Abstract
N -benzyl substitution markedly enhances the affinity of phenethylamine hallucinogens at the 5-HT2A receptor. N-benzyl substituted derivatives of 2,5-dimethoxy-4-iodophenethylamine (2C-I), such as N-(2-methoxybenzyl)-2,5-dimethoxy-4-iodophenethylamine (25I-NBOMe) and N-(2,3-methylenedioxybenzyl)-2,5-dimethoxy-4-iodophenethylamine (25I-NBMD), have appeared recently as designer drugs, but have not been characterized behaviorally. The head twitch response (HTR) is induced by 5-HT2A receptor activation in rats and mice, and is widely used as a behavioral proxy for hallucinogen effects in humans. Nevertheless, it is not clear whether phenethylamine hallucinogens reliably provoke this behavior. Hence, we investigated whether 2C-I, 25I-NBOMe and 25I-NBMD induce head twitches in C57BL/6J mice. The HTR was assessed using a head-mounted magnet and a magnetometer coil. 2C-I (1–10 mg/kg SC), 25I-NBOMe (0.1–1 mg/kg SC), and 25I-NBMD (1–10 mg/kg SC) induced the HTR. 25I-NBOMe displayed 14-fold higher potency than 2C-I, and the selective 5-HT2A antagonist M100,907 completely blocked the HTR induced by all three compounds. These findings show that phenethylamine hallucinogens induce the HTR by activating 5-HT2A receptors. Our results demonstrate that 25I-NBOMe is a highly potent derivative of 2C-I, confirming previous in vitro findings that N-benzyl substitution increases 5-HT2A affinity. Given the high potency and ease of synthesis of N-benzylphenethylamines, it is likely that the recreational use of these hallucinogens will become more widespread in the future.
Keywords: psychedelic, 5-HT2A, head-twitch, LSD
1. INTRODUCTION
Serotonergic hallucinogens belong to two chemical classes: indoleamines and phenylalkylamines. Members of the phenylalkylamine hallucinogen class can be further subdivided into phenethylamines (including mescaline, 2,5-dimethoxy-4-bromophenethylamine (2C-B), and 2,5-dimethoxy-4-iodophenethylamine (2C-I; Fig. 1)) and phenylisopropylamines (such as 2,5-dimethoxy-4-iodoamphetamine (DOI) and 2,5-dimethoxy-4-methylamphetamine (DOM)). All serotonergic hallucinogens act as 5-HT2A agonists, and the phenylalkylamines are selective for 5-HT2A and 5-HT2C sites (reviewed by: Nichols, 2004; Halberstadt and Geyer, 2011). It is generally accepted that the characteristic subjective and behavioral effects of hallucinogens in both humans and animals are mediated by activation of the 5-HT2A receptor (Vollenweider et al., 1998; Geyer and Vollenweider, 2008).
Figure 1.
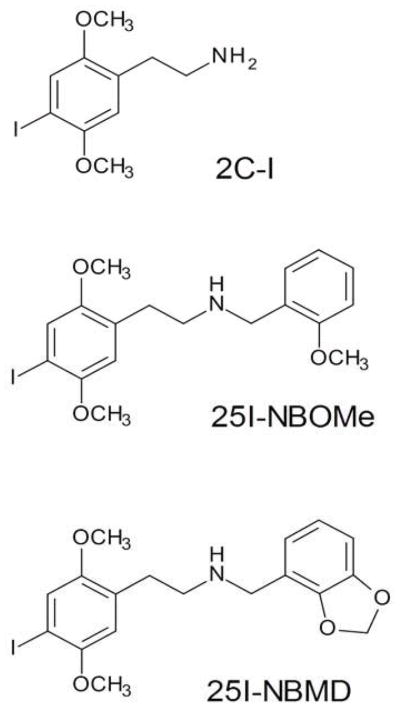
Chemical structures of phenethylamines.
N-benzyl substituted phenethylamines are a new class of serotonergic hallucinogens that are currently being marketed online and distributed as “research chemicals” (Zuba and Sekula, 2012; Zuba et al., 2013; Rose et al., 2013; Hill et al., 2013). These compounds include N-(2-methoxybenzyl)-2,5-dimethoxy-4-iodophenethylamine (25I-NBOMe, Fig. 1), N-(2-methoxybenzyl)-2,5-dimethoxy-4-chlorophenethylamine (25C-NBOMe), and N-(2-methoxybenzyl)-2,5-dimethoxy-4-methylphenethylamine (25D-NBOMe). The addition of an N-benzyl substituent to phenethylamine hallucinogens dramatically increases 5-HT2A receptor affinity (Braden et al., 2006). For example, the 5-HT2A affinity of 25I-NBOMe (Ki= 0.044 nM) is more than an order of magnitude greater than that of 2C-I (Ki= 0.73 nM). 25I-NBOMe is a potent and highly efficacious 5-HT2A agonist that is selective for 5-HT2 sites (Braden et al., 2006; Nichols et al., 2008; Ettrup et al., 2010, 2011). The increase in 5-HT2A affinity appears to be a consequence of a specific π-π interaction between the N-benzyl moiety and the aromatic ring of Phe339 (Braden et al., 2006). The high affinity and efficacy of these N-benzylphenethylamines has made them attractive agents for development as radioligands (Nichols et al., 2008) and Positron Emission Tomography (PET) radiotracers (Ettrup et al., 2010, 2011, 2013), but also indicates that they may have abuse potential. Anecdotal reports indicate that these compounds produce hallucinogenic effects and are highly potent when ingested sublingually, buccally, or by insufflation, but have very low oral bioavailability. For example, 25I-NBOMe (“25-I”, “N-Bomb”, “Smiles”) appears to be active at doses as low as 50–250 μg, and is typically used at dosages ranging from 500 to 800 μg (Erowid, 2013). The duration of action of 25I-NBOMe varies depending on the route of administration, ranging from 4–6 h (insufflation) to 6–10 h (sublingual). Several fatalities and hospitalizations have occurred as a consequence of the recreational use of 25I-NBOMe. Overdoses of 25I-NBOMe can produce tachycardia, hypertension, seizures, bizarre behavior, and agitation persisting for up to three days (Kelly et al., 2012; Rose et al., 2012, 2013; Hill et al., 2013; Spellpflug et al., 2013). Currently, 25I-NBOMe is controlled in four U.S. states (Virginia, Louisiana, Florida, and Georgia), but none of the N-benzylphenethylamines are scheduled federally or subject to international controls. Despite the widespread use of these compounds, very little is known about their behavioral or toxicological effects.
