Abstract
Rationale
The head-twitch response (HTR) is a rapid side-to-side rotational head movement that occurs in rats and mice after administration of serotonergic hallucinogens and other 5-HT2A agonists. The HTR is widely used as a behavioral assay for 5-HT2A activation and to probe for interactions between the 5-HT2A receptor and other transmitter systems.
Objective
High-speed video recordings were used to analyze the head movement that occurs during head twitches in C57BL/6J mice. Experiments were also conducted in C57BL/6J mice to determine whether a head-mounted magnet and a magnetometer coil could be used to detect the HTR induced by serotonergic hallucinations based on the dynamics of the response.
Results
Head movement during the HTR was highly rhythmic and occurred within a specific frequency range (mean reciprocation frequency of 90.3 Hz). Head twitches produced wave-like oscillations of magnetometer coil voltage that matched the frequency of head movement during the response. The magnetometer coil detected the HTR induced by the serotonergic hallucinogens 2,5-dimethoxy-4-iodoamphetamine (DOI; 0.25, 0.5, and 1.0 mg/kg, IP) and lysergic acid diethylamide (LSD; 0.05, 0.1, 0.2, and 0.4 mg/kg, IP) with extremely high sensitivity and specificity. Magnetometer coil recordings demonstrated that the non-hallucinogenic compounds (+)-amphetamine (2.5 and 5.0 mg/kg, IP) and lisuride (0.8, 1.6, and 3.2 mg/kg, IP) did not induce the HTR.
Conclusions
These studies confirm that a magnetometer coil can be used to detect the HTR induced by hallucinogens. The use of magnetometer-based HTR detection provides a high-throughput, semi-automated assay for this behavior, and offers several advantages over traditional assessment methods.
The 5-HT2A receptor, a Gq/11-coupled receptor that is widely distributed in the central nervous system, is targeted by several classes of psychoactive drugs, including antipsychotics, hallucinogens, and antidepressants. Atypical antipsychotic agents such as clozapine and risperidone display 5-HT2A antagonist or inverse agonist activity (Weiner et al., 2001), and there is evidence that 5-HT2A blockade contributes to their therapeutic efficacy. Serotonergic hallucinogens such as lysergic acid diethylamide (LSD), psilocybin, and 2,5-dimethoxy-4-iodoamphetamine (DOI) act as 5-HT2A agonists, and most of the effects of these compounds are mediated by 5-HT2A receptor activation (reviewed by: Nichols 2004; Halberstadt and Geyer, 2011). For example, a significant correlation exists between the 5-HT2A receptor affinity of hallucinogens and their potency in vivo. Furthermore, 5-HT2A antagonists block most of the behavioral effects of hallucinogens in both animals and humans.
The head-twitch response (HTR) is a rhythmic paroxysmal rotational head movement that occurs in mice and rats in response to 5-HT2A receptor activation (Halberstadt and Geyer, 2011; Canal and Morgan, 2012). Head twitches in rats are sometimes referred to as wet-dog shakes because in that species the behavior frequently involves the head, neck, and trunk (Bedard and Pycock, 1977). The HTR was first observed in mice after administration of the 5-HT precursor 5-hydroxytryptophan (Corne et al., 1963), and was later shown to be induced by a variety of serotonergic hallucinogens, including LSD and DOI (Corne and Pickering, 1967; Yamamoto and Ueki, 1975; Bedard and Pycock, 1977; Arnt and Hyttel, 1989; Darmani et al., 1990). Although multiple receptor and neurotransmitter systems can regulate or modulate expression of the HTR, numerous studies have confirmed that this behavior is specifically linked to 5-HT2A activation. Most importantly, selective 5-HT2A antagonists block the HTR induced by DOI and other hallucinogens (Schreiber et al., 1995; Fantegrossi et al., 2005, 2006, 2008, 2010), and hallucinogens do not induce the HTR in 5-HT2A knockout mice (Gonzalez-Maeso et al., 2003, 2007; Keiser et al., 2009; Halberstadt et al., 2011). Although phencyclidine (Nabeshima et al., 1987), benzodiazepines (Tadano et al., 2001), the CB1 antagonist SR 14176A (Darmani and Pandya, 2000), the 5-HT1A antagonist WAY-100635 (Darmani, 1998), and thyrotropin-releasing hormone analogs (Fone et al., 1989) induce the HTR, these effects are blocked by 5-HT2A receptor antagonists. Because of this specificity, this behavior has been widely adopted as an animal behavioral assay for 5-HT2A activation and hallucinogen-like effects. Over the last decade, the HTR has been used to characterize the downstream signaling pathways that are coupled to the 5-HT2A receptor (Garcia et al., 2007; Gonzalez-Maeso et al., 2007; Schmid et al., 2008; Abbas et al., 2009; Schmid and Bohn, 2010), and to investigate the regulation of 5-HT2A signaling by other transmitter systems (Klodzinska et al., 2002; Marek et al., 2003, 2009; Benneyworth et al., 2007; Zhang and Marek, 2008; Egashira et al., 2011; Moreno et al., 2011a). It has also been proposed that the HTR may model the tics that occur in Tourette’s syndrome (Dursun and Handley, 1996; Handley and Dursun, 1992).
Two methods are currently used to assess the HTR in laboratory studies. Most commonly, a trained rater observes the animals directly and counts the number of head twitches (Schmid et al., 2008; Marek, 2009; Fox et al., 2010; Canal et al., 2010). Alternatively, the behavior of the test animals may be video recorded for off-line assessment (Fantegrossi et al., 2010; Klein et al., 2010; Moreno et al., 2011b; Halberstadt et al., 2011). In both cases, HTR experiments are time-consuming and potentially susceptible to inter-rater variability, have a low degree of temporal precision, and are not amenable to high-throughput screening. Furthermore, these methods do not allow the dynamics of the response to be studied, and are impractical for studies of the behavior over very long durations.
Siegel and colleagues (1972) attempted to automate detection of the HTR using a head-mounted magnet and a magnetometer coil. Head movements altered the magnetic field adjacent to the coil, inducing a change in coil voltage. The principle of HTR detection was based on the amplitude of the response; compared to other behaviors, head twitches tended to produce larger voltage deflections because they involve faster head movements. Unfortunately, because there was some overlap between the amplitude of the responses induced by head twitches and other behaviors, this detection method only identified 81% of the head twitches. In addition to the high failure rate, there were several other problems with this methodology. First, the recorded response (a voltage deflection) was not specific to the HTR, and therefore it was not possible to confirm that head twitches were actually being recorded. This limitation is particularly problematic because electromagnetic interference, as well as high-intensity movements such as jumping or seizures, can induce voltage deflections that are indistinguishable from head twitches. Second, the magnitude of the response to head-twitches was dependent on physical dimensions of the coil and the size of the magnet, making it difficult to standardize analysis. Third, there was no record of the individual head movements during head twitches, and therefore it was not possible to analyze response biophysics or distributions. For these and other reasons, the system developed by Siegel and colleagues has not replaced the traditional methods routinely used to assess the HTR in the laboratory.
Because the HTR is a highly rhythmic behavior, presumably involving head movements within a specific frequency range, it should be possible to detect this behavior based on the specific dynamics of the response. To test this hypothesis, we first analyzed the kinematics of head twitches using high-speed video recordings. We also conducted experiments to investigate whether a magnetometer coil can be used to detect head twitches induced by hallucinogens based on the specific sequences of head movement that occur during this behavior. The effects of DOI and LSD were compared to (+)-amphetamine and lisuride, compounds that do not induce the HTR in rodents (Bedard and Pycock, 1977; Gonzalez-Maeso et al., 2003, 2007) but do increase motor activity (Adams and Geyer, 1985; Young et al., 2010). We were interested in testing the effects of lisuride because this structural analog of LSD is a 5-HT2A agonist but is non-hallucinogenic in humans (Gonzalez-Maeso et al., 2007; Halberstadt and Geyer, 2010). These experiments confirmed that a magnetometer-based assessment can be used to detect the HTR with extremely high reliability and specificity, providing a high-throughput, semi-automated assay for this behavior.
