Abstract
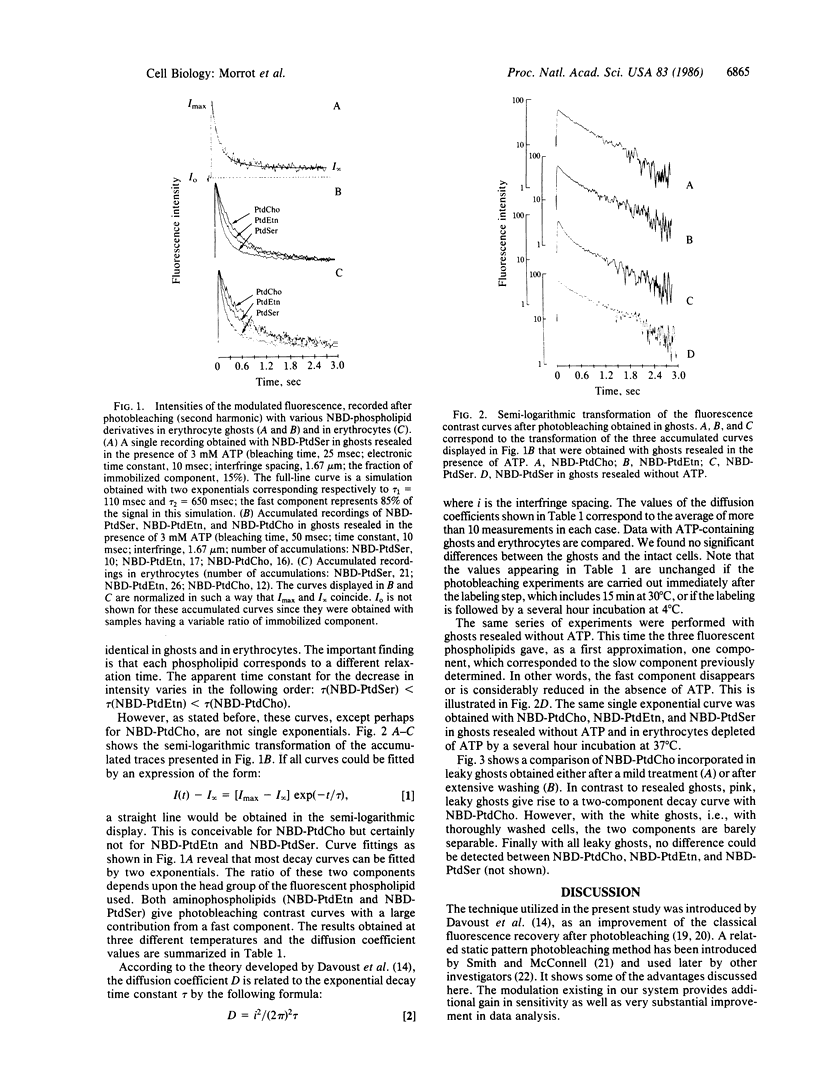
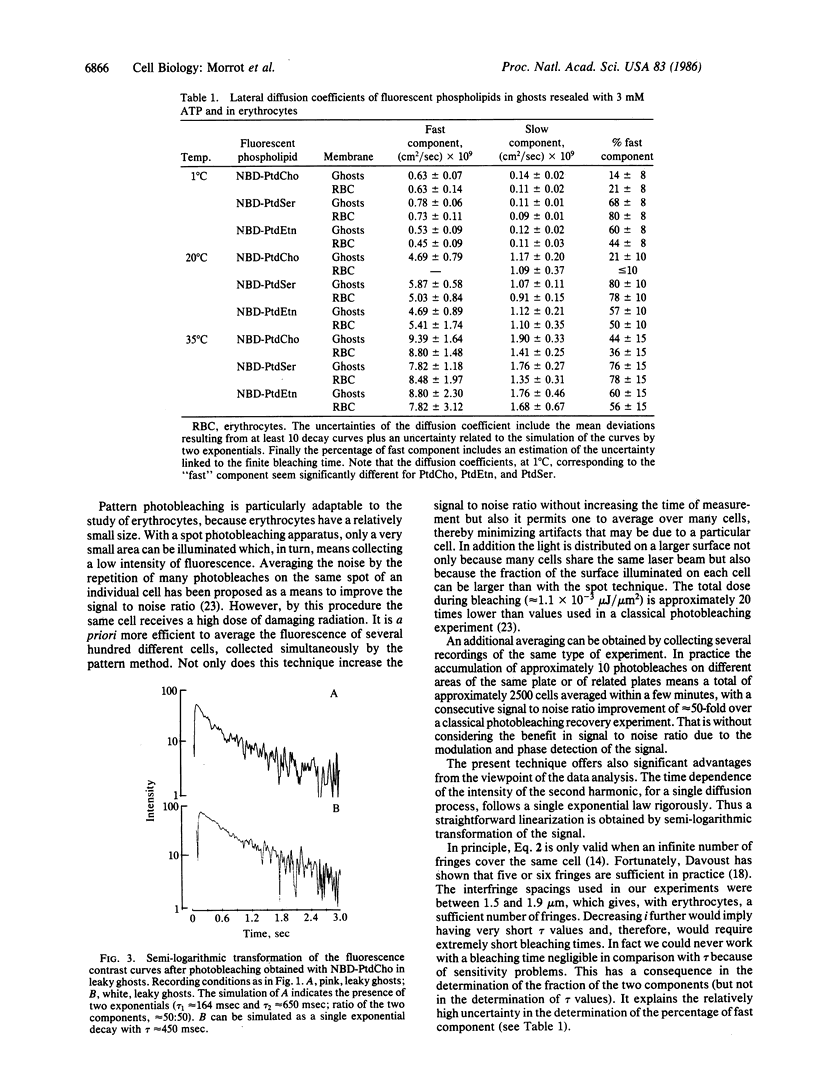
The fluorescent phospholipid 1-acyl-2-[12-(7-nitrobenz-2-oxa-1,3-diazol-4- yl)aminododecanoyl]phosphatidylcholine (NBD-phosphatidylcholine) and the corresponding aminophospholipid derivatives (NBD-phosphatidylethanolamine and NBD-phosphatidylserine) were introduced in the human erythrocyte membrane by a nonspecific phospholipid exchange protein purified from corn. The lateral mobility of the fluorescent phospholipids was measured by using an extension of the classical photobleaching recovery technique that takes advantage of a modulated fringe pattern and provides a high sensitivity. In intact erythrocytes and in ghosts resealed in the presence of ATP, the fluorescence-contrast curves after photobleaching decayed biexponentially corresponding to two lateral diffusion constants. With NBD-phosphatidylcholine, the majority of the signal corresponded to a "slow" component (1.08 X 10(-9) cm2/sec at 20 degrees C), whereas with the amino derivatives the majority of the signal corresponded to a "fast" component (5.14 X 10(-9) cm2/sec at 20 degrees C). If the ghosts were resealed without ATP, the fast component of the aminophospholipids disappeared. We interpret these results as follows: (i) Provided the cells or the ghosts contain ATP, the three fluorescent phospholipids distribute spontaneously between inner and outer leaflets as endogenous phospholipids, namely NBD-phosphatidylcholine is located in the outer leaflet, while both aminophospholipids are preferentially located in the inner leaflet. (ii) The viscosity of the inner leaflet of human erythrocyte membranes is lower than that of the outer leaflet.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom J. A., Webb W. W. Lipid diffusibility in the intact erythrocyte membrane. Biophys J. 1983 Jun;42(3):295–305. doi: 10.1016/S0006-3495(83)84397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comfurius P., Zwaal R. F. The enzymatic synthesis of phosphatidylserine and purification by CM-cellulose column chromatography. Biochim Biophys Acta. 1977 Jul 20;488(1):36–42. doi: 10.1016/0005-2760(77)90120-5. [DOI] [PubMed] [Google Scholar]
- Daleke D. L., Huestis W. H. Incorporation and translocation of aminophospholipids in human erythrocytes. Biochemistry. 1985 Sep 24;24(20):5406–5416. doi: 10.1021/bi00341a019. [DOI] [PubMed] [Google Scholar]