The head twitch response (HTR), a high-frequency paroxysmal head rotation that occurs in rats and mice after 5-HT2A receptor activation (Halberstadt and Geyer, 2011, 2013; Canal and Morgan, 2012), is widely used as a behavioral proxy in rodents for human hallucinogen effects. González-Maeso and colleagues compared multiple behavioral paradigms and found that the HTR is one of only a few behaviors that can reliably distinguish hallucinogenic and non-hallucinogenic 5-HT2A agonists (González-Maeso et al., 2007). These and other workers have used the HTR to identify 5-HT2A-coupled signaling pathways, downstream neurochemical effects, and interactions with other transmitter systems that are specifically relevant to hallucinogenesis (Marek 2003, 2009; Benneyworth et al., 2007; González-Maeso et al., 2007; Schmid et al., 2008; Schmid and Bohn, 2010; Egashira et al., 2011; Moreno et al., 2012). If the HTR is a valid animal model of hallucinogen effects in humans, it is critical to confirm that all classes of hallucinogens induce the behavior. Phenylisopropylamine and indoleamine hallucinogens reliably induce the HTR (reviewed: Halberstadt and Geyer, 2011), but there is some disagreement in the literature regarding the effectiveness of phenethylamine hallucinogens. Several studies have confirmed that mescaline induces the HTR in rats and mice (Silva and Calil, 1975; Yamamoto and Ueki, 1981; González-Maeso et al., 2007), and 2,5-dimethoxy-4-n-propylthiophenethylamine (2C-T-7) has been shown to induce head twitches in mice (Fantegrossi et al., 2005). By contrast, it has been reported that even high doses of 2C-I, 2C-B, and 2,5-dimethoxy-4-methylphenethylamine (2C-D) fail to induce the HTR in rats (Moya et al., 2007). If the latter report is correct, the inactivity of these phenethylamine hallucinogens strongly argues against the use of the HTR as a behavioral proxy for human hallucinogen effects. Therefore, additional studies with phenethylamine hallucinogens are necessary to assess the validity of the HTR behavioral model.
Since very little is known about the behavioral pharmacology of N-benzylphenethylamines and they have not been directly compared to their unsubstituted parent compounds in vivo, we conducted a series of experiments to characterize the effects of these compounds. We compared the effects of 2C-I and two N-benzyl substituted derivatives (25I-NBOMe and 25I-NBMD; Fig. 1) on HTR in C57BL/6J mice. HTR was assessed using a magnetometer coil-based system that can detect the behavior objectively with extremely high sensitivity and reliability (Halberstadt and Geyer, 2013). We also tested whether the 5-HT2A receptor is responsible for mediating the HTR to 2C-I, 25I-NBOMe, and 25I-NBMD. The experiments demonstrated that these phenethylamines induce the HTR in mice by activating the 5-HT2A receptor, and confirmed that 25I-NBOMe is extremely potent in vivo.
2. MATERIALS AND METHODS
2.1. Subjects
Mice were housed in a vivarium at the University of California San Diego (UCSD), an AAALAC-approved animal facility that meets Federal and State requirements for care and treatment of laboratory animals. Male C57BL/6J mice (6–8 weeks old) were obtained from Jackson Labs (Bar Harbor, ME, USA) and were allowed to acclimate for 1 week after arrival. Mice were housed up to 4 per cage in a climate-controlled room with a reversed light-cycle (lights on at 19:00 hours, off at 07:00 hours). Food and water were provided ad libitum, except during behavioral testing. Testing occurred between 10:00 and 18:00 hours. Experiments were conducted in accord with NIH guidelines and were approved by the UCSD animal care committee. All efforts were made to minimize animal suffering and the number of animals used.
2.2. Detection of the Head Twitch Response
The HTR was assessed using a head-mounted magnet and a magnetometer coil (see Halberstadt and Geyer, 2013). Briefly, mice were anesthetized using a mixture of ketamine (100 mg/kg IP) and acepromazine (5 mg/kg IP), and a small neodymium magnet (4.57 × 4.57 × 2.03 mm, 375 mg) was attached to the center of the dorsal surface of the cranium with dental resin; the magnet was mounted with the axis of the N-S poles parallel to the dorsoventral plane of the head. HTR experiments were conducted beginning 2 weeks after magnet implantation, and mice were tested repeatedly with at least 7 days between test sessions. Head movements were recorded and the HTR was analyzed as described previously (Halberstadt and Geyer, 2013). The mice were placed in a 12.5-cm glass cylinder surrounded by a magnetometer coil, and coil voltage was filtered (5–10 KHz low-pass), amplified, and digitized (40 kHz sampling rate) using a Powerlab/8SP with LabChart v.7.3.2 (ADInstruments, Colorado Springs, CO, USA). The LabChart data was filtered digitally (40–200 Hz band-pass) to remove extraneous head movements and high-frequency interference, and responses were identified by manually searching for individual sinusoidal wavelets with the following characteristics: (1) waveform and spectrum consistent with 40–160 Hz activity; (2) > 2 bipolar peaks; (3) amplitude exceeding the background noise level; and (4) duration <0.15 s (coil voltage should be stable during the period immediately before and after the response). In a previous study of head twitches in mice (Halberstadt and Geyer, 2013), events were counted as head twitches if their duration was <0.12 s. Extended testing, however, has demonstrated that some mice occasionally display head twitches with slightly longer duration.
In some experiments, the magnetometer-based assessment of head twitch was combined with simultaneous video recordings. The behavior of the mice within the magnetometer coil was captured at 30 Hz using a CCD video camera, digitized, and stored on a PC as an AVI file. Head twitches were counted by an observer blind to the treatment and the magnetometer data. Potential responses were analyzed frame-by-frame using VirtualDub v.1.9.11 and were counted as head twitches if there was evidence of torsional head movement over consecutive frames.
2.3. Procedures
Mice were transferred to the testing room under black cloth 1 h before testing. The experiments were conducted in a well-lit room. The test beakers were cleaned thoroughly between test sessions. For dose-response studies, mice were treated with vehicle or 2C-I (0.3, 1, 3, or 10 mg/kg; n=5–6/group, 26 total; Experiment 1), vehicle or 25I-NBOMe (0.03, 0.1, 0.3, or 1 mg/kg; n=5, 25 total; Experiment 2), or vehicle or 25I-NBMD (0.3, 1, 3, or 10 mg/kg; n=5, 25 total; Experiment 3), and HTR was assessed for 30 min. For antagonist blockade studies, mice were pretreated with vehicle or M100,907 (0.001, 0.01, or 0.1 mg/kg) prior to treatment with 3 mg/kg 2C-I (n=6, 24 total; Experiment 4), 0.3 mg/kg 25I-NBOMe (n=5–6, 23 total; Experiment 5), or 3 mg/kg 25I-NBMD (n=5–6, 21 total; Experiment 6), and HTR was assessed for 20 min. Animals were tested immediately after administration of 2C-I, 25I-NBOMe, and 25I-NBMD, and 30 min after administration of M100,907.
