would be expected chemically.
Refinement of the SOS occupancy had shown that the map of the complex represented a weighted average of bound and unbound structures. The full extent of conformational changes might therefore be better revealed after subtraction of the unbound component (ρAAV) from that of the complex (ρAAV+SOS) in a difference map (ρΔ) that is weighted according to the ligand occupancy (OSOS) (Smith et al., 1986):
ρΔ − ρAAV+SOS − [1 − OSOS] · ρAAV
The traditional difference maps discussed earlier (Δρ = ρAAV+SOS − ρAAV), should show the ligand in positive density and conformational changes to the target with paired positive and negative density features. By contrast, the “high order” difference map (ρΔ) is designed to show the ligand and changed target structure with positive density of equal strength, without contributions from the unbound conformation (Smith et al., 1986). A known drawback is a reduced signal-to-noise ratio in protein regions, because nearly equal densities are subtracted unless the occupancy is high. When the model of the complex was subjected to an additional round of refinement against this map, differences with respect to the crystal structures increased to 1.5 Å (backbone) and 1.8 Å (all atom). Even though such differences are modest at 4.8 Å resolution, visual inspection showed that all but one of the changes greater than 2 Å occurred in loops where there were breaks in the density in the high-order difference map (ρΔ). The one exception, with density supporting the displaced structure, was near Arg587. Thus, just residues 584 to 589 were refined against the high order ρΔ difference map, then incorporated as a local second conformer for final refinement of the whole structure against the higher quality reconstruction of the complex (ρAAV+SOS).
Figures were generated using PyMol (DeLano, 2002).
Results
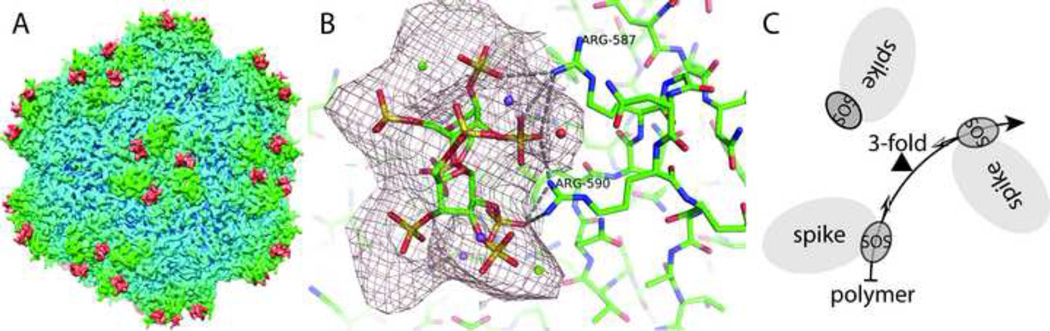
The SOS binding site was clear in the Δρ difference map. The peak density is at 13 RMSD (σ), equal to the highest peak within the viral protein, and twice the height of any other peak on the outside surface. The full width at half-maximum (FWHM) corresponds to the dimensions of SOS, whereas other peaks at 6.5σ are less than 3 Å wide, finer than the 4.8 Å resolution and indicative of noise. In vivo, AAV binds to GAG polymers, so it was conceivable that multiple SOS disaccharides would be bound along the path of an extended polymer. However only one SOS is seen (per viral subunit), presumably where the viral-GAG interactions are strongest. Partial occupancy is indicated by SOS maximal density that is 45% of the maximal protein value in the reconstruction of the complex (ρAAV+SOS). Contoured at 6σ, the Δρ difference map shows both saccharide rings and protrusions for all 8 sulfates (Figure 1). Differences between the glucosyl and fructosyl rings of the sucrose cannot be distinguished, and 4 of 32 sulfate oxygens protrude from the density, limitations to be expected at 4.8 Å resolution. However, the locations of the sulfates are clear, providing validation that the density corresponds to the ligand rather than noise or an artifact, and revealing the molecular interactions of the SOS with the virus.
Figure 1.
3D cryo-EM reconstruction of AAV-DJ complexed with SOS at 4.8 Å resolution. A: Peaks (segmented in red) are seen at the SOS binding sites in the map of the complex on the side of each spike that surrounds each viral 3-fold axis. B: The Δρ difference map (complex – native), contoured at 6σ shows, the SOS sulfate locations clearly, even at this resolution. Three of the sulfates appear to form salt bridge interactions with Arg587 and Arg590 from one of the exterior loops of the virus structure. C: Schematic of the central part of panel A showing the binding sites on three adjacent spikes related by icosahedral symmetry. As indicated by the two-tone arrows emanating from each SOS and pointing towards the 3-fold axis (triangle), a disaccharide could be bound at each site in the same orientation with respect to the local virus environment. The 8 Å resolution structure of the AAV-2 heparin complex (O'Donnell et al., 2009) shows diffuse density bridging between adjacent sites, consistent with a single polysaccharide passing through both sites. In such a case, the direction of the polysaccharide backbone in one site, relative to the local protein environment, would be the opposite of the direction at the adjacent site. This suggests that a virus that had evolved for polyvalent binding would have local binding sites tolerant of two GAG orientations.