MATERIALS AND METHODS
Animals
Mice were housed in a vivarium at the University of California San Diego (UCSD), an AAALAC-approved animal facility that meets Federal and State requirements for care and treatment of laboratory animals. Male C57BL/6J mice (6–8 weeks old) were obtained from Jackson Labs (Bar Harbor, ME, USA) and were allowed to acclimate for 1 week after arrival. Mice were housed up to 4 per cage in a climate-controlled room with a reversed light-cycle (lights on at 19:00 hours, off at 07:00 hours). Food and water were provided ad libitum, except during behavioral testing. Testing occurred between 10:00 and 18:00 hours. Experiments were conducted in accord with NIH guidelines and were approved by the UCSD animal care committee. All efforts were made to minimize animal suffering and the number of animals used.
Detection of the Head-Twitch Response
420-Hz video assessment
Mice were anesthetized with 2.5% isoflurane. After shaving the hair on the top of the head, a circular white plastic marker (6.3 mm diameter, 0.22 mm thick, 22 mg) was glued to the scalp with cyanoacrylate, centered between the eyes and the ears. The mice were allowed to recover for 48 h prior to testing. Immediately after administration of DOI (2.0 mg/kg IP), the mice were placed in a 15.5-cm diameter glass beaker, and their behavior was recorded for 10 min at 420 Hz using a Casio EX-FH100 camera. The AVI files were subsequently transferred to a PC for analysis. The location of the center of the marker (in pixel x,y coordinates) over sequential frames was tracked and recorded using the Video Spot Tracker program v.7.01 (Computer-Integrated Systems for Microscopy and Manipulation, University of North Carolina, NC, USA). The sequences of head movement were then reconstructed using SigmaPlot v.10.0. The videos were also analyzed by a trained observer to confirm the accuracy of the head-tracking algorithm. For each HTR, the average reciprocation frequency (in cycles per second) was determined using the formula f = n/t, where n equals the number of head rotations made during the response and t equals the duration of the response.
Magnetometer detection
Mice were anesthetized using a mixture of ketamine (100 mg/kg IP) and acepromazine (5 mg/kg IP). A rostrocaudal incision was made in the scalp, and a small neodymium magnet (4.57 mm × 4.57 mm × 2.03 mm, 375 mg) was attached to the center of the dorsal surface of the cranium using cyanoacrylate or dental resin; the magnet was mounted with the axis of the N-S poles parallel to the dorsoventral plane of the head. The mice were allowed to recover for 1–2 weeks prior to testing. After magnet implantation, mice were tested multiple times over 4–6 weeks, with a minimum of 1–2 weeks between tests; importantly, it was previously reported that administration of a 5-HT2A agonist at weekly intervals does not alter HTR sensitivity (Gewirtz and Marek, 2000). To record head movements, the mice were placed in a 12.5 cm diameter glass beaker surrounded by ~150 turns of #30 enameled magnet wire. The output of the coil was recorded using a Powerlab/8SP with LabChart v.7.3.2 (ADInstruments, Colorado Springs, CO, USA). Coil voltage was amplified, low-pass filtered (10 kHz cut-off frequency) to remove radiofrequency interference, and sampled at 40 kHz. Data were imported into the Matlab programming environment (MathWorks, Natick, MA, USA) for Fourier analysis. Voltage and frequency peaks were detected using the LabChart cyclic measurements analysis module. For HTR analysis, the LabChart data were digitally band-pass filtered (40–200 Hz), and responses were identified by manually searching for sinusoidal wavelets with the following characteristics: (1) waveform and spectrum consistent with 40–160 Hz activity; (2) contain more than 2 bipolar peaks; (3) amplitude exceeds the background noise level; and (3) duration <120 ms.
In most experiments, the magnetometer-based assessment of head twitch was combined with simultaneous video recordings. The behavior of the mice within the magnetometer coil was captured at 30-Hz using a CCD video camera located above the glass beaker, digitized, and stored on a PC as an AVI file. Subsequently, head twitches were counted by an observer blind to the treatment and the magnetometer data (Halberstadt et al., 2011). Potential responses were analyzed frame-by-frame using VirtualDub v.1.9.11 and were counted as head twitches if there was evidence of torsional head movement over consecutive frames. For the experiment with SKF38393, the duration of each grooming bout (including licking the paws, legs, fur, body, tail, or genitals, and washing the head, face, and ears) was assessed by an observer blind to the treatment.
Procedures
Mice were transferred to the testing room 1 h before testing. The test beakers were cleaned thoroughly between test sessions. The details of the specific experiments are shown in Table 1.
Table 1.
Experimental design
| Experiment | Treatment | Interval between treatment and testing | Test duration | Animals | Design |
|---|---|---|---|---|---|
| DOI dose-response | Vehicle or DOI (0.25, 0.5, 1.0mg/kg) | 0 min | 10 min | n = 13 | 4-way semi-randomized crossover with 1week between tests |
| (+)- Amphetamine dose-response | Vehicle or (+)-amphetamine (2.5, 5.0 mg/kg) | 20 min | 10 min | n = 5–7 (18 total) | Between-subjects |
| SKF38393 | Vehicle or 10 mg/kg SKF38393 | 20 min | 10 min | n = 5 (10 total) | Between-subjects |
| LSD dose-response | Vehicle or LSD (50, 100, 200, 400 μg/kg) | 0 min | 30 min | n = 5 (25 total) | Between-subjects |
| Lisuride dose-response | Vehicle or lisuride (0.8, 1.6, 3.2 mg/kg) | 0 min | 30 min | n = 5 (20 total) | Between-subjects |
Drugs
Drugs used were (+)-amphetamine hemisulfate; (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI) (Sigma-Aldrich, St. Louis, MO, USA); (+)-lysergic acid diethylamide tartrate (LSD) (National Institute on Drug Abuse, Rockville, MD, USA); R-lisuride hydrogen maleate (98.9% purity; IVAX Pharmaceuticals, Opava, Czech Republic); and 1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol (SKF38393) (RBI, Natick, MA, USA). Drug doses are expressed as the salt form of the drug, except for (+)-amphetamine, which refers to the freebase weight. All drugs were dissolved in isotonic saline and administered IP in a volume of 5 ml/kg.
Analysis
For studies with a between-subjects design, data were analyzed by using one-way analyses of variance (ANOVAs) with drug treatment as the between-subject variable. When appropriate, time was included as a repeated measure. Specific post hoc comparisons between selected groups were done using Tukey’s studentized range method. Significance was demonstrated by surpassing an α-level of 0.05. For the DOI crossover study, data were analyzed using ANOVAs with DOI dose as a repeated measure. ANOVAs between specific groups were used for post hoc analysis of the crossover study. For all experiments, specific post hoc comparisons were only performed when the ANOVAs revealed significant main effects or interactions with time.