2.4. Drugs
Drugs used were 2,5-dimethoxy-4-iodophenethylamine hydrochloride (2C-I), N-(2-methoxybenzyl)-2,5-dimethoxy-4-iodophenethylamine hydrochloride (25I-NBOMe), N-(2,3-methylenedioxybenzyl)-2,5-dimethoxy-4-iodophenethylamine hydrochloride (25I-NBMD; Cayman Chemical, Ann Arbor, MI, USA), and (R)-(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol (M100,907; donated by Hoechst Marion Roussel Inc., Kansas City, MO, USA). Doses of 2C-I, 25I-NBOMe, and 25I-NBMD are expressed as the salt form of the drug; doses of M100,907 are expressed as the freebase. 2C-I and 25I-NBOMe were dissolved in isotonic saline; 25I-NBMD was dissolved in isotonic saline containing 2% Tween 80; M100,907 was dissolved in water containing 5% Tween 80. All drugs were administered subcutaneously at a volume of 5 mL/kg body weight.
2.6. Data Analysis
Head twitch counts were analyzed using one- or two-way analyses of variance (ANOVAs) with drug treatment as the between-subject factor. When appropriate, time was included as a repeated measure. Specific post hoc comparisons between selected groups were done using Tukey’s studentized range method. Significance was demonstrated by surpassing an α-level of 0.05. ED50, ID50, and 95% confidence limits (95% C.L.) were calculated using non-linear regression.
3. RESULTS
3.1. Detection of the head twitch response using a magnetometer coil
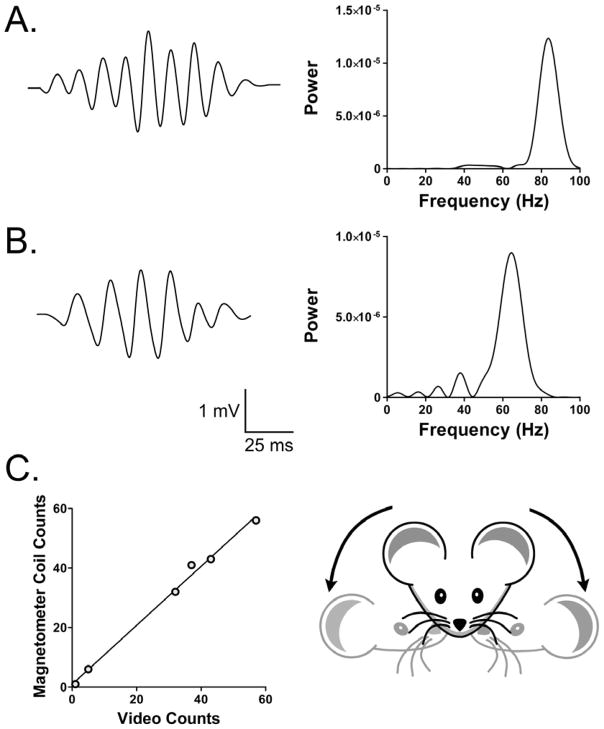
Head twitches induced brief sinusoidal oscillations of magnetometer coil voltage. As we previously reported (Halberstadt and Geyer, 2013), the frequency of the vast majority of responses peaked between 80–100 Hz (Fig. 2A), with some responses including a 40–50 Hz sub-harmonic peak. However, in a minority of responses, the frequency peaked between 50 and 80 Hz (Fig. 2B). To validate the magnetometer data, the results in a subset of animals (n = 6) were compared with HTR counts obtained by video analysis; the results obtained using the two methods (179 magnetometer responses; 175 video responses) were highly correlated (r=0.997, F(1,5)=625.9244, p<0.0001; Fig. 2C). Figure 3 shows the temporal distribution of head twitches after administration of different doses of 2C-I.
Figure 2.
(A, B) Magnetometer coil responses induced by head twitches. The left panels show the voltage response of the magnetometer coil, and the right panels are periodograms showing the spectral density of the responses. The response in (A) peaked at 83.8 Hz and the response in (B) peaked at 64.4 Hz. The magnetometer recordings were band-pass filtered (40–200 Hz) to attenuate the response to extraneous head movements and high-frequency noise. (C) Correlation between head-twitch counts in simultaneous video and the magnetometer coil recordings from 6 mice (r=0.997).
Figure 3.
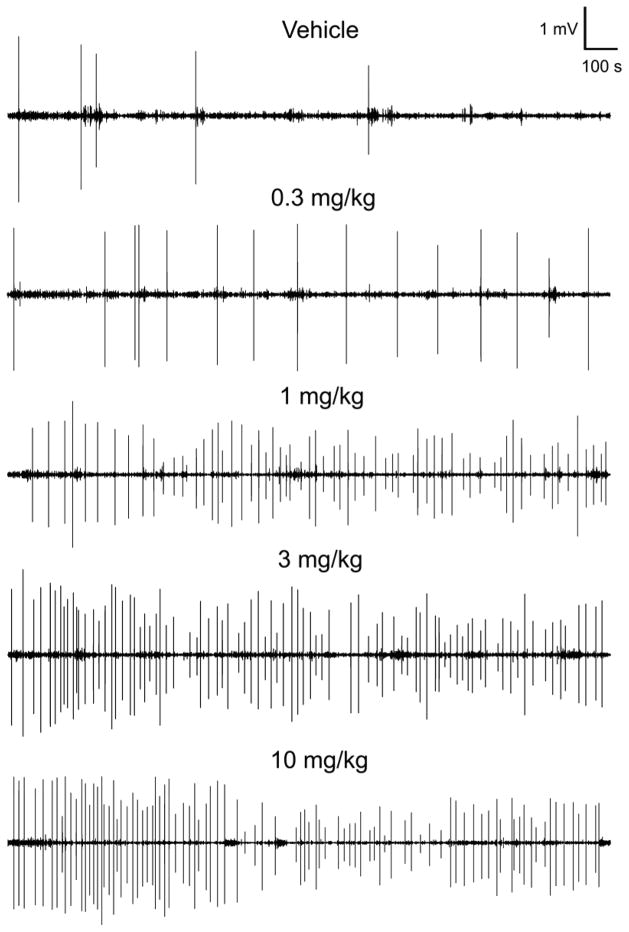
Plots of magnetometer coil voltage responses showing the temporal distribution of head twitches induced by 2C-I during the 30-min test sessions. At this time resolution, head twitches appear as high-amplitude bipolar deflections. The numbers of head twitches detected were 5 (vehicle), 16 (0.3 mg/kg), 65 (1 mg/kg), 84 (3 mg/kg), and 89 (10 mg/kg).