Systematically sampling all possible SOS orientations, structures were refined from 12 starting points, two converging to a single orientation with a local model-map correlation coefficient of 0.73 (7% above the next best). Subtle distinction between orientations arises from pseudo-symmetry in the ligand. It also likely arises from attachment sites that might have evolved for interactions with polysaccharides bound in two local orientations. There is some evidence that symmetry-related adjacent sites bind to different parts of the same polysaccharide chain in AAV (O'Donnell et al., 2009) and other viruses (Fry et al., 1999), presumably to increase binding avidity. As illustrated in Figure 1C, the implication is that a single GAG polymer bound at two adjacent sites on the virus has opposite polarities relative to the local protein environment in each site. Thus, there is likely some intrinsic disorder in the binding interactions. The reconstruction reflects a superposition of bound configurations, of which one might hope to model the predominant.
The predominant SOS configuration was optimized against the difference density, and then the entire structure was refined against the reconstruction of the complex using a combination of gradient descent and torsion angle simulated annealing refinement (Chapman et al., 2013). The resulting local map correlation coefficient was 0.90 with a refined SOS occupancy of 0.35. An upper-bound estimate of coordinate error comes from comparison to a homology model constructed from the crystallographic structures of the parent AAV-2/-8/and -9 strains of the DJ chimera (DiMattia et al., 2012; Nam et al., 2007; Xie et al., 2002). The RMS differences are 1.0 Å (backbone) and 1.4 Å (all-atom). An accuracy (1.4 Å) better than the nominal EM resolution (4.8 Å) is possible through the use of stringent stereochemical restraints. For comparison, stereochemical restraints in crystallography yield structures with accuracies typically 3 to 9-fold better than the resolution. High accuracy was also easier to achieve starting from the high resolution structure of a homolog (AAV-2) than it would have been if model building were ab initio. The ångström-level precision is more than sufficient to identify binding-site residues, and provides a starting point for modeling of ligand interactions.
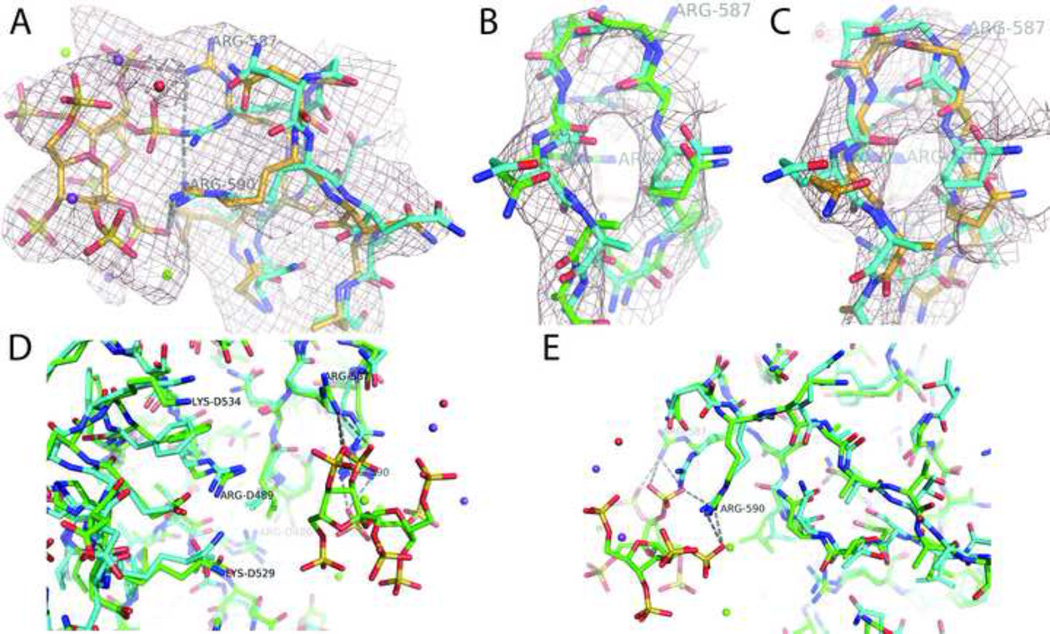
Changes induced by SOS are local (Figure 2D, E). Relative to the AAV-2 crystal structure, the guanidinium of Arg587 is displaced 4.7 Å, while Arg590 interacts without moving. Local displacements of the backbone (up to 1.0 Å) might be underestimated by refinement against the map of the complex (Figure 2B), which represents a mixture of 35% bound and 65% unbound protein. In the ρΔ difference map of Figure 2A & C, contributions from the unbound configuration should be subtracted out, leaving just the bound form. This map has a poorer signal-to-noise ratio, but can still be used for refinement, yielding similar but slightly larger backbone displacement of 1.5 to 2.1 Å for residues 484-9 in the loop closest to the SOS (Figure 2A,C). This is the only region where density indicates a significant SOS-induced displacement. Indeed, structures independently refined against the complex (ρAAV+SOS) and native AAV-DJ (ρAAV) reconstructions differ overall by only 0.8 Å (RMS, backbone) and 0.9 Å (all-atom).