RESULTS
High speed video recording studies
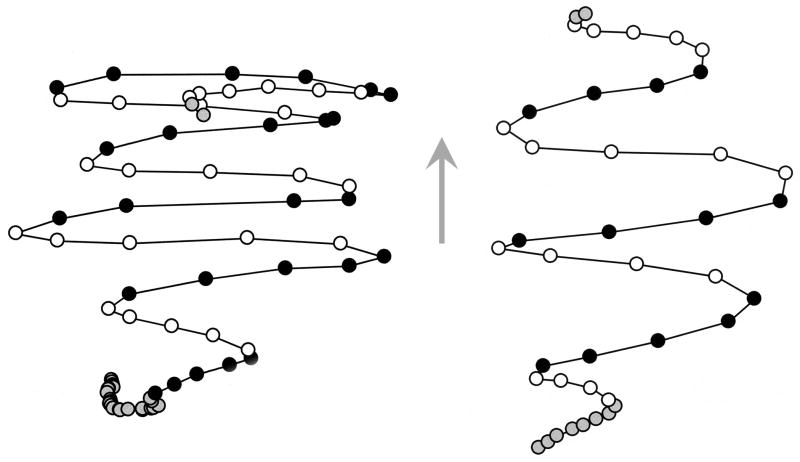
To characterize the dynamics of head movement during the HTR, we treated C57BL/6J mice (n = 5) with 2 mg/kg DOI and then recorded their behavior at 420 Hz using a high-speed video camera. The video recordings captured a total of 160 head twitches (see Table 2), and the sequences of head movement during the responses were reconstructed using video spot tracking. The recordings demonstrated that the HTR consists of a rapid series of reciprocal, side-to-side radial head movements. Figure 1 shows reconstructions of the sequences of head movements made during characteristic responses. Each HTR consisted of 5–11 head rotations (individual side-to-side head movements), with the average number of rotations per HTR being 7.2±0.1 (mean±S.E.M.), and the direction of rotation alternating at an average frequency of 90.3±0.3 Hz (see Table 2). The mice initiated the HTR while balanced on their hind paws or in a quadruped stance, typically with the body slightly hunched back and the head extended forward. The trunk and neck became progressively elongated over the course of the response, increasing the extension of the head from the body. Torsional movement during the HTR was confined to the neck unless the behavior occurred in combination with a body shake. In the latter case, the rotation started with the head and neck and then progressively rolled over the trunk of the animal toward the tail. Often, head twitches were initiated after the mice made a slow head rotation to one side. Over the course of individual head twitches, the rotational distance and angular velocity of head movement increased and then decayed, with the last rotation often being the slowest.
Table 2.
Dynamics of DOI-induced HTRs in 420-Hz video recordings.
| Reciprocation frequency (Hz) | Head rotations per response | ||||
|---|---|---|---|---|---|
|
| |||||
| Mouse | # HTRs analyzed | Mean±S.E.M. | Range | Mean±S.E.M. | Range |
|
| |||||
| 1 | 45 | 90.7±0.7 | 77.4–96.9 | 6.8±0.2 | 5–9 |
| 2 | 34 | 91.0±0.7 | 81.7–98.0 | 7.2±0.2 | 5–11 |
| 3 | 40 | 90.5±0.6 | 81.9–98.0 | 7.4±0.1 | 6–9 |
| 4 | 12 | 93.4±0.9 | 89.1–98.0 | 7.0±0.1 | 6–8 |
| 5 | 29 | 87.1±0.6 | 81.7–93.3 | 7.4±0.2 | 6–9 |
|
| |||||
| Total: | 160 | 90.3±0.31 | 7.2±0.11 | ||
Calculated as the mean of all analyzed responses.
Figure 1.
Reconstructions of head movement sequences during head-twitches in mice after administration of 2 mg/kg DOI. Two representative plots are shown. Head position was sampled every 2.38 ms. The circles show the position of the center of the head in x,y coordinates over successive video frames. Open and filled circles represent rotational head movements toward the left or right side of the mouse, respectively; grey circles represent movements immediately preceding or subsequent to the HTR. The arrow shows the direction the head was facing during the responses.
DOI Dose-Response
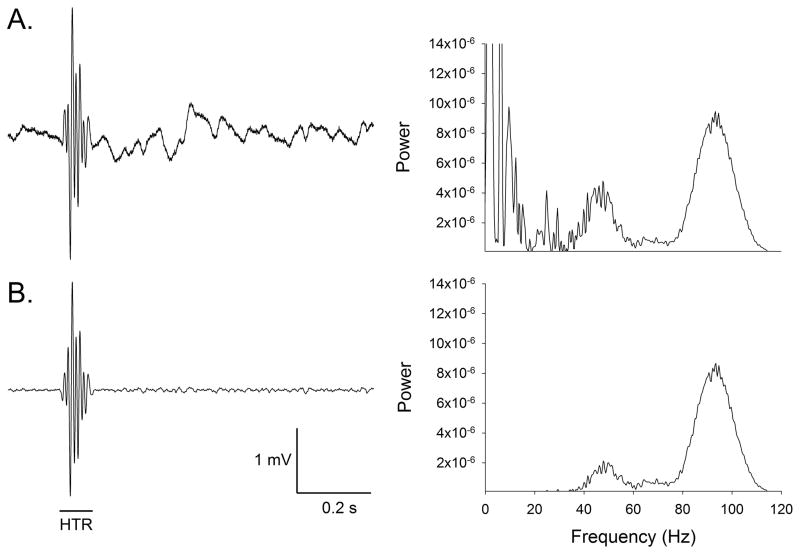
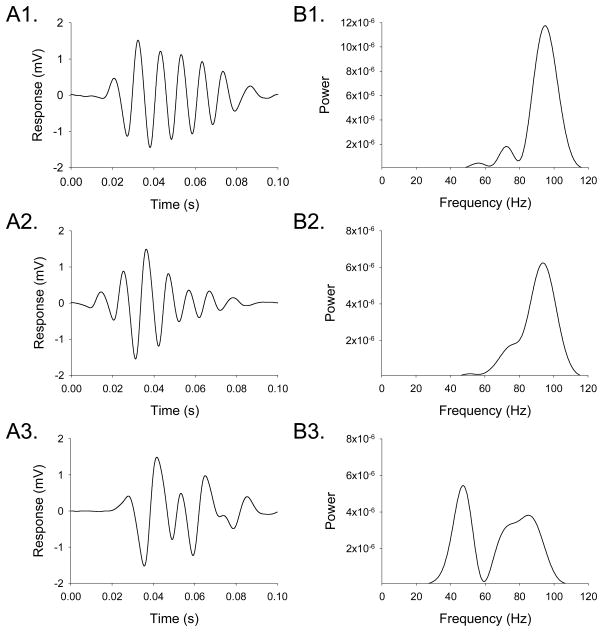
The high-speed video recordings demonstrated that head movement in mice during the HTR is highly rhythmic and occurs within a specific frequency range. Because spontaneous non-HTR head movement in mice occurs at ≤ 20 Hz, with most activity ≤ 4 Hz (Beraneck et al., 2008), these data indicate that it should be possible to detect the HTR and discriminate it from other head movements based on its frequency and dynamics. Experiments using an indwelling skull-mounted magnet and a magnetometer coil demonstrated that this arrangement can be used to detect the rhythmic head movement that occurs during head twitches induced by DOI, and confirmed that the HTR can be distinguished from other types of head movement. Although the raw output of the magnetometer coil contains a large amount of low-frequency background noise due to non-HTR head movements (Fig 2A), the noise from extraneous head movements can be removed by high-pass filtering (40 Hz cut-off frequency), allowing head twitches to be detected against a stable background (Fig 2B). Head twitches produced brief (< 0.12 s) wave-like oscillations of magnetometer coil voltage (Fig. 3A). Fourier analysis confirmed that the voltage response to non-HTR head movements had very little frequency content > 20 Hz, whereas the voltage response induced by head twitches peaked between 80–100 Hz, sometimes with a harmonic in the 40–50 Hz range (Fig. 3B). The 420-Hz video recordings failed to identify any individual head rotations during head twitches that lasted longer than 16.7 ms (i.e., a reciprocation frequency < 60 Hz; data not shown), indicating that the 40–50 Hz signals are likely an artifact. The most likely explanation for these lower-frequency responses is that the magnetometer coil sometimes responds weakly to head rotations in one direction. Comparison of simultaneous magnetometer coil and 420-Hz video recordings in a pilot experiment confirmed that each side-to-side head rotation induces a 360° change in magnetometer coil voltage (data not shown).
Figure 2.
Effect of filtering on magnetometer coil responses. The left panels show the voltage response of the magnetometer coil, and the right panels are periodograms showing the spectral density of the responses. (A) Unfiltered magnetometer coil responses. (B) Output of the magnetometer coil after band-pass filtering (40–200 Hz).
Figure 3.
Characteristic magnetometer coil responses induced by head-twitches. (A) Response of the magnetometer coil to head-twitches. (B) Periodograms showing the spectral density of the responses. The magnetometer recordings were band-pass filtered (40–200 Hz) to attenuate the response to extraneous head movements and high-frequency noise.