3.2. 2C-I dose-response
Administration of 2C-I produced a dose-dependent increase in HTR (F(4,21) = 20.34, p<0.0001). As shown in Figure 4, the 1, 3, and 10 mg/kg doses of 2C-I significantly increased the number of head twitches (p<0.01, Tukey’s test). The 3 mg/kg dose of 2C-I was maximally effective, producing 93.8 ± 7.4 (mean ± SEM) head twitches during the 30-min observation period. The ED50 (95% C.L.) for 2C-I was 0.83 (0.50–1.38) mg/kg.
Figure 4.
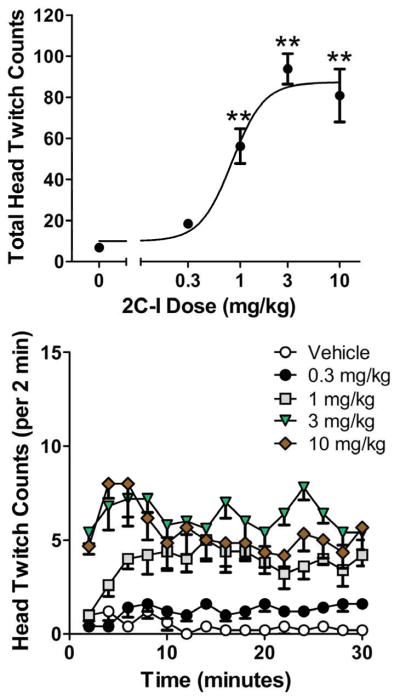
Effect of 2C-I on the head twitch response. (Top panel) Total counts over the 30-min test session. (Bottom panel) Time-course of the head twitch response induced by 2C-I (in 2-min blocks). Data are presented as group means±SEM. **p<0.01, significant difference from the vehicle control group.
HTR counts were also analyzed in 2-min bins to examine the time-course of the response to 2C-I (Fig. 4). There was a significant interaction between 2C-I treatment and time (Drug × Time: F(56,294)=2.11, p<0.0001). Inspection of the data showed that 3 and 10 mg/kg 2C-I induced a relatively constant level of HTR across the entire test session, whereas the 1 mg/kg dose did not induce a significant HTR until the sixth time block (ie., 10–12 min after administration).
Experiment 1 initially included 30 mg/kg 2C-I, but this dose was only administered to 3 mice, which were subsequently excluded from analysis. Administration of 30 mg/kg 2C-I produced a high rate of HTR during the first ~5 min of testing, but very few responses were observed subsequently and the mice became completely inactive. Furthermore, the dynamics of some of the head twitches induced by 30 mg/kg 2C-I were atypical, with duration exceeding 0.16–0.2 s (data not shown).
3.3. 25I-NBOMe dose-response
The 0.1, 0.3, and 1 mg/kg doses of 25I-NBOMe significantly increased the HTR rate (F(4,20)=55.66, p<0.0001)(p<0.01, Tukey’s test; Fig 5). The ED50 for 25I-NBOMe was 0.078 (0.054–0.112) mg/kg. Based on molecular weight, 25I-NBOMe was 14-fold more potent than 2C-I (F(1,43)=9.662, p<0.004; Table 1).
Figure 5.
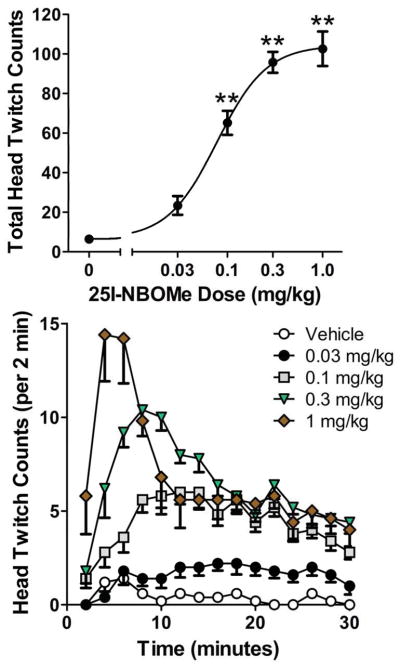
Effect of 25I-NBOMe on the head twitch response. (Top panel) Total counts over the 30-min test session. (Bottom panel) Time-course of the head twitch response induced by 25I-NBOMe (in 2-min blocks). Data are presented as group means±SEM. **p<0.01, significant difference from the vehicle control group.
Table 1.
Head twitch response (HTR) induced by substituted phenethylamines.
| Compound code | ED50 (μmol/kg) | Relative potency | ED50 (mg/kg) | 95% C.L. (mg/kg) | Max HTR counts (mean±SEM)1 | Dose (mg/kg)2 |
|---|---|---|---|---|---|---|
| 2C-I | 2.42 | 1.0 | 0.83 | 0.50–1.38 | 93.8±7.4 | 3 |
| 25I-NBOMe | 0.17 | 14.2 | 0.078 | 0.054–0.11 | 102.6±8.7 | 1 |
| 25I-NBMD | 2.36 | 1.0 | 1.13 | 0.68–1.86 | 80.2±9.5 | 10 |
Counts per 30 min.
Dose producing the maximal response.
Analysis of the 30-min test session in 2-min bins demonstrated that the response to 25I-NBOMe was temporally dependent (Drug × Time: F(56,280)=6,29, p<0.0001; Fig. 5). The interval between drug administration and maximal effect on HTR decreased as dosage increased, with the effect of 0.1 mg/kg peaking 10–14 min post-administration, the effect of 0.3 mg/kg peaking 6–8 min post-administration, and the effect of 1 mg/kg peaking 2–4 min post-administration.
3.4. 25I-NBMD dose-response
The response to 25I-NBMD is shown in Figure 6. Compared to vehicle treatment, administration of 1, 3, and 10 mg/kg significantly increased the head twitch rate (F(4,20)=36.63, p<0.0001). The ED50 for 25I-NBMD was 1.13 (0.68–1.86) mg/kg. There was no difference between the potencies of 2C-I and 25I-NBMD (F(1,43)=0.2153).