Figure 2.
Local changes induced by SOS-binding. The largest conformational changes are in the SOS-proximal loop containing residues 584 – 592. A: The “high order” ρΔ difference map is designed to show just the bound conformation, with contributions from unbound virus subtracted (Smith et al., 1986). Equal strength density for ligand and protein (contoured at 4.5 σ) indicates that the estimated occupancy is approximately correct. The model of the complex (gold carbons), refined against this map, shows a maximal backbone displacement of 2.1 Å, relative to the SOS-free AAV-2 crystal structure (cyan carbons) (Xie et al., 2002). B: Density for the initial reconstruction (ρAAV+SOS contoured at 9σ to show backbone) appears to have a stronger signal-to-noise ratio than the ρΔ difference map, but corresponds to a mixture of bound and unbound forms. Nevertheless, the model with green carbons that was refined against this map shows much of the induced change when compared to the SOS-free AAV-2 structure (cyan carbons). C: The ρΔ difference map of panel A is shown in the same orientation as the ρAAV+SOS reconstruction in B. The difference map, contoured at 6 σ, is noisier, but clearly shows change away from the SOS-free AAV-2 structure (cyan carbons). Noting that ρΔ should show only the bound configuration, the model (gold carbons) refined against this map shows displacements that are slightly greater, but mostly in the same direction as those refined against the ρAAV+SOS reconstruction of panel B (green carbons).
D & E: orthogonal views of the binding site environment, comparing the refined EM-based structure of the SOS complex (green carbons) to the homology model built from the SOS-free crystal structures of the parental strains (cyan carbons). Changes are modest, even close to the ligand, and are smaller farther away. Much of the overall RMS difference of 1.4 Å can be attributed to experimental error at 4.8 Å resolution. It is therefore concluded that conformational changes are not propagated away from the binding site. Residues from a 3-fold related neighbor are numbered with the preface “D”.
SOS interacts with the virus primarily through salt bridge interactions, particularly with Arg587 and Arg590. Arg590 forms salt bridges with sulfates 2 and 3’, and a longer-range (4 Å) coulombic interaction with sulfate 3. (Sulfates are numbered by hexose oxygen, using a prime for the fructofuranose ring.) In its changed rotamer, Arg587 salt bridges to sulfates 3’ and 4’ (Figure 1). The corresponding residues in AAV-2 (Arg585 and Arg588) are among 5 positively charged residues in a region predicted to be involved from the crystal structure (Xie et al., 2002) and among those implicated in heparin binding and cell attachment by mutagenesis (Kern et al., 2003). They were also the residues in contact with the strongest density in the 8 Å resolution AAV-2/heparin complex (O'Donnell et al., 2009). Thus, the current 4.8 Å AAVDJ-SOS complex differs from the AAV2-heparin complex studied at 18 Å resolution by Levy et al. (2009), but is fully consistent with the EM structure of O’Donnell et al. at 8 Å resolution (2009) and with the available AAV-2 genetic data (Kern et al., 2003; Opie et al., 2003). Charged residues other than Arg587 and Arg590 come from a symmetry-equivalent neighbor (Figure 2). Lys534 and Arg486 are too distant (7–8 Å) to interact with the SOS, but could, perhaps with neighboring residues in a GAG polymer. This is true for Arg489 and Lys529, lying 4 and 6 Å from SOS sulfates, but weak density also hints at an alternative rotamer for sulfate 14, which would be able to salt-bridge to both.