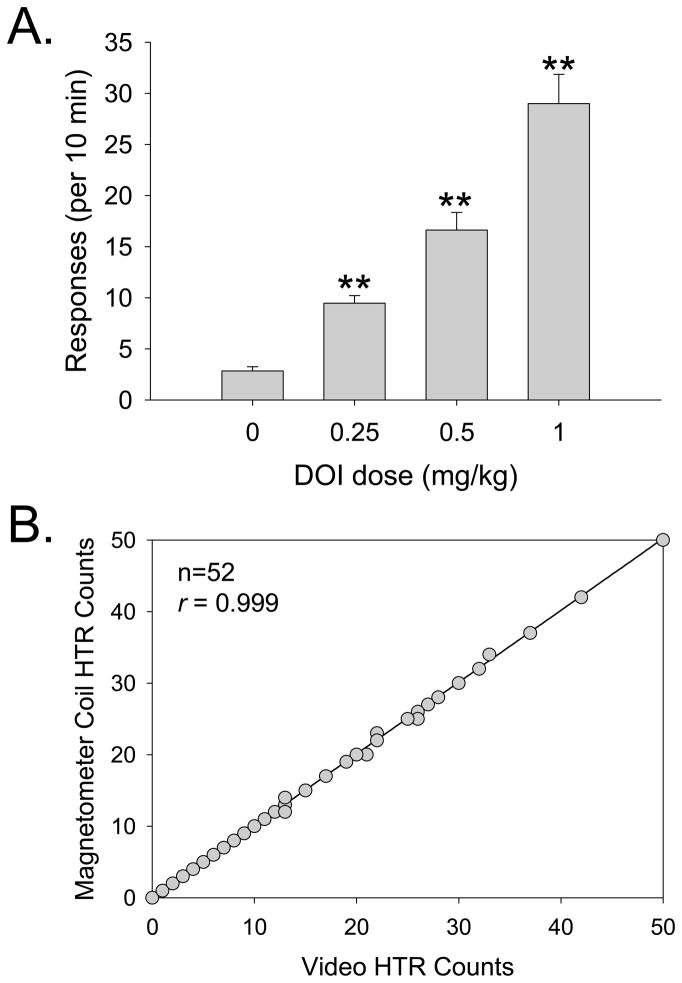
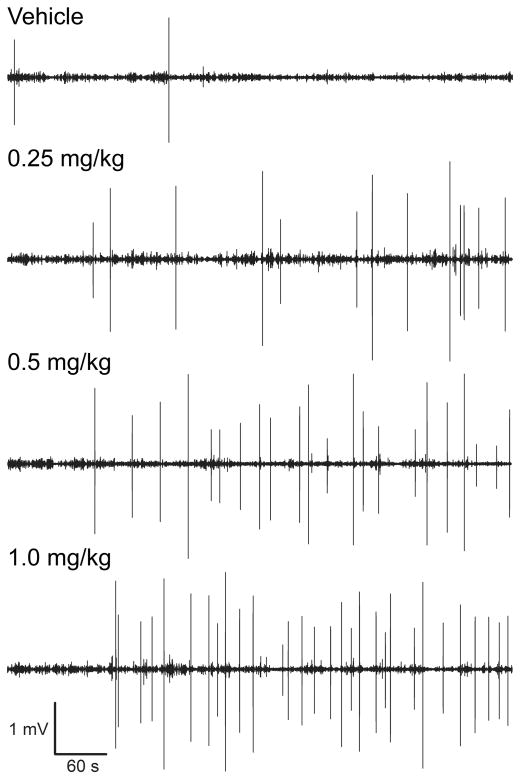
To validate the use of a magnetometer-based assessment of the HTR, we conducted a dose-response study with DOI (n = 13) and compared the results obtained using a magnetometer coil versus a standard 30-Hz video assessment (e.g., Halberstadt et al., 2011). The magnetometer data were analyzed by identifying individual sinusoidal wavelets that match the rhythmicity, frequency, and duration characteristic of head twitches. As shown in Figure 4A, the video recordings confirmed that the HTR induced by DOI is dose-dependent (Drug effect: F(3,36)=58.05, p<0.0001). Video and magnetometer HTR counts were highly correlated (r = 0.999, F(1,51)=48448.32, p<0.0001; Fig. 4B); of the 753 head twitches observed in the video recordings during the 10-min observation period, 749 (99.5%) were detected by blind assessment of the magnetometer data. Five additional head twitches were identified in the magnetometer data but were not observed in the videos (a false-positive detection rate of 0.01 responses/min); because there was some evidence of head movement in the videos at those time points, these responses were most likely head twitches that were not identified in the video recordings because the capture rate (30 fps) was too low (i.e., the head movements occurred during the interval between video frames). Figure 5 shows the temporal distribution of head twitches in the magnetometer data from one mouse treated with 0–1.0 mg/kg DOI over multiple test sessions; head twitches were evenly distributed throughout the 10 min test sessions, except during the first 1–2 min when the response rate was very low.
Figure 4.
Effect of DOI on the head-twitch response. (A) Manually scored head-twitch counts from video recordings. Data are presented as group means±S.E.M. **p<0.01, significant difference from vehicle control group. (B) Correlation between head-twitch counts in the videos and the magnetometer coil recordings.
Figure 5.
Plots of magnetometer coil voltage responses showing the temporal distribution of head twitches induced by DOI. A single C57BL/6J mouse was treated with vehicle and DOI (0.25, 0.5, and 1.0 mg/kg, i.p.), with 1-week between test sessions. At this time resolution, head twitches appear as high-amplitude bipolar deflections. The numbers of head twitches detected were 2 (vehicle), 13 (0.25 mg/kg), 22 (0.5 mg/kg), and 30 (1.0 mg/kg).
Direct and indirect DA agonist studies
To determine whether high levels of activity can interfere with HTR detection using a magnetometer coil, (+)-amphetamine was tested at doses that produce robust increases in locomotor activity in C57BL/6J mice (Young et al., 2010). There was no difference between the number of HTRs detected by the magnetometer coil and by video analysis (data not shown). Compared with vehicle treatment, 2.5 and 5.0 mg/kg amphetamine did not produce a significant change in the spontaneous HTR rate (F(2,17)=2.07).
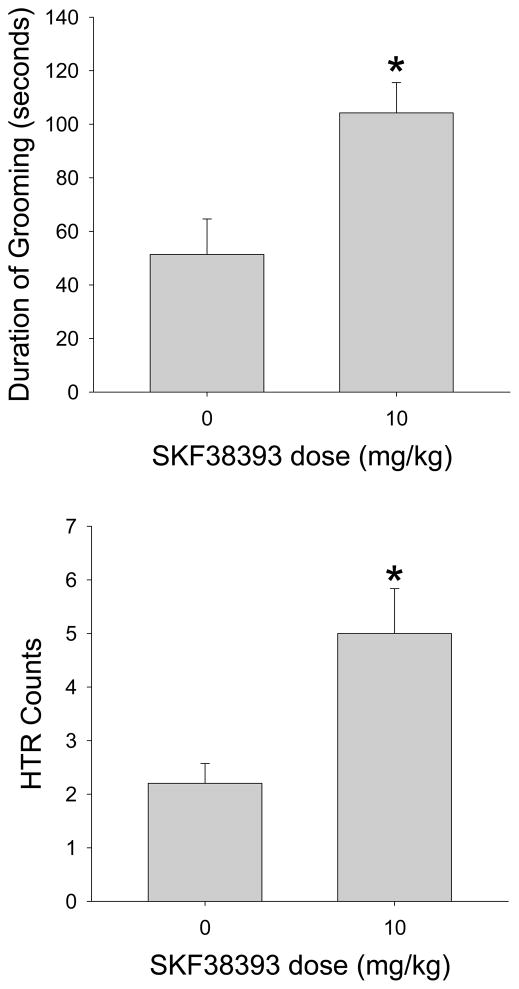
Mice make frequent head movements while grooming, and hence this behavior could potentially interfere with the magnetometer-based assessment of HTR. To determine whether grooming is a confounding factor, we assessed the HTR rate in mice treated with a dose of the partial D1 agonist SKF38393 that was previously reported to significantly increase the time spent grooming (Starr & Starr, 1986). As shown in Figure 6, administration of 10 mg/kg SKF38393 increased the duration of grooming (F(1,9)=9.191, p<0.02). SKF38393 also produced a slight but significant increase in HTR (F(1,9)=9.333, p<0.02). Importantly, grooming did not cause false-positive HTR detections by the magnetometer coil, and there was no difference between the number of head twitches detected by the magnetometer coil (36 counts) and the video analysis. Furthermore, frequency analysis demonstrated that head movement during grooming produces 0–20 Hz responses (Supplemental Figure 1), which is too low to interfere with detection of the HTR. Therefore, it appears that SKF38393 increases the HTR rate. Importantly, the experiment with SKF38393 confirms that our detection methodology can distinguish the HTR from the head movements that occur during grooming.