Figure 6.
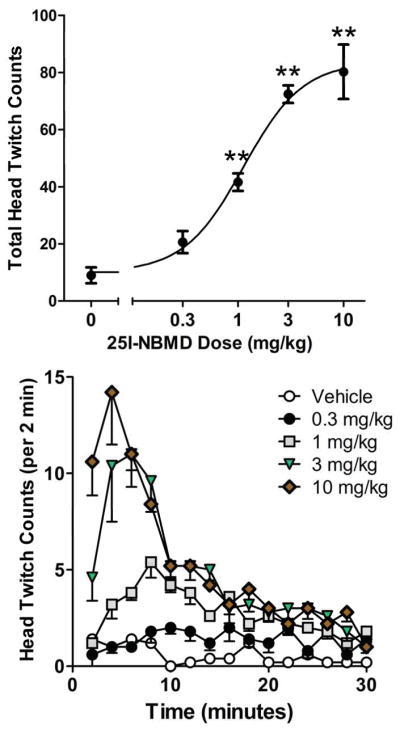
Effect of 25I-NBMD on the head-twitch response. (Top panel) Total counts over the 30-min test session. (Bottom panel) Time-course of the head twitch response induced by 25I-NBMD (in 2-min blocks). Data are presented as group means±SEM. **p<0.01, significant difference from the vehicle control group.
As was found with 2C-I and 25I-NBOMe, the response to 25I-NBMD was time-dependent (Drug × Time: F(56,280)=8.05, p<0.0001; Fig. 6). Similar to 25I-NBOMe, the interval between 25I-NBMD administration and maximal effect on HTR decreased as dosage increased, with the effect of 1 mg/kg peaking 8–10 min post-administration, the effect of 3 mg/kg peaking 6–8 min post-administration, and the effect of 10 mg/kg peaking 2–4 min post-administration.
3.5. Effect of M100,907 on the head twitch response induced by phenethylamines
As shown in Fig. 7, M100,907 was a highly potent antagonist of the HTR induced by 3 mg/kg 2C-I (F(3,20)=80.90, p<0.0001), blocking the response with an ID50 of 0.0045 (0.0021–0.0097) mg/kg. The effect of 2C-I was completely blocked by 0.1 mg/kg M100,907 and partially attenuated by 0.01 mg/kg (p<0.01, Tukey’s test). M100,907 also dose-dependently antagonized the HTR induced by 0.3 mg/kg 25I-NBOMe (F(3,19)=17.46, p<0.0001), with the ID50 calculated as 0.0062 (0.0017–0.0222) mg/kg. As was found with 2C-I, 0.1 mg/kg M100,907 completely blocked the HTR induced by 25I-NBOMe, and 0.01 mg/kg partially attenuated the response (p<0.01, Tukey’s test; Fig. 7). The HTR induced by 3 mg/kg 25I-NBMD was blocked by M100,907 (ID50 = 0.0015 (0.0006–0.0036) mg/kg; F(3,17)=40.74, p<0.0001). The two lowest doses of M100,907 (0.001 and 0.01 mg/kg) significantly attenuated the response to 25I-NBMD, and 0.1 mg/kg completely blocked the response (p<0.01, Tukey’s test; Fig. 7).
Figure 7.
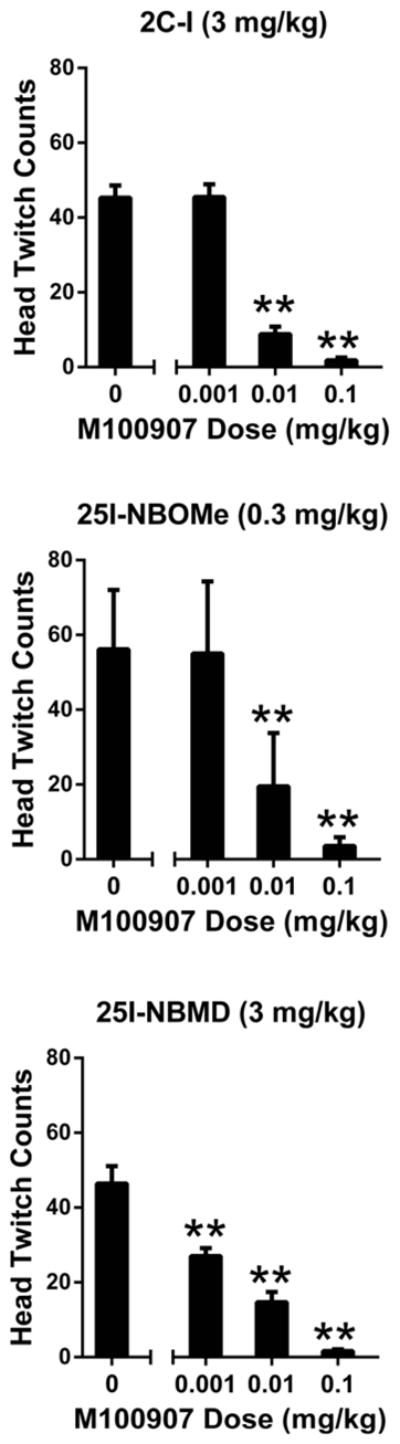
Effect of pretreatment with the 5-HT2A antagonist M100,907 on the head twitch response induced by phenethylamines. Mice were pretreated with varying doses of M100,907 and then treated with 3 mg/kg 2C-I (top panel), 0.3 mg/kg 25I-NBOMe (middle panel), or 3 mg/kg 25I-NBMD (bottom panel). Data are presented as group means±SEM for the entire 20-min test sessions. **p < 0.01, significant difference from 2C-I, 25I-NBOMe, or 25I-NBMD alone.
4. DISCUSSION
The present investigation was conducted to assess the behavioral effects of 25I-NBOMe, a novel N-benzyl substituted derivative of 2C-I that is an extremely potent 5-HT2A agonist and a member of a relatively new class of hallucinogens that are used recreationally. Our studies demonstrated that 2C-I and 25I-NBOMe induce the HTR, and confirmed that this effect is mediated by the 5-HT2A receptor. We also found that 25I-NBMD, the 2′,3′-methylenedioxy homolog of 25I-NBOMe, induces the HTR. Taken together, these findings are consistent with the reports that 25I-NBOMe and other N-benzylphenethylamines act as hallucinogens.
