Abstract
Species-specific Quantitative Real Time PCR (qPCR) alone and combined with the use of propidium monoazide (PMA) were used along with the plate count method to evaluate the survival of the probiotic strains Lactobacillus acidophilus La-5 and Bifidobacterium animalis subsp. lactis Bb-12, and the bacteriocinogenic and potentially probiotic strain Lactobacillus sakei subsp. sakei 2a in synbiotic (F1) and probiotic (F2) petit-suisse cheeses exposed throughout shelf-life to in vitro simulated gastrointestinal tract conditions. The three strains studied showed a reduction in their viability after the 6 h assay. Bb-12 displayed the highest survival capacity, above 72.6 and 74.6% of the initial populations, respectively, by plate count and PMA-qPCR, maintaining population levels in the range or above 6 log CFU/g. The prebiotic mix of inulin and FOS did not offer any additional protection for the strains against the simulated gastrointestinal environment. The microorganisms' populations were comparable among the three methods at the initial time of the assay, confirming the presence of mainly viable and culturable cells. However, with the intensification of the stress induced throughout the various stages of the in vitro test, the differences among the methods increased. The qPCR was not a reliable enumeration method for the quantification of intact bacterial populations, mixed with large numbers of injured and dead bacteria, as confirmed by the scanning electron microscopy results. Furthermore, bacteria plate counts were much lower (P<0.05) than with the PMA-qPCR method, suggesting the accumulation of stressed or dead microorganisms unable to form colonies. The use of PMA overcame the qPCR inability to differentiate between dead and alive cells. The combination of PMA and species-specific qPCR in this study allowed a quick and unequivocal way of enumeration of viable closely related species incorporated into probiotic and synbiotic petit-suisse cheeses and under stress conditions.
Introduction
Lactic acid bacteria (LAB) comprise a group of several genera of microorganisms, which display lactic acid as their major end product of fermentation [1]. LAB are indigenous members of the intestinal microbiota of animals and are also associated with plants, and fermented food, including meat and dairy products [2]. These microorganisms produce different antimicrobial peptides, known as bacteriocins, which are useful to improve the safety and biopreservation of foods [3].
Probiotic microorganisms are defined as ‘live microorganisms, which when administered in adequate amounts confer health benefits to the host’ [4]. The consumption of probiotics are related to diverse human health benefits, including the homeostasis of intestinal microbiota, anticarcinogenesis, hypocholesterolemic effect, reduction in the risk of diarrhea caused by bacteria and virus, alleviation of lactose malabsorption, and allergy [5], [6]. Species of Lactobacillus and Bifidobacterium are most commonly used as probiotics. Among them, Bifidobacterium animalis subsp. lactis Bb-12 and Lactobacillus acidophilus La-5 strains have been widely studied with several health benefits associated to their consumption [7], [8]. Bacteriocin-producing Lactobacillus species might play a dual role by acting as agents for food preservation and safety as well as presenting a potential to confer health benefits [9]. The Lactobacillus sakei subsp. sakei 2a strain, isolated from Brazilian sausage, was found to produce a bacteriocin which is active against Listeria monocytogenes and Staphylococcus aureus [10]. On the other hand, prebiotics are fermentable ingredients that allow specific changes in the composition and/or activity of the gastrointestinal microbiota leading to benefits in host health and well-being [11].
Functional foods, including pro- and prebiotics, beyond basic nutritional functions, show physiological benefits and can help to maintain the gut health [12]. Dairy matrices are traditionally used as vehicles for probiotics delivery. Among them, cheeses are of special importance, since, in comparison to yogurts and fermented milks, they present higher pH, less acidity, higher buffering capacity, higher lipid content and nutrients availability, and lower oxygen levels, resulting in a higher degree of protection to the microorganisms during their passage throughout the harsh conditions found in the gastrointestinal tract (GIT) [13]. Due to these characteristics, the successful use of cheeses supplemented with prebiotics to deliver probiotic cultures has been reported [14], [15].
The probiotic potential of a microorganism might be assessed using in vitro models that mimic the physical–chemical conditions found in the human GIT tract, including survival at low pH (stomach environment) and the presence of bile salts and digestive enzymes (simulating the duodenum characteristics) [16]. Interestingly, according to van Bokhorst-van de Veen et al. [17], there is a considerable variation in the GIT survival when different strains of a same microorganism are evaluated.
Although there is not a standardized amount of live microorganisms that must reach the colon, it is believed that this number correlates with the health benefits intended [18] and, therefore, it is important to quantify their survival under the different steps of the gastric and enteric conditions. For the detection and quantification of microbial populations, plating on selective media has been traditionally used. However, culture-dependent techniques have been questioned due to their inability to differentiate viable cells from non-culturable ones. The depletion of nutrients, low pH, and the enzymatic activity are conditions able to promote injury and may alter the cell metabolic activity, limiting the ability of the microorganisms to form colonies, and this fact may impair the identification