Discussion
The structure reveals the molecular interactions of Arg587 and Arg590 that are important in cell attachment. Intriguingly, these arginines are conserved only in AAV-DJ and AAV-2, the AAVs with strongest heparin-binding (Lerch et al., 2012). It is the corresponding arginines that on mutation, most reduce heparin-binding in AAV-2, and that on substitution into the sequence of AAV-3B lead to increased heparin-binding, cell attachment and transgene expression of AAV-3B vectors (Lerch and Chapman, 2012). Thus, the structure is consistent with growing evidence of the importance of these residues in AAV2-like strains. It further highlights the paradox that the arginines are not even conserved among human heparin-binding AAVs. It has been suggested that this situation might have arisen through the evolution of mechanistically analogous binding sites comprised of distinct amino acids in the different serotypes, due to the selective pressure for surface modulation to escape neutralizing antibodies (Lerch and Chapman, 2012). Diversity in the viral binding site might also lead to differential heparanoid affinities. Comparative analysis of protein interactions like those studied will be important in achieving a complete understanding of viral-cell specificities and for cell targeting in gene therapies.
SOS had been chosen as a readily-available chemically homogeneous analog of the natural GAGs which are heterogeneous in chemical structure (Conrad, 1998). The modest occupancy (0.35) in the AAV complex was initially surprising, but can be rationalized in several ways. Sucrose only approximates the structure of a GAG, and the higher sulfonation may not be optimal for binding. In a concurrent analysis by surface plasmon resonance, examining the inhibition of AAV binding to a heparin chip (Zhang et al., 2013), disaccharides are noticeably less inhibitory than tetra- and hexa-saccharides. They are much less inhibitory, binding less strongly than oligomers exceeding 16-mers, which presumably achieve higher avidity by bridging to a second symmetry-equivalent site (Figure 1C). The same study showed that the level and type of sulfonation affected binding, inhibition being strongest with heparin and chondroitin sulfate E, averaging 2 to 2.5 sulfates per disaccharide. SOS was commensurate with the more weakly inhibiting GAGs. Thus, while SOS is a commonly used analog that has the advantage of homogeneity, its binding is weaker than the natural AAV ligands. The natural heparanoids are of variable sequence, but average 2 or 3 sulfates per disaccharide in various ring locations. Thus, the four sulfate interactions seen with SOS represent a compendium of the possible interactions that could occur with natural GAGs, subsets of which will be in-play for each specific region of a GAG bound in each site.
The SOS-AAVDJ complex shows no evidence of induced conformational changes propagated away from the binding site. Conformational changes had been invoked to reconcile the distance between Arg585/Arg588 and density features that were interpreted as a bound heparin fragment in the 18 Å resolution cryo-EM study of AAV-2 (Levy et al., 2009). This work has been widely cited, because it was attractive to speculate that far-reaching conformational changes, expected during endosomal entry, were induced/triggered by heparin attachment, even though there were contrary indications from a complex with heparin at 8 Å resolution (O'Donnell et al., 2009). Now at 4.8 Å resolution, and with a chemically uniform ligand, the expected SOS stereochemistry can be discerned, validating interpretation of features in the difference map. The SOS location, corresponding to part of the 8 Å heparin density in AAV-2, and the observed direct interactions with the two arginines, refute the argument that large conformational changes are needed for these residues to participate in binding. While widespread conformational changes are still expected at a later point during viral entry, the current 4.8 Å structure supersedes inferences made at 18 Å resolution: the preponderance of evidence is that heparin-binding is not the trigger for large conformational changes.
How does the absence of large GAG-induced conformational change impact our understanding of AAV’s cellular entry? GAGs were the first host molecules associated with entry (Summerford and Samulski, 1998). It is perhaps for historical reasons that GAGs have become known as the primary receptors, although the perception of an active role has also been strengthened by the impact of changes to the binding site in cell specificity and tissue tropism (Asokan et al., 2010; Bell et al., 2012; Choi et al., 2005; Perabo et al., 2006; Work et al., 2006; Wu et al., 2006). The now-refuted GAG-induced conformational changes appeared consistent with a very active role of the GAG in entry. However, perhaps the association should be considered more of a passive attachment, anchoring the virus near the cell surface ready for down-stream events, perhaps more like other viruses such as HIV (Dimitrov, 1997; Patel et al., 1993). The role of additional proteins termed co- or secondary receptors is well established, if not the identity of which, if any, has a dominant role in vivo (Akache et al., 2006; Asokan et al., 2006; Kashiwakura et al., 2005; Qing et al., 1999; Qiu and Brown, 1999; Summerford et al., 1999). Co-receptor interaction is required for endosomal-mediated entry (Bartlett et al., 2000), and it is possible that this is a trigger for conformational change. Substantial conformational changes are required to externalize, through narrow pores at the 5-fold axes, the N-termini of VP1/VP2 which contain a phospholipase A2 domain and nuclear localization/import motifs that are exposed after acidification and prior to passage of the virus into the cytoplasm (Sonntag et al., 2006). At most hints of conformational change have been seen in pH-dependent structural studies of AAV-8 (Nam et al., 2011). Studies of autonomous parvoviruses have led to the suggestion that conformational changes may be a multi-step process that could involve an initial induced transition from a stable to metastable capsid, rendering it sensitive to subsequent environmental or molecular triggers of more sweeping change (Cotmore and Tattersall, 2007; Mani et al., 2006; Ros et al., 2006; Vihinen-Ranta and Parrish, 2006). None of these states has been characterized, so ideas remain speculative. The current work shows that GAG-attachment is not the primary trigger, but there are many subsequent opportunities for the conformational transitions to be induced prior to endosomal release.