Figure 6.
Effects of SKF38393 (10 mg/kg) on (A) the duration of grooming, and (B) the head-twitch response. Data are presented as group means±S.E.M. Five C57BL/6J mice were used per group. *p<0.05, significant difference from vehicle control group.
LSD dose-response study
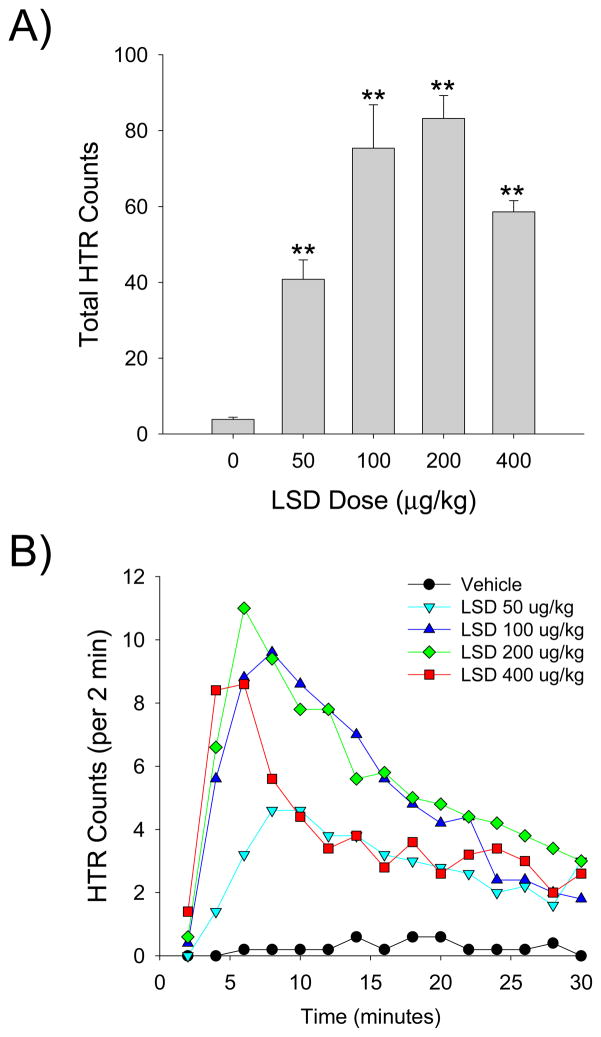
As shown by the magnetometer data, LSD produced a dose-dependent increase in HTR with an inverted U-shaped dose-response function (Drug effect: F(4,20)=25.28, p<0.0001; Fig. 7A). The 200 μg/kg dose of LSD was maximally effective, inducing 83.8±5.9 head twitches during the 30-min test session. The ED50 of LSD was calculated as 52.9 μg/kg (95% CL = 38.9–72.0 μg/kg) by nonlinear regression. To validate the magnetometer data, the results in a subset of animals (n = 6) were compared with HTR counts obtained by video analysis; the results obtained using the two methods (337 magnetometer responses; 339 video responses) were highly correlated (r = 0.9975, F(1,5)=810.1986, p<0.001).
Figure 7.
Effect of LSD on the head-twitch response. (A) Total counts over the 30-min test session. Data are based on the magnetometer recordings, and are presented as group means±S.E.M. (B) Time-course of the HTR induced by LSD, in 2-min blocks. Data are based on the magnetometer recordings, and are presented as group means. Five C57BL/6J mice were used per group. *p<0.05, **p<0.01, significant difference from vehicle control group.
HTR counts were also analyzed in 2-min bins to examine the time-course of the response to LSD. The effect of LSD was highly temporally dependent (Drug × Time: F(56,280)=7.00, p<0.0001). Depending on the dose administered, the maximal response to LSD occurred during either the third (200 and 400 μg/kg) or fourth (50 and 100 μg/kg) time block (i.e., 4–10 min after administration; Fig. 7B). Although the response to LSD declined over the course of the session, all four doses of LSD induced a significant response during the last time block (p<0.05, 0.01 versus vehicle, Tukey’s test).
Lisuride dose-response study
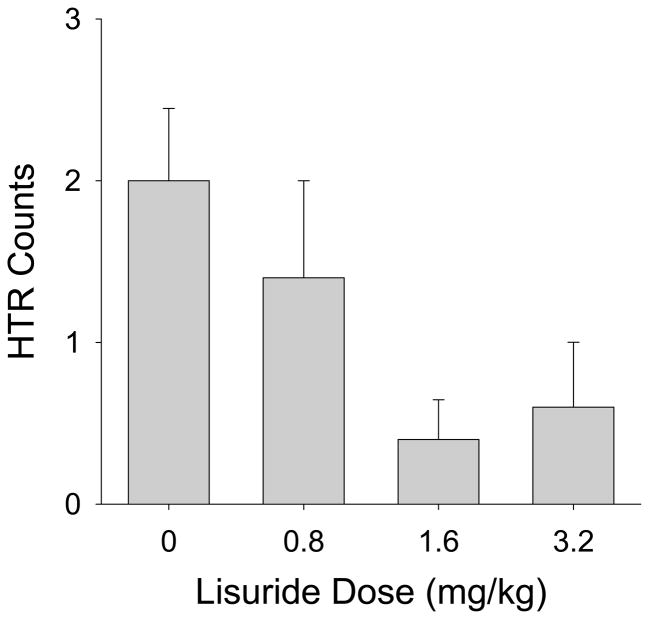
Even high doses of lisuride (1.6 and 3.2 mg/kg) failed to induce the HTR in mice (Fig. 8). Lisuride reduced the baseline level of HTR, although the effect was not significant (F(3,16)=2.80, p<0.08). These findings demonstrate that lisuride is at least 64-fold less potent than LSD and 13-fold less potent than DOI.
Figure 8.
Effect of lisuride on the head-twitch response. Data are based on the magnetometer recordings over the 30-min test session. Five C57BL/6J mice were used per group.
DISCUSSION
These experiments demonstrated that head movement during the HTR in mice is highly rhythmic, with the direction of head movement changing at relatively high frequency. Specifically, each HTR consists of a rapid sequence of reciprocating, side-to-side head movements. Using a head-mounted magnet and a magnetometer coil, we were able to detect the head movement that occurs during the HTR as a characteristic, sinusoidal waveform. Importantly, the frequency of the magnetometer response induced by head twitches is higher than the frequency associated with other spontaneous head movements, and therefore with suitable filtering it is possible to detect and discriminate the HTR with an extremely high degree of specificity and reliability. Using a magnetometer coil to assess the HTR, we conducted pharmacological studies with DOI and LSD, hallucinogens that are commonly used to induce the HTR; these experiments confirmed that DOI and LSD induce the HTR dose-dependently. By contrast, neither (+)-amphetamine nor the non-hallucinogenic 5-HT2A agonist lisuride induced the HTR, confirming findings previously reported in the literature (Bedard and Pycock, 1977; Gonzalez-Maeso et al., 2003, 2007). We also found that high levels of grooming (induced by the D1 agonist SKF38393) did not interfere with assessment of the HTR or result in false-positive detections. In summary, a magnetometer coil can be used to detect the HTR in mice with high sensitivity and specificity, yielding a high-throughput, semi-automated assay for this behavior.