- Davoust J., Devaux P. F., Leger L. Fringe pattern photobleaching, a new method for the measurement of transport coefficients of biological macromolecules. EMBO J. 1982;1(10):1233–1238. doi: 10.1002/j.1460-2075.1982.tb00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler V., Haest C. W., Plasa G., Deuticke B., Erusalimsky J. D. Stabilizing factors of phospholipid asymmetry in the erythrocyte membrane. Biochim Biophys Acta. 1984 Aug 22;775(2):189–196. doi: 10.1016/0005-2736(84)90170-6. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Golan D. E., Alecio M. R., Veatch W. R., Rando R. R. Lateral mobility of phospholipid and cholesterol in the human erythrocyte membrane: effects of protein-lipid interactions. Biochemistry. 1984 Jan 17;23(2):332–339. doi: 10.1021/bi00297a024. [DOI] [PubMed] [Google Scholar]
- Henis Y. I., Rimon G., Felder S. Lateral mobility of phospholipids in turkey erythrocytes. Implications for adenylate cyclase activation. J Biol Chem. 1982 Feb 10;257(3):1407–1411. [PubMed] [Google Scholar]
- Heyn M. P. Determination of lipid order parameters and rotational correlation times from fluorescence depolarization experiments. FEBS Lett. 1979 Dec 15;108(2):359–364. doi: 10.1016/0014-5793(79)80564-5. [DOI] [PubMed] [Google Scholar]
- Kader J. C., Julienne M., Vergnolle C. Purification and characterization of a spinach-leaf protein capable of transferring phospholipids from liposomes to mitochondria or chloroplasts. Eur J Biochem. 1984 Mar 1;139(2):411–416. doi: 10.1111/j.1432-1033.1984.tb08020.x. [DOI] [PubMed] [Google Scholar]
- Kapitza H. G., Sackmann E. Local measurement of lateral motion in erythrocyte membranes by photobleaching technique. Biochim Biophys Acta. 1980;595(1):56–64. doi: 10.1016/0005-2736(80)90247-3. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P. A localized pattern photobleaching method for the concurrent analysis of rapid and slow diffusion processes. Biophys J. 1983 Aug;43(2):175–181. doi: 10.1016/S0006-3495(83)84338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimon G., Meyerstein N., Henis Y. I. Lateral mobility of phospholipids in the external and internal leaflets of normal and hereditary spherocytic human erythrocytes. Biochim Biophys Acta. 1984 Sep 5;775(3):283–290. doi: 10.1016/0005-2736(84)90182-2. [DOI] [PubMed] [Google Scholar]