The current study shows that, at least in favorable conditions, electron microscopy can elucidate the interactions of macromolecules with small ligands, providing an alternative to x-ray studies when ligand-binding interferes with a crystal lattice. Several technological advances have made this possible. Automated instrumentation and improved processing methods make tractable the collection of matched complex and native data sets at sufficient resolution. New methods for refining atomic structures against EM reconstructions (Chapman et al., 2013) improve not only final model precision, but provide a means to calibrate the magnification and density scaling, reducing artifacts in difference maps. The current application benefitted from icosahedral symmetry facilitating particle alignment and data processing, from a well-ordered protein target to which the ligand bound, and from the availability of high resolution structures of homologs to initiate refinement. However, increasing numbers of non-viral complexes are yielding commensurate resolution (Hashem et al., 2013; Ludtke et al., 2008; Mills et al., 2013) due to advances in the automation, optics and detectors for electron microscopes (Bai et al., 2013; Milne et al., 2013). Furthermore, future applications may be free of some of the limitations of the current study: the partial occupancy and intrinsic disorder of the ligand studied here. Thus, it can be hoped that an increasing number of macromolecular complexes will prove amenable to electron microscopic analysis of ligand-binding by the approaches used here.
Acknowledgements
The atomic model and EM reconstruction will be available from the EMDataBank (http://www.ebi.ac.uk/pdbe/emdb/). Software used in the structure refinement is available from http://xtal.ohsu.edu. Data processing and reconstruction was performed with help from the Florida State University shared High-Performance Computing facility. The research was supported by grants from the National Institutes of Health (R01GM66875 & R01GM78538) and a Pilot Project Grant from the OHSU Center for Spatial Systems Biomedicine (OCSSB) to MSC, and a grant partially supporting the purchase of an FEI Titan Krios microscope (S10-RR025080 to Kenneth A. Taylor at FSU). TFL was supported by the Interactions at Microbe-Host Interface training grant from the National Institutes of Health (T32AI007472).
Abbreviations
- AAV
Adeno-Associated Virus
- CTF
contrast transfer function
- EM
Electron Microscopy
- FSC
Fourier shell correlation
- HBD
heparin-binding domain
- HSPG
heparan sulfate proteoglycan
- VLP
Virus-like particle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akache B, Grimm D, Pandey K, Yant SR, Xu H, et al. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Mol Ther. 2012;20:699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asokan A, Hamra JB, Govindasamy L, Agbandje-McKenna M, Samulski RJ. Adeno-associated virus type 2 contains an integrin alpha5beta1 binding domain essential for viral cell entry. J Virol. 2006;80:8961–8969. doi: 10.1128/JVI.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asokan A, Conway JC, Phillips JL, Li C, Hegge J, et al. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat Biotechnol. 2010;28:79–82. doi: 10.1038/nbt.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai XC, Fernandez IS, McMullan G, Scheres SH. Ribosome structures to near-atomic resolution from thirty thousand cryo-EM particles. Elife. 2013;2:e00461. doi: 10.7554/eLife.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JS, Wilcher R, Samulski RJ. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J Virol. 2000;74:2777–2785. doi: 10.1128/jvi.74.6.2777-2785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CL, Gurda BL, Van Vliet K, Agbandje-McKenna M, Wilson JM. Identification of the galactose binding domain of the AAV9 capsid. J Virol. 2012 doi: 10.1128/JVI.00448-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher B, Wynne SA, Crowther RA. Determination of the fold of the core protein of hepatitis B virus by electron cryomicroscopy. Nature. 1997;386:88–91. doi: 10.1038/386088a0. [DOI] [PubMed] [Google Scholar]
- Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, et al. Crystallography and NMR system: A new software system for macromolecular structure determination. Acta Crystallographica. 1998;D54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- Carter BJ, Burstein H, Peluso RW. Adeno-associated Virus and AAV Vectors for Gene delivery. In: Templeton NS, editor. Gene and cell therapy: therapeutic mechanisms and strategies. Boca Raton: CRC Press; 2008. pp. 115–156. p. 115–156. [Google Scholar]
- Chapman MS, Trzynka A, Chapman BK. Atomic modeling of cryo-electron microscopy reconstructions - Joint refinement of model and imaging parameters. J Struct Biol. 2013;182:10–21. doi: 10.1016/j.jsb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi VW, McCarty DM, Samulski RJ. AAV hybrid serotypes: improved vectors for gene delivery. Curr Gene Ther. 2005;5:299–310. doi: 10.2174/1566523054064968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad HE. Heparin-Binding Proteins. 1st ed. San Diego: Academic Press; 1998. [Google Scholar]
- Cotmore SF, Tattersall P. Parvoviral host range and cell entry mechanisms. Adv Virus Res. 2007;70:183–232. doi: 10.1016/S0065-3527(07)70005-2. [DOI] [PubMed] [Google Scholar]
- DeLano WL. The PyMOL Molecular Graphics System. San Carlos, CA, USA: DeLano Scientific; 2002. [Google Scholar]
- DiMattia MA, Nam HJ, Van Vliet K, Mitchell M, Bennett A, et al. Structural insight into the unique properties of adeno-associated virus serotype 9. J Virol. 2012;86:6947–6958. doi: 10.1128/JVI.07232-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov DS. How do viruses enter cells? The HIV coreceptors teach us a lesson of complexity. Cell. 1997;91:721–730. doi: 10.1016/s0092-8674(00)80460-2. [DOI] [PubMed] [Google Scholar]
- DiPrimio N, McPhee SW, Samulski RJ. Adeno-associated virus for the treatment of muscle diseases: toward clinical trials. Curr Opin Mol Ther. 2010;12:553–560. [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez JJ, Luque D, Caston JR, Carrascosa JL. Sharpening high resolution information in single particle electron cryomicroscopy. J Struct Biol. 2008;164:170–175. doi: 10.1016/j.jsb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Fry EE, Lea SM, Jackson T, Newman JW, Ellard FM, et al. The structure and function of a foot-and-mouth disease virus-oligosaccharide receptor complex. Embo J. 1999;18:543–554. doi: 10.1093/emboj/18.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorieff N. Resolution measurement in structures derived from single particles. Acta Crystallogr D Biol Crystallogr. 2000;56:1270–1277. doi: 10.1107/s0907444900009549. [DOI] [PubMed] [Google Scholar]
- Grigorieff N. FREALIGN: high-resolution refinement of single particle structures. J Struct Biol. 2007;157:117–125. doi: 10.1016/j.jsb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Grimm D, Lee JS, Wang L, Desai T, Akache B, et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol. 2008;82:5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harauz G, van Heel M. Exact filters for general geometry 3-dimensional reconstruction. Optik. 1986;73:146–156. [Google Scholar]
- Hashem Y, des Georges A, Fu J, Buss SN, Jossinet F, et al. High-resolution cryo-electron microscopy structure of the Trypanosoma brucei ribosome. Nature. 2013;494:385–389. doi: 10.1038/nature11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R, Moffat JK. The Difference Fourier Technique in Protein Crystallography: Errors and their Treatment. Acta Cryst. 1971;B 27 [Google Scholar]
- Johnson LN, Phillips DC. Structure of some crystalline lysozyme-inhibitor complexes determined by X-ray analysis at 6 Angstrom resolution. Nature. 1965;206:761–763. doi: 10.1038/206761a0. [DOI] [PubMed] [Google Scholar]
- Kaludov N, Brown KE, Walters RW, Zabner J, Chiorini JA. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J Virol. 2001;75:6884–6893. doi: 10.1128/JVI.75.15.6884-6893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwakura Y, Tamayose K, Iwabuchi K, Hirai Y, Shimada T, et al. Hepatocyte growth factor receptor is a coreceptor for adeno-associated virus type 2 infection. J Virol. 2005;79:609–614. doi: 10.1128/JVI.79.1.609-614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern A, Schmidt K, Leder C, Muller OJ, Wobus CE, et al. Identification of a heparin-binding motif on adeno-associated virus type 2 capsids. J Virol. 2003;77:11072–11081. doi: 10.1128/JVI.77.20.11072-11081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander GC, Stagg SM, Voss NR, Cheng A, Fellmann D, et al. Appion: an integrated, database-driven pipeline to facilitate EM image processing. J Struct Biol. 2009;166:95–102. doi: 10.1016/j.jsb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch TF, Chapman MS. Identification of the heparin binding site on adeno-associated virus serotype 3B (AAV-3B) Virology. 