As far as we are aware, the present investigation is the first to examine the dynamics of head twitches induced by hallucinogens in mice. The 420-Hz video recordings demonstrated that mice make 5–11 head movements during a HTR, with the direction of head rotation changing reciprocally at approximately 78–98 Hz. These findings are supported by the magnetometer recordings, with the frequency content of HTR-induced responses typically peaking between 80 Hz and 100 Hz. Electromyography (EMG) studies have characterized the head movement that occurs during spontaneous head twitches in cats (Richmond et al., 1992) and infant pigs (Wentzel et al., 2011). In cats, the head makes 1–5 full oscillatory cycles during a head twitch, with each cycle completed in approximately 100–150 ms (Richmond et al., 1992). Likewise, for head twitches in pigs, the head makes 2–8 full oscillations, each lasting for ~100 ms (Wentzel et al., 2011). It is important to note that the EMG studies analyzed the responses in terms of oscillatory cycles (one full oscillation consisting of a head roll to one side and then back to the opposite side), whereas our studies counted the number of individual head movements and quantified the reciprocation frequency. One reason why we did not characterize the HTR in terms of oscillations is that the mice did not always make an even number of head movements during the response; more importantly, the magnetometer coil responds to individual head movements and not to oscillatory cycles. In terms of the conventions used in our studies, cats make 2–10 reciprocal rotational movements during head twitches, alternating direction at a frequency of ~13–20 Hz, whereas pigs make 4–16 head rotations, with a reciprocation frequency of ~20 Hz. We have also conducted preliminary magnetometer studies of the HTR in Long-Evans rats, and in that species the head movement displays a reciprocation frequency of ~30–40 Hz (A.L. Halberstadt, unpublished observations). Studies characterizing the kinematics of wet-dog shakes in a variety of species demonstrate that the oscillation frequency of body shaking scales with mass as f ~ M−0.22 (Dickerson et al., 2012). Based on the results obtained in mice, rats, and cats, it appears that the frequency of head shaking during the HTR is also inversely proportional to the mass of the head. Dickerson et al. (2012) reported that wet-dog shakes in adult mice occur with a mean oscillation frequency of 29 Hz (i.e., a reciprocation frequency of ~ 58 Hz). The higher frequency values observed in our studies likely reflect inherent differences between the frequencies of head twitches and body shakes, due to the head having a much smaller mass than the body. Indeed, when the fact that the head accounts for only about 10–15% of the total weight of an adult mouse (Mason et al., 1955) is taken into account, the observed HTR oscillation frequency is entirely consistent with the mass scaling rule formulated by Dickerson and colleagues.
Compared with the laboratory methods that are used currently to assess the HTR, there are several advantages to using a magnetometer coil-based assessment. First, because the technique does not require direct observation of behavior and can be used to test multiple animals simultaneously, the time required to complete HTR experiments is markedly reduced. Second, the use of a magnetometer coil allows the HTR to be detected using specific, objective criteria. Because of the brief duration of head twitches (often <0.1 s), measurement of the response by visual monitoring is highly subjective, potentially reducing the reliability of head-twitch measures. Although some investigators have reported anecdotally that it is possible to assess the HTR with high inter-rater reliability (Garcia et al., 2007; Canal and Morgan, 2012), one study that specifically addressed this issue found relatively low levels of inter-observer agreement (Silva and Calil, 1975). A third advantage of this assessment method is that it records the individual head movements that occur during each HTR. In addition to altering the number of head twitches made, it is possible that certain pharmacological treatments or neurochemical manipulations may alter the dynamics of the response (including the frequency, intensity, or duration of movement). Hence, this technique enables the HTR to be studied both quantitatively and qualitatively. Furthermore, while previously it was not possible to assess the HTR with high temporal precision, the magnetometer recordings provide a detailed record of when head twitches occur. Increasing the temporal resolution of HTR detection could potentially facilitate identification of the neuroanatomical site(s) mediating this behavior using electrophysiological techniques. Indeed, there is some disagreement in the literature regarding whether the 5-HT2A receptors that mediate the HTR are located in prefrontal cortex (Willins and Meltzer, 1997; González-Maeso et al., 2007) or in other brain regions (Bedard and Pycock, 1977; Lucki and Minugh-Purvis, 1987; Watson and Gorzalka, 1992).
Most investigators should be able to implement a magnetometer-based assessment of HTR with little difficulty. Subcutaneous magnet implantation can be completed very quickly (requiring ~5 min per mouse, excluding the time required for recovery from anesthesia), and the mice can be maintained and tested for several months post-implantation. In order to detect the HTR, it is necessary to amplify and analyze the output of the magnetometer coil. Although we used a commercially available data-acquisition system, a low-noise preamplifier and a personal computer with a soundcard could be used for data collection. To detect head twitches in the magnetometer coil recordings, we used a manual detection strategy where potential responses were identified in the data and then classified as being head twitches or extraneous movements. Inspection of Figure 5 shows that it is very easy to identify potential responses in the recordings. This procedure was highly effective, as evidenced by the fact that video and magnetometer HTR counts were highly correlated. Although it is possible to automate the HTR detection process using an algorithm, in the present studies we detected responses manually to ensure that detection was as accurate as possible. If possible, future studies will make use of an automated detection algorithm, potentially reducing analysis time.
Although most 5-HT2A agonists induce the HTR in mice and rats, one notable exception is the non-hallucinogenic LSD analog lisuride (Gerber et al., 1985; González-Maeso et al., 2003, 2007). It was recently proposed that the behavioral differences between LSD and lisuride may be due to 5-HT2A functional selectivity, whereby lisuride activates the 5-HT2A receptor but does not recruit the specific signaling mechanisms necessary to induce the HTR and provoke hallucinogenesis (González-Maeso et al., 2007). Alternatively, as we have discussed previously (Halberstadt and Geyer, 2010), lisuride is a weak 5-HT2A partial agonist (Cussac et al., 2008), and it is possible that lisuride does not activate the receptor with sufficient efficacy to induce the HTR. The fact that lisuride has been found to induce the HTR in the least shrew (Cryptotis parva), a non-rodent species that is reportedly highly sensitive to 5-HT2A agonists (Darmani, 1994), is consistent with the latter hypothesis. Although previous HTR studies in mice tested LSD and lisuride at comparable doses (0.06–0.96 and 0.1–0.8 mg/kg, respectively; González-Maeso et al., 2003, 2007), lisuride induced the HTR in the least shrew with lower potency than DOI (Darmani, 1994), indicating that lisuride is likely to be substantially less potent than LSD in mice. Given the potency of DOI in mice (see Fig. 4A), we reasoned that if the partial agonist hypothesis is correct, doses of lisuride >1 mg/kg may be required to induce the HTR. In our experiments, however, even 3.2 mg/kg lisuride failed to induce the HTR. While these findings do not completely eliminate the possibility that the behavioral inactivity of lisuride is due to partial agonist activity, they do confirm that lisuride does not induce the HTR in mice.
Grooming behavior in rodents involves frequent head movements that could potentially interfere with HTR detection by the magnetometer coil or induce false-positive responses. Importantly, head movement during grooming produces 0–20 Hz magnetometer responses, whereas head twitches induce higher frequency responses, Hence, these two behaviors are distinguished easily based on their frequency characteristics. Administration of SKF38393 confirmed that high levels of grooming do not result in false-positive responses or interfere with HTR detection. This is an extremely important finding because it demonstrates that the detection of head twitches is not compromised by high levels of extraneous head movement.