- Schachter D., Cogan U., Abbott R. E. Asymmetry of lipid dynamics in human erythrocyte membranes studied with permanent fluorophores. Biochemistry. 1982 Apr 27;21(9):2146–2150. doi: 10.1021/bi00538a025. [DOI] [PubMed] [Google Scholar]
- Schreier S., Polnaszek C. F., Smith I. C. Spin labels in membranes. Problems in practice. Biochim Biophys Acta. 1978 Dec 15;515(4):395–436. doi: 10.1016/0304-4157(78)90011-4. [DOI] [PubMed] [Google Scholar]
- Schwoch G., Passow H. Preparation and properties of human erythrocyte ghosts. Mol Cell Biochem. 1973 Dec 15;2(2):197–218. doi: 10.1007/BF01795474. [DOI] [PubMed] [Google Scholar]
- Seigneuret M., Devaux P. F. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneuret M., Zachowski A., Hermann A., Devaux P. F. Asymmetric lipid fluidity in human erythrocyte membrane: new spin-label evidence. Biochemistry. 1984 Sep 11;23(19):4271–4275. doi: 10.1021/bi00314a002. [DOI] [PubMed] [Google Scholar]
- Smith B. A., McConnell H. M. Determination of molecular motion in membranes using periodic pattern photobleaching. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2759–2763. doi: 10.1073/pnas.75.6.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. I., Ohnishi S. Heterogeneity in the fluidity of intact erythrocyte membrane and its homogenization upon hemolysis. Biochim Biophys Acta. 1976 Mar 5;426(2):218–231. doi: 10.1016/0005-2736(76)90333-3. [DOI] [PubMed] [Google Scholar]
- Thompson N. L., Axelrod D. Reduced lateral mobility of a fluorescent lipid probe in cholesterol-depleted erythrocyte membrane. Biochim Biophys Acta. 1980 Mar 27;597(1):155–165. doi: 10.1016/0005-2736(80)90159-5. [DOI] [PubMed] [Google Scholar]
- Tilley L., Cribier S., Roelofsen B., Op den Kamp J. A., van Deenen L. L. ATP-dependent translocation of amino phospholipids across the human erythrocyte membrane. FEBS Lett. 1986 Jan 1;194(1):21–27. doi: 10.1016/0014-5793(86)80044-8. [DOI] [PubMed] [Google Scholar]
- Williamson P., Bateman J., Kozarsky K., Mattocks K., Hermanowicz N., Choe H. R., Schlegel R. A. Involvement of spectrin in the maintenance of phase-state asymmetry in the erythrocyte membrane. Cell. 1982 Oct;30(3):725–733. doi: 10.1016/0092-8674(82)90277-x. [DOI] [PubMed] [Google Scholar]
- van Deenen L. L. Topology and dynamics of phospholipids in membranes. FEBS Lett. 1981 Jan 12;123(1):3–15. doi: 10.1016/0014-5793(81)80007-5. [DOI] [PubMed] [Google Scholar]