2012;423:6–13. doi: 10.1016/j.virol.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch Thomas F, O'Donnell Jason K, Meyer Nancy L, Xie Q, Taylor Kenneth A, et al. Structure of AAV-DJ, a Retargeted Gene Therapy Vector: Cryo-Electron Microscopy at 4.5 Å Resolution. Structure. 2012;20:1310–1320. doi: 10.1016/j.str.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy HC, Bowman VD, Govindasamy L, McKenna R, Nash K, et al. Heparin binding induces conformational changes in Adeno-associated virus serotype 2. J Struct Biol. 2009;165:146–156. doi: 10.1016/j.jsb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtke SJ, Baldwin PR, Chiu W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J Struct Biol. 1999;128:82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- Ludtke SJ, Baker ML, Chen DH, Song JL, Chuang DT, et al. De novo backbone trace of GroEL from single particle electron cryomicroscopy. Structure. 2008;16:441–448. doi: 10.1016/j.str.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Maheshri N, Koerber JT, Kaspar BK, Schaffer DV. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nature Biotechnology. 2006;24:198–204. doi: 10.1038/nbt1182. [DOI] [PubMed] [Google Scholar]
- Mallick SP, Carragher B, Potter CS, Kriegman DJ. ACE: automated CTF estimation. Ultramicroscopy. 2005;104:8–29. doi: 10.1016/j.ultramic.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Mani B, Baltzer C, Valle N, Almendral JM, Kempf C, et al. Low pH-dependent endosomal processing of the incoming parvovirus minute virus of mice virion leads to externalization of the VP1 N-terminal sequence (N-VP1), N-VP2 cleavage, and uncoating of the full-length genome. J Virol. 2006;80:1015–1024. doi: 10.1128/JVI.80.2.1015-1024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCraw DM, O'Donnell JK, Taylor KA, Stagg SM, Chapman MS. Structure of adeno-associated virus-2 in complex with neutralizing monoclonal antibody A20. Virology. 2012;431:40–49. doi: 10.1016/j.virol.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills DJ, Vitt S, Strauss M, Shima S, Vonck J. De novo modeling of the F420-reducing [NiFe]-hydrogenase from a methanogenic archaeon by cryo-electron microscopy. Elife. 2013;2:e00218. doi: 10.7554/eLife.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JL, Borgnia MJ, Bartesaghi A, Tran EE, Earl LA, et al. Cryo-electron microscopy--a primer for the non-microscopist. FEBS J. 2013;280:28–45. doi: 10.1111/febs.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty NW, Grosse-Kunstleve RW, Adams PD. electronic Ligand Builder and Optimization Workbench (eLBOW): a tool for ligand coordinate and restraint generation. Acta Crystallogr D Biol Crystallogr. 2009;65:1074–1080. doi: 10.1107/S0907444909029436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HJ, Gurda BL, McKenna R, Potter M, Byrne B, et al. Structural studies of adeno-associated virus serotype 8 capsid transitions associated with endosomal trafficking. J Virol. 2011;85:11791–11799. doi: 10.1128/JVI.05305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HJ, Lane MD, Padron E, Gurda B, McKenna R, et al. Structure of adeno-associated virus serotype 8, a gene therapy vector. J Virol. 2007;81:12260–12271. doi: 10.1128/JVI.01304-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell J, Taylor KA, Chapman MS. Adeno-associated virus-2 and its primary cellular receptor - Cryo-EM structure of a heparin complex. Virology. 2009;385:434–443. doi: 10.1016/j.virol.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie SR, Warrington KH, Jr, Agbandje-McKenna M, Jr, Zolotukhin S, Muzyczka N. Identification of amino acid residues in the capsid proteins of adeno-associated virus type 2 that contribute to heparan sulfate proteoglycan binding. J Virol. 2003;77:6995–7006. doi: 10.1128/JVI.77.12.6995-7006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Yanagishita M, Roderiquez G, Bou-Habib DC, Oravecz T, et al. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res Hum Retroviruses. 1993;9:167–174. doi: 10.1089/aid.1993.9.167. [DOI] [PubMed] [Google Scholar]