The present studies demonstrated that 25I-NBOMe induces the HTR with 14-fold greater potency than 2C-I (see Table 1). The relative potencies of these two phenethylamines are consistent with in vitro binding data and anecdotal reports from individuals using them recreationally. For example, in a cell line expressing the human 5-HT2A receptor, 25I-NBOMe displaced [125I]DOI binding with 16-fold higher affinity than 2C-I (Braden et al., 2006). Likewise, 2C-I is active in humans at dosages of 14–22 mg (Shulgin and Shulgin, 1991), so it is more than 10-fold less potent than 25I-NBOMe. The HTR data show that 25I-NBOMe is highly potent; in fact, 25I-NBOMe (ED50 = 78 μg/kg (0.17 μmol/kg)) is only slightly less potent than (+)-lysergic acid diethylamide (LSD) (ED50 = 52.9 μg/kg (0.13 μmol/kg); Halberstadt and Geyer, 2013). Similar to our findings with 25I-NBOMe, it was reported recently that the 4-bromo analog 25B-NBOMe induces the HTR when administered to mice at 0.5 mg/kg but not at 0.05 mg/kg (Ettrup et al., 2013). Unfortunately, Ettrup and colleagues (2013) only tested two doses of 25B-NBOMe, and they did not confirm that 25B-NBOMe acts via the 5-HT2A receptor. Taken together, these findings demonstrate that N-benzylphenethylamines are behaviorally active and highly potent.
Pretreatment with M100,907 (also known as MDL 100,907 or volinanserin), a potent 5-HT2A antagonist that is >200-fold selective for 5-HT2A compared with 5-HT2B or 5-HT2C sites (Sorensen et al., 1993; Dekeyne et al., 2002), blocked the HTR to 2C-I, 25I-NBOMe, and 25I-NBMD. These findings confirm that these phenethylamine hallucinogens induce the HTR by activating the 5-HT2A receptor, consistent with a large body of evidence that the HTR is mediated by 5-HT2A. M100,907 was highly potent in these studies, blocking the behavioral effects of 2C-I, 25I-NBOMe, and 25I-NBMD with ID50 values of ~0.002–0.006 mg/kg (see Fig. 7). These findings are notable because Schreiber has reported that M100,907 blocks the HTR to DOI in rats with an ID50 of 0.005 mg/kg (Schreiber et al., 1995). Interestingly, slightly higher doses of M100,907 (ID50 = 0.03 mg/kg) are required to prevent the HTR induced by the hallucinogen 5-methoxy-N,N-dimethyltryptamine in mice (Kekne et al., 1996). Although M100,907 does have modest affinity for 5-HT2C and α1-adrenergic sites (Kehne et al., 1996), it is likely that the doses of M100,907 that were used in these experiments are selective for 5-HT2A receptors. Administration of 0.1 mg/kg SC M100,907 failed to block the hyperlocomotion induced by the 5-HT2C agonist m-chlorophenylpiperazine in rats (Gleason et al., 2001), and the discriminative stimulus evoked by the 5-HT2C agonist Ro 60-0175 is not blocked by doses of M100,907 up to 0.63 mg/kg SC (Dekeyne et al., 1999). In rats trained to discriminate 0.16 mg/kg M100,907 from saline, stimulus generalization did not occur when a variety of 5-HT2C antagonists were tested at doses known to block 5-HT2C sites (Dekeyne et al., 2002). There is also evidence that M100,907 produces negligible occupation of central α1-adrenergic receptors in mice at 1 mg/kg SC (Patel et al., 2001), and that doses as high as 16 mg/kg fail to block the lethality induced by the α1 agonist phenylephrine (Kehne et al., 1996).
Our experiments confirmed that the phenethylamine hallucinogens 2C-I, 25I-NBOMe, and 25I-NBMD induce the HTR in mice. Although the HTR is widely used as a rodent model of human hallucinogenic effects, there is some evidence that phenethylamine hallucinogens may not induce this behavior reliably. Specifically, it was reported that 2C-I and two other 4-substituted 2,5-dimethoxyphenethylamines do not evoke head twitches in rats (Moya et al., 2007). One potential explanation for these discrepant findings is that mice are more sensitive than rats to the HTR response induced by certain compounds. For example, 1-(3-trifluoromethylphenyl)piperazine (TFMPP) induces head twitches in mice (Yarosh et al., 2007) but not in rats (Arnt and Hyttel, 1989; Schreiber et al., 1995). Similar species differences have been reported for the D1 partial agonist SKF 38393 (Schreiber et al., 1995; Halberstadt and Geyer, 2013). Given the relatively low intrinsic activity of 2C-I at the 5-HT2A receptor (Acuña-Castillo et al., 2002; Parrish et al., 2005; Braden et al., 2006; Moya et al., 2007), it may not have high enough efficacy to elicit head twitches in rats. Nevertheless, our findings confirm that 2C-I has sufficient 5-HT2A efficacy to induce the HTR in mice. In addition to analyzing aggregate HTR counts, we also assessed the time-course of the response. The effects of 25I-NBOMe and 25I-NBMD peak during the first 10 min and then progressively decline, whereas the response to 2C-I is relatively flat. The absence of a peak with 2C-I may reflect its relatively low lipophilicity, potentially slowing its ability to partition into the CNS. Alternatively, the maximal response to 2C-I may be limited by its weak 5-HT2A efficacy. It was previously shown for a series of 5-HT2A agonists that a significant positive correlation exists between 5-HT2A efficacy and the maximum number of head twitches observed (Vickers et al., 2001).
We assessed the HTR using a head-mounted magnet and a magnetometer coil, a technique that can detect the behavior with extremely high sensitivity and reliability (Halberstadt and Geyer, 2013). Indeed, the dose-response data shown in Figs. 4–7 are extremely orderly, and we confirmed that there is a robust correlation between HTR counts obtained using the magnetometer coil vs. video recordings (see Fig. 2C). Analysis of the kinematics of DOI-induced head twitches using high-speed video recordings revealed that the response involves repetitive side-to-side head movement, with the head movement occurring at an average frequency of 77–98 Hz (Halberstadt and Geyer, 2013). However, in the present experiments the magnetometer recordings indicated that the head movement frequency during head twitches sometimes occurs at frequencies below 77 Hz. There was no obvious relationship between the presence of the lower frequency responses and the interval between surgery and testing, the number of previous test sessions, the age of the mice, or the drug that was tested. We did find that certain mice were more likely than others to display lower frequency head movements, but this did not occur consistently in all experiments. Compared with our previous studies, we implanted the magnets in this group of mice using a larger amount of resin to reduce the incidence of magnet loss or damage; nevertheless, it is unlikely that the lower frequency head twitches are an artifact due to the weight of the resin because we would expect the frequency to be altered consistently across multiple experiments. These experiments sampled a large number of head twitches (>5,700) and involved a larger cohort of mice, so it is possible that the present data may more accurately reflect the normal range of HTR head movement frequencies that occur in mice. Nevertheless, it is important to note that our previous studies identified head twitches based on the presence of sinusoidal waveforms with frequency ranging from 40 Hz to 160 Hz (Halberstadt and Geyer, 2013), which is compatible with the present data.