The experiment with the D1 partial agonist SKF38393 demonstrated that this compound produced a small but significant HTR. This finding is unexpected because it was previously reported that SKF38393 does not induce the HTR in rats (Schreiber et al., 1995). Because SKF38393 binds to [3H]ketanserin-labeled 5-HT2A receptors with low-affinity (Ki > 10,000 nM; Neumeyer et al., 2003), it is unlikely that this compound is inducing the HTR by directly activating 5-HT2A receptors. One possible explanation for the effect of SKF38393 is that D1 receptor activation may modulate endogenous 5-HT2A signaling, increasing the spontaneous rate of HTR. The HTR induced by DOI in rats is potently attenuated by D1 antagonists such as SCH 23390 and SCH 39166 (Schreiber et al., 1995; Dursun and Handley, 1996). Likewise, in rabbits, SCH 23390 blocks the ability of DOI to induce head bobs (Schindler et al., 2012), a 5-HT2A- mediated behavior that is similar to the HTR. Since the HTR induced by DOI is mediated by the 5-HT2A receptor (Schreiber et al., 1995; Fantegrossi et al., 2010) and DOI has negligible D1 affinity (Ki = 16,908 nM; Schindler et al., 2012), these findings indicate that the D1 receptor can modulate the behavioral response to 5-HT2A receptor activation. It is unlikely that D1 receptor activation could induce the HTR directly because SKF38393 (via the D1 receptor) and DOI (via the 5-HT2A receptor) have opposite effects on network activity in the prefrontal cortex (Lambe and Aghajanian, 2007), the brain region that is thought to mediate the HTR to DOI (Willins and Meltzer, 1997). Future studies will determine whether the HTR induced by SKF38393 is dependent on the 5-HT2A receptor, and will investigate whether the D1 receptor can modulate the HTR induced by 5-HT2A activation.
These experiments demonstrate that it is possible to detect the HTR using a magnetometer coil and a head-mounted magnet. Not only does this technique simplify assessment of a widely studied behavior, but it provides a high-resolution qualitative assessment that was heretofore unavailable. To date, assessment of the HTR has been highly subjective. Because the magnetometer-based assessment detects head twitches using objective criteria, it enables HTR analysis to be standardized, potentially increasing the reliability and reproducibility of these measures. Given that the HTR is commonly used to assess whether compounds display 5-HT2A agonist and antagonist activity in vivo, the development of a magnetometer-based assessment could potentially facilitate the high-throughput screening of compounds for interactions with the 5-HT2A receptor.
Supplementary Material
Supplementary Figure 1. Magnetometer coil responses induced by grooming behavior. (A) Voltage response of the magnetometer coil during grooming. (B) Periodogram showing the spectral density of the magnetometer response to grooming.
Acknowledgments
Supported by National Institute on Drug Abuse Awards R01 DA002925 and F32 DA025412, and the Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center.
References
- Abbas AI, Yadav PN, Yao WD, Arbuckle MI, Grant SG, Caron MG, Roth BL. PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci. 2009;29:7124–7136. doi: 10.1523/JNEUROSCI.1090-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams LM, Geyer MA. Patterns of exploration in rats distinguish lisuride from lysergic acid diethylamide. Pharmacol Biochem Behav. 1985;23:461–468. doi: 10.1016/0091-3057(85)90022-x. [DOI] [PubMed] [Google Scholar]
- Arnt J, Hyttel J. Facilitation of 8-OHDPAT-induced forepaw treading of rats by the 5-HT2 agonist DOI. Eur J Pharmacol. 1989;161:45–51. doi: 10.1016/0014-2999(89)90178-7. [DOI] [PubMed] [Google Scholar]
- Bedard P, Pycock CJ. “Wet-dog” shake behaviour in the rat: a possible quantitative model of central 5-hydroxytryptamine activity. Neuropharmacology. 1977;16:663–670. doi: 10.1016/0028-3908(77)90117-4. [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol. 2007;72:477–484. doi: 10.1124/mol.107.035170. [DOI] [PubMed] [Google Scholar]
- Beraneck M, McKee JL, Aleisa M, Cullen KE. Asymmetric recovery in cerebellar-deficient mice following unilateral labyrinthectomy. J Neurophysiol. 2008;100:945–958. doi: 10.1152/jn.90319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Morgan D. Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal. 2012 doi: 10.1002/dta.1333. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC. The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology. 2010;209:163–174. doi: 10.1007/s00213-010-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW. A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia. 1967;11:65–78. doi: 10.1007/BF00401509. [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW, Warnet BT. A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Br J Pharmacol Chemother. 1963;20:106–120. doi: 10.1111/j.1476-5381.1963.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussac D, Boutet-Robinet E, Ailhaud MC, Newman-Tancredi A, Martel JC, Danty N, Rauly-Lestienne I. Agonist-directed trafficking of signaling at serotonin 5-HT2A, 5-HT2B and 5-HT2C-VSV receptors mediated Gq/11 activation and calcium mobilisation in CHO cells. Eur J Pharmacol. 2008;594:32–38. doi: 10.1016/j.ejphar.2008.07.040. [DOI] [PubMed] [Google Scholar]
- Darmani NA. The silent and selective 5-HT1A antagonist, WAY 100635, produces via an indirect mechanism, a 5-HT2A receptor-mediated behaviour in mice during the day but not at night. J Neural Transm. 1998;105:635–643. doi: 10.1007/s007020050085. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav. 1990;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Mock OB, Towns LC, Gerdes CF. The head twitch response in the least shrew (Cryptotis parva) is a 5-HT2- and not a 5-HT1C-mediated phenomenon. Pharmacol Biochem Behav. 1994;48:383–396. doi: 10.1016/0091-3057(94)90542-8. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Pandya DK. Involvement of other neurotransmitters in behaviors induced by the cannabinoid CB1 receptor antagonist SR 141716A in naive mice. J Neural Transm. 2000;107:931–945. doi: 10.1007/s007020070043. [DOI] [PubMed] [Google Scholar]
- Dickerson AK, Mills ZG, Hu DL. Wet mammals shake at tuned frequencies to dry. J Royal Soc Interface. 2012 doi: 10.1098/rsif.2012.0429. in press. http://dx.doi.org/10.1098/rsif.2012.0429. [DOI] [PMC free article] [PubMed]
- Dursun SM, Handley SL. Similarities in the pharmacology of spontaneous and DOI- induced head shakes suggest 5-HT2A receptors are active under physiological conditions. Psychopharmacology. 1996;128:198–205. doi: 10.1007/s002130050125. [DOI] [PubMed] [Google Scholar]
- Egashira N, Shirakawa A, Okuno R, Mishima K, Iwasaki K, Oishi R, Fujiwara M. Role of endocannabinoid and glutamatergic systems in DOI-induced head-twitch response in mice. Pharmacol Biochem Behav. 2011;99:52–58. doi: 10.1016/j.pbb.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology. 2005;181:496–503. doi: 10.1007/s00213-005-0009-4. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav. 2006;83:122–129. doi: 10.1016/j.pbb.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Reissig CJ, Katz EB, Yarosh HL, Rice KC, Winter JC. Hallucinogen-like effects of N,N-dipropyltryptamine (DPT): possible mediation by serotonin 5-HT1A and 5-HT2A receptors in rodents. Pharmacol Biochem Behav. 2008;88:358–365. doi: 10.1016/j.pbb.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, Woods JH. Interaction of 5-HT2A and 5-HT2C receptors in R(−)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J Pharmacol Exp Ther. 2010;335:728–734. doi: 10.1124/jpet.110.172247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone KC, Johnson JV, Bennett GW, Marsden CA. Involvement of 5-HT2 receptors in the behaviours produced by intrathecal administration of selected 5-HT agonists and the TRH analogue (CG 3509) to rats. Br J Pharmacol. 1989;96:599–608. doi: 10.1111/j.1476-5381.1989.tb11858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Stein AR, French HT, Murphy DL. Functional interactions between 5-HT2A and presynaptic 5-HT1A receptor-based responses in mice genetically deficient in the serotonin 5-HT transporter (SERT) Br J Pharmacol. 2010;159:879–887. doi: 10.1111/j.1476-5381.2009.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia EE, Smith RL, Sanders-Bush E. Role of Gq protein in behavioral effects of the hallucinogenic drug 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane. Neuropharmacology. 