- Perabo L, Goldnau D, White K, Endell J, Boucas J, et al. Heparan sulfate proteoglycan binding properties of adeno-associated virus retargeting mutants and consequences for their in vivo tropism. J Virol. 2006;80:7265–7269. doi: 10.1128/JVI.00076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing K, Mah C, Hansen J, Zhou S, Dwarki V, et al. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- Qiu J, Brown KE. Integrin alphaVbeta5 is not involved in adeno-associated virus type 2 (AAV2) infection. Virology. 1999;264:436–440. doi: 10.1006/viro.1999.0010. [DOI] [PubMed] [Google Scholar]
- Ros C, Gerber M, Kempf C. Conformational changes in the VP1-unique region of native human parvovirus B19 lead to exposure of internal sequences that play a role in virus neutralization and infectivity. J Virol. 2006;80:12017–12024. doi: 10.1128/JVI.01435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal PB, Henderson R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J Mol Biol. 2003;333:721–745. doi: 10.1016/j.jmb.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Shen S, Bryant KD, Brown SM, Randell SH, Asokan A. Terminal N-linked galactose is the primary receptor for adeno-associated virus 9. J Biol Chem. 2011;286:13532–13440. doi: 10.1074/jbc.M110.210922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Bryant KD, Sun J, Brown SM, Troupes A, et al. Glycan binding avidity determines the systemic fate of adeno-associated virus type 9. J Virol. 2012;86:10408–10417. doi: 10.1128/JVI.01155-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigler PB, Jeffery BA, Matthews BW, Blow DM. An x-ray diffraction study of inhibited derivatives of alpha-chymotrypsin. J Mol Biol. 1966;15:175–192. doi: 10.1016/s0022-2836(66)80219-x. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Kremer MJ, Luo M, Vriend G, Arnold E, et al. The Site of Attachment in Human Rhinovirus 14 for Antiviral Agents that Inhibit Uncoating. Science. 1986;233:1286–1293. doi: 10.1126/science.3018924. [DOI] [PubMed] [Google Scholar]
- Sonntag F, Bleker S, Leuchs B, Fischer R, Kleinschmidt JA. Adeno-associated virus type 2 capsids with externalized VP1/VP2 trafficking domains are generated prior to passage through the cytoplasm and are maintained until uncoating occurs in the nucleus. J Virol. 2006;80:11040–11054. doi: 10.1128/JVI.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa D, Grigorieff N. Ab initio resolution measurement for single particle structures. J Struct Biol. 2007;157:201–210. doi: 10.1016/j.jsb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Stewart PL, Fuller SD, Burnett RM. Difference imaging of adenovirus: bridging the resolution gap between X-ray crystallography and electron microscopy. EMBO Journal. 1993;12:2589–2599. doi: 10.1002/j.1460-2075.1993.tb05919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L, Kendrew JC, Watson HC. The Mode of Attachment of the Azide Ion to Sperm Whale Metmyoglobin. J Mol Biol. 1964;8:96–104. doi: 10.1016/s0022-2836(64)80152-2. [DOI] [PubMed] [Google Scholar]
- Suloway C, Pulokas J, Fellmann D, Cheng A, Guerra F, et al. Automated molecular microscopy: the new Leginon system. J Struct Biol. 2005;151:41–60. doi: 10.1016/j.jsb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerford C, Bartlett JS, Samulski RJ. AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- Vihinen-Ranta M, Parrish CR. Cell infection processes of autonomous parvoviruses. In: Kerr JR, et al., editors. Parvoviruses. London: Hodder Arnold, Ltd.; 2006. pp. 157–163. p. 157–163. [Google Scholar]
- Work LM, Buning H, Hunt E, Nicklin SA, Denby L, et al. Vascular bed-targeted in vivo gene delivery using tropism-modified adeno-associated viruses. Mol Ther. 2006;13:683–693. doi: 10.1016/j.ymthe.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Wu Z, Asokan A, Grieger JC, Govindasamy L, Agbandje-McKenna M, et al. Single Amino Acid Changes Can Influence Titer, Heparin Binding, and Tissue Tropism in Different Adeno-Associated Virus (AAV) Serotypes. J Virol. 2006;80:11393–11397. doi: 10.1128/JVI.01288-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, et al. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:10405–10410. doi: 10.1073/pnas.162250899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Aguilera J, Beaudet JM, Xie Q, Lerch TF, et al. Characterization of Interactions between Heparin/Glycosaminoglycan and Adeno-Associated Virus. Biochemistry. 2013 doi: 10.1021/bi4008676. [DOI] [PMC free article] [PubMed] [Google Scholar]