In conclusion, 25I-NBOMe produces hallucinogen-like behavioral effects in mice and is highly potent. This finding is consistent with anecdotal reports that 25I-NBOMe induces hallucinogenic effects at sub-milligram doses and is used recreationally. Several 25I-NBOMe overdoses and fatalities occurred in 2012 and 2013 (Kelly et al., 2012; Rose et al., 2012, 2013; Hill et al., 2013) possibly as a consequence of the high potency of the drug. Some suppliers may be attempting to mitigate these risks, as it is apparently now common for 25I-NBOMe and other N-benzylphenethylamines to be distributed on blotter paper, similar to LSD (Hill et al., 2013). While this packaging could potentially reduce the incidence of accidental overdose, it may facilitate the misrepresentation of 25I-NBOMe as LSD, which has a higher safety index. 25I-NBOMe is synthesized from 2C-I by reductive alkylation using a relatively simple one- or two-step procedure (Heim, 2003). Although 2C-I was scheduled in the United States in 2012, it is unlikely that this action will reduce the availability of 25I-NBOMe because 2C-I is not controlled internationally and continues to be available from numerous suppliers. Because a simple chemical modification of 2C-I increases its potency by more than an order of magnitude, there is an economic incentive favoring the distribution of 25I-NBOMe versus 2C-I. These factors indicate that the availability and use of 25I-NBOMe is likely to increase in the future.
HIGHLIGHTS.
N- Benzyl substitution increases the 5-HT2A affinity of phenethylamine hallucinogens
N- Benzyl substituted derivatives of 2C-I are used as recreational drugs
The phenethylamines induce head twitches in mice by activating 5-HT2A receptors
Acknowledgments
This work was supported by National Institute on Drug Abuse Award DA002925, the Brain & Behavior Research Foundation, and the Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acuña-Castillo C, Villalobos C, Moya PR, Sáez P, Cassels BK, Huidobro-Toro JP. Differences in potency and efficacy of a series of phenylisopropylamine/phenylethylamine pairs at 5-HT2A and 5-HT2C receptors. Br J Pharmacol. 2002;136:510–519. doi: 10.1038/sj.bjp.0704747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnt J, Hyttel J. Facilitation of 8-OHDPAT-induced forepaw treading of rats by the 5-HT2 agonist DOI. Eur J Pharmacol. 1989;161:45–51. doi: 10.1016/0014-2999(89)90178-7. [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol. 2007;72:477–484. doi: 10.1124/mol.107.035170. [DOI] [PubMed] [Google Scholar]
- Braden MR, Parrish JC, Naylor JC, Nichols DE. Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol Pharmacol. 2006;70:1956–1964. doi: 10.1124/mol.106.028720. [DOI] [PubMed] [Google Scholar]
- Canal CE, Morgan D. Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal. 2012;4:556–576. doi: 10.1002/dta.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekeyne A, Girardon S, Millan MJ. Discriminative stimulus properties of the novel serotonin (5-HT)2C receptor agonist, RO 60-0175: a pharmacological analysis. Neuropharmacology. 1999;38:415–423. doi: 10.1016/s0028-3908(98)00203-2. [DOI] [PubMed] [Google Scholar]
- Dekeyne A, Iob L, Hautefaye P, Millan MJ. The selective serotonin2A receptor antagonist, MDL100,907, elicits a specific interoceptive cue in rats. Neuropsychopharmacology. 2002;26:552–556. doi: 10.1016/S0893-133X(01)00389-X. [DOI] [PubMed] [Google Scholar]
- Egashira N, Shirakawa A, Okuno R, Mishima K, Iwasaki K, Oishi R, Fujiwara M. Role of endocannabinoid and glutamatergic systems in DOI-induced head-twitch response in mice. Pharmacol Biochem Behav. 2011;99:52–58. doi: 10.1016/j.pbb.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Erowid 2013 25I-NBOMe (2C-I-NBOMe) Dosehttp://www.erowid.org/chemicals/2ci_nbome/2ci_nbome_dose.shtml (Retrieved March 30 2013).
- Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, Lehel S, Herth MM, Madsen J, Kristensen J, Begtrup M, Knudsen GM. Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers. Eur J Nucl Med Mol Imaging. 2011;38:681–693. doi: 10.1007/s00259-010-1686-8. [DOI] [PubMed] [Google Scholar]
- Ettrup A, Holm S, Hansen M, Wasim M, Santini MA, Palner M, Madsen J, Svarer C, Kristensen JL, Knudsen GM. Preclinical Safety Assessment of the 5-HT2A Receptor Agonist PET Radioligand [(11)C]Cimbi-36. Mol Imaging Biol. 2013 doi: 10.1007/s11307-012-0609-4. in press. http://dx.doi.org/10.1007/s11307-012-0609-4. [DOI] [PubMed]
- Ettrup A, Palner M, Gillings N, Santini MA, Hansen M, Kornum BR, Rasmussen LK, Någren K, Madsen J, Begtrup M, Knudsen GM. Radiosynthesis and evaluation of 11C-CIMBI-5 as a 5-HT2A receptor agonist radioligand for PET. J Nucl Med. 2010;51:1763–1770. doi: 10.2967/jnumed.109.074021. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology. 2005;181:496–503. doi: 10.1007/s00213-005-0009-4. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Vollenweider FX. Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci. 2008;29:445–453. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Gleason SD, Lucaites VL, Shannon HE, Nelson DL, Leander JD. m-CPP hypolocomotion is selectively antagonized by compounds with high affinity for 5-HT2C receptors but not 5-HT2A or 5-HT2B receptors. Behav Pharmacol. 2001;12:613–620. doi: 10.1097/00008877-200112000-00005. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61:364–381. doi: 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Characterization of the head-twitch response induced by hallucinogens in mice: Detection of the behavior based on the dynamics of head movement. Psychopharmacology. 2013;227:727–739. doi: 10.1007/s00213-013-3006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim R. Entwicklung eines neuen struktur-wirkungskonzepts. Berlin: Freien Universität Berlin; 2003. Synthese und pharmakologie potenter 5-HT2A-rezeptoragonisten mit N-2-methoxybenzyl-partialstruktur. http://www.diss.fu-berlin.de/diss/receive/FUDISS_thesis_000000001221 (Retrieved April 14 2013) [Google Scholar]