2007;52:1671–1677. doi: 10.1016/j.neuropharm.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber R, Barbaz BJ, Martin LL, Neale R, Williams M, Liebman JF. Antagonism of L-5-hydroxytryptophan-induced head-twitching in rats by lisuride: a mixed 5-hydroxytryptamine agonist-antagonist? Neurosci Lett. 1985;60:207–213. doi: 10.1016/0304-3940(85)90245-9. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology. 2000;23:569–576. doi: 10.1016/S0893-133X(00)00136-6. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5- HT2A receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23:8836–8843. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. LSD but not lisuride disrupts prepulse inhibition in rats by activating the 5-HT2A receptor. Psychopharmacology. 2010;208:179–189. doi: 10.1007/s00213-009-1718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61:364–381. doi: 10.1016/j.neuropharm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Koedood L, Powell SB, Geyer MA. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J Psychopharmacol. 2011;25:1548–1561. doi: 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley SL, Dursun SM. Serotonin and Tourette’s syndrome: movements such as head-shakes and wet-dog shakes may model human tics. Adv Biosci. 1992;85:235–253. [Google Scholar]
- Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, Jensen NH, Kuijer MB, Matos RC, Tran TB, Whaley R, Glennon RA, Hert J, Thomas KL, Edwards DD, Shoichet BK, Roth BL. Predicting new molecular targets for known drugs. Nature. 2009;462:175–181. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AB, Santini MA, Aznar S, Knudsen GM, Rios M. Changes in 5-HT2A-mediated behavior and 5-HT2A- and 5-HT1A receptor binding and expression in conditional brain-derived neurotrophic factor knock-out mice. Neuroscience. 2010;169:1007–1016. doi: 10.1016/j.neuroscience.2010.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kłodzinska A, Bijak M, Tokarski K, Pilc A. Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharmacol Biochem Behav. 2002;73:327–332. doi: 10.1016/s0091-3057(02)00845-6. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. Prefrontal cortical network activity: Opposite effects of psychedelic hallucinogens and D1/D5 dopamine receptor activation. Neuroscience. 2007;145:900–910. doi: 10.1016/j.neuroscience.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I, Minugh-Purvis N. Serotonin-induced head shaking behavior in rats does not involve receptors located in the frontal cortex. Brain Res. 1987;42:403–406. doi: 10.1016/0006-8993(87)91265-0. [DOI] [PubMed] [Google Scholar]
- Marek GJ. Behavioral evidence for mu-opioid and 5-HT2A receptor interactions. Eur J Pharmacol. 2003;474:77–83. doi: 10.1016/s0014-2999(03)01971-x. [DOI] [PubMed] [Google Scholar]
- Marek GJ. Activation of adenosine1 (A1) receptors suppresses head shakes induced by a serotonergic hallucinogen in rats. Neuropharmacology. 2009;56:1082–1087. doi: 10.1016/j.neuropharm.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason HC, Mason BT, Moos WS. Total-head (brain) X irradiation of mice and primary factors involved. Br J Radiology. 1955;28:495–507. doi: 10.1259/0007-1285-28-333-495. [DOI] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Albizu L, Sealfon SC, González-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett. 2011a;493:76–79. doi: 10.1016/j.neulet.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Kurita M, Holloway T, López J, Cadagan R, Martínez-Sobrido L, García-Sastre A, González-Maeso J. Maternal influenza viral infection causes schizophrenia-like alterations of 5-HT2A and mGlu2 receptors in the adult offspring. J Neurosci. 2011b;31:1863–1872. doi: 10.1523/JNEUROSCI.4230-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima T, Ishikawa K, Yamaguchi K, Furukawa H, Kameyama T. Phencyclidine-induced head-twitch responses as 5-HT2 receptor-mediated behavior in rats. Neurosci Lett. 1987;76:335–338. doi: 10.1016/0304-3940(87)90425-3. [DOI] [PubMed] [Google Scholar]
- Neumeyer JL, Kula NS, Bergman J, Baldessarini RJ. Receptor affinities of dopamine D1 receptor-selective novel phenylbenzazepines. Eur J Pharmacol. 2003;474:137–140. doi: 10.1016/s0014-2999(03)02008-9. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Contarino A. Gender- and morphine dose-linked expression of spontaneous somatic opiate withdrawal in mice. Behav Brain Res. 2006;170:110–118. doi: 10.1016/j.bbr.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Richmond FJ, Loeb GE. Electromyographic studies of neck muscles in the intact cat. II. Reflexes evoked by muscle nerve stimulation. Exp Brain Res. 1992;88:59–66. doi: 10.1007/BF02259128. [DOI] [PubMed] [Google Scholar]
- Schindler EA, Dave KD, Smolock EM, Aloyo VJ, Harvey JA. Serotonergic and dopaminergic distinctions in the behavioral pharmacology of (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and lysergic acid diethylamide (LSD) Pharmacol Biochem Behav. 2012;101:69–76. doi: 10.1016/j.pbb.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a β-arrestin2/Src/Akt signaling complex in vivo. J Neurosci. 2010;30:13513–13524. doi: 10.1523/JNEUROSCI.1665-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Raehal KM, Bohn LM. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci USA. 2008;105:1079–1084. doi: 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–112. [PubMed] [Google Scholar]
- Siegel RK, Lee MA, Jarvik ME. A device for analyzing drug-induced responses in freely moving mice. J Exp Anal Behav. 1972;18:415–418. doi: 10.1901/jeab.1972.18-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MT, Calil HM. Screening hallucinogenic drugs: systematic study of three behavioral tests. Psychopharmacologia. 1975;42:163–171. doi: 10.1007/BF00429548. [DOI] [PubMed] [Google Scholar]
- Starr BS, Starr MS. Grooming in the mouse is stimulated by the dopamine D1 agonist SKF 38393 and by low doses of the D1 antagonist SCH 23390, but is inhibited by dopamine D2 agonists, D2 antagonists and high doses of SCH 23390. Pharmacol Biochem Behav. 1986;24:837–839. doi: 10.1016/0091-3057(86)90421-1. [DOI] [PubMed] [Google Scholar]
- Tadano T, Hozumi M, Satoh N, Oka R, Hishinuma T, Mizugaki M, Arai Y, Yasuhara H, Kinemuchi H, Niijima F, Nakagawasai O, Tan-no K, Kisara K. Central serotonergic mechanisms on head twitch response induced by benzodiazepine receptor agonists. Pharmacology. 2001;62:157–162. doi: 10.1159/000056089. [DOI] [PubMed] [Google Scholar]
- Watson NV, Gorzalka BB. Concurrent wet dog shaking and inhibition of male rat copulation after ventromedial brainstem injection of the 5-HT2 agonist DOI. Neurosci Lett. 1992;141:25–29. doi: 10.1016/0304-3940(92)90326-3. [DOI] [PubMed] [Google Scholar]
- Wei E. Brain lesions attenuating “wet shake” behavior in morphine-abstinent rats. Life Sci. 1973;12:385–392. [Google Scholar]
- Weiner DM, Burstein ES, Nash N, Croston GE, Currier EA, Vanover KE, Harvey SC, Donohue E, Hansen HC, Andersson CM, Spalding TA, Gibson DF, Krebs-Thomson K, Powell SB, Geyer MA, Hacksell U, Brann MR. 5-hydroxytryptamine2A receptor inverse agonists as antipsychotics. J Pharmacol Exp Ther. 2001;299:268–276. [PubMed] [Google Scholar]
- Wentzel SE, Konow N, German RZ. Regional differences in hyoid muscle activity and length dynamics during mammalian head shaking. J Exp Zool A Ecol Genet Physiol. 2011;315:111–120. doi: 10.1002/jez.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- Yamamoto T, Ueki S. Behavioral effects of 2,5-dimethoxy-4-methylamphetamine (DOM) in rats and mice. Eur J Pharmacol. 1975;32:156–162. doi: 10.1016/0014-2999(75)90278-2. [DOI] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology. 2010;208:443–454. doi: 10.1007/s00213-009-1744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Marek GJ. AMPA receptor involvement in 5-hydroxytryptamine2A receptor-mediated pre-frontal cortical excitatory synaptic currents and DOI-induced head shakes. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:62–71. doi: 10.1016/j.pnpbp.2007.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Magnetometer coil responses induced by grooming behavior. (A) Voltage response of the magnetometer coil during grooming. (B) Periodogram showing the spectral density of the magnetometer response to grooming.