- Hill SL, Doris T, Gurung S, Katebe S, Lomas A, Dunn M, Blain P, Thomas SHL. Severe clinical toxicity associated with analytically confirmed recreational use of 25I-NBOMe: case series. Clinical Toxicol. 2013 doi: 10.3109/15563650.2013.802795. in press. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, et al. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther. 1996;277:968–981. [PubMed] [Google Scholar]
- Kelly A, Eisenga B, Riley B, Judge B. Case series of 25I-NBOMe exposures with laboratory confirmation. Clin Toxicol. 2012;50:702. [Google Scholar]
- Marek GJ. Behavioral evidence for mu-opioid and 5-HT2A receptor interactions. Eur J Pharmacol. 2003;474:77–83. doi: 10.1016/s0014-2999(03)01971-x. [DOI] [PubMed] [Google Scholar]
- Marek GJ. Activation of adenosine1 (A1) receptors suppresses head shakes induced by a serotonergic hallucinogen in rats. Neuropharmacology. 2009;56:1082–1087. doi: 10.1016/j.neuropharm.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Muguruza C, Umali A, Mortillo S, Holloway T, Pilar-Cuéllar F, Mocci G, Seto J, Callado LF, Neve RL, Milligan G, Sealfon SC, López-Giménez JF, Meana JJ, Benson DL, González-Maeso J. Identification of three residues essential for 5-hydroxytryptamine 2A-metabotropic glutamate 2 (5-HT2A·mGlu2) receptor heteromerization and its psychoactive behavioral function. J Biol Chem. 2012;287:44301–44319. doi: 10.1074/jbc.M112.413161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya PR, Berg KA, Gutiérrez-Hernandez MA, Sáez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP. Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Pharmacol Exp Ther. 2007;321:1054–1061. doi: 10.1124/jpet.106.117507. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Frescas SP, Chemel BR, Rehder KS, Zhong D, Lewin AH. High specific activity tritium-labeled N-(2-methoxybenzyl)-2,5-dimethoxy-4-iodophenethylamine (INBMeO): a high-affinity 5-HT2A receptor-selective agonist radioligand. Bioorg Med Chem. 2008;16:6116–6123. doi: 10.1016/j.bmc.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish JC, Braden MR, Gundy E, Nichols DE. Differential phospholipase C activation by phenylalkylamine serotonin 5-HT2A receptor agonists. J Neurochem. 2005;95:1575–1584. doi: 10.1111/j.1471-4159.2005.03477.x. [DOI] [PubMed] [Google Scholar]
- Patel S, Fernandez-Garcia E, Hutson PH, Patel S. An in vivo binding assay to determine central α1-adrenoceptor occupancy using [3H]prazocin. Brain Res Protocols. 2001;8:191–198. doi: 10.1016/s1385-299x(01)00110-6. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Rose SR, Cumpston KL, Stromberg PE, Wills BK. Severe poisoning following self-reported use of 25-I, a novel substituted amphetamine. Clin Toxicol. 2012;50:707–708. [Google Scholar]
- Rose SR, Poklis JL, Poklis A. A case of 25I-NBOMe (25-I) intoxication: a new potent 5-HT2A agonist designer drug. Clin Toxicol. 2013;51:174–177. doi: 10.3109/15563650.2013.772191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a β-arrestin2/Src/Akt signaling complex in vivo. J Neurosci. 2010;30:13513–13524. doi: 10.1523/JNEUROSCI.1665-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Raehal KM, Bohn LM. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci USA. 2008;105:1079–1084. doi: 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–112. [PubMed] [Google Scholar]
- Shulgin A, Shulgin A. PIHKAL: A Chemical Love Story. Berkeley, CA: Transform Press; 1991. [Google Scholar]
- Silva MT, Calil HM. Screening hallucinogenic drugs: systematic study of three behavioral tests. Psychopharmacologia. 1975;42:163–171. doi: 10.1007/BF00429548. [DOI] [PubMed] [Google Scholar]
- Sorensen SM, Kehne JH, Fadayel GM, Humphreys TM, Ketteler HJ, Sullivan CK, Taylor VL, Schmidt CJ. Characterization of the 5-HT2 receptor antagonist MDL 100907 as a putative atypical antipsychotic: Behavioral, electrophysiological and neurochemical studies. J Pharmacol Exp Ther. 1993;266:684–691. [PubMed] [Google Scholar]
- Stellpflug SJ, Kealey SE, Hegarty CB, Janis GC. 2-(4-Iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine (25I-NBOMe): clinical case with unique confirmatory testing. J Med Toxicol. 2013 doi: 10.1007/s13181-013-0314-y. in press. http://dx.doi.org/10.1007/s13181-013-0314-y. [DOI] [PMC free article] [PubMed]
- Tanaka S, Young JW, Halberstadt AL, Masten VL, Geyer MA. Four factors underlying mouse behavior in an open field. Behav Brain Res. 2012;233:55–61. doi: 10.1016/j.bbr.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, Kennett GA. Modulation of 5-HT2A receptor-mediated head-twitch behavior in the rat by 5-HT2C receptor agonists. Pharmacol Biochem Behave. 2001;69:643–652. doi: 10.1016/s0091-3057(01)00552-4. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ueki S. Behavioral effects of 2,5-dimethoxy-4-methylamphetamine (DOM) in rats and mice. Eur J Pharmacol. 1975;32:156–162. doi: 10.1016/0014-2999(75)90278-2. [DOI] [PubMed] [Google Scholar]
- Yarosh HL, Katz EB, Coop A, Fantegrossi WE. MDMA-like behavioral effects of N-substituted piperazines in the mouse. Pharmacol Biochem Behav. 2007;88:18–27. doi: 10.1016/j.pbb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuba D, Sekuła K. Analytical characterization of three hallucinogenic N-(2-methoxy)benzyl derivatives of the 2C-series of phenethylamine drugs. Drug Test Anal. 2012 doi: 10.1002/dta.1397. in press. [DOI] [PubMed] [Google Scholar]
- Zuba D, Sekuła K, Buczek A. 25C-NBOMe - New potent hallucinogenic substance identified on the drug market. Forensic Sci Int. 2013;227:7–14. doi: 10.1016/j.forsciint.2012.08.027. [DOI] [PubMed] [Google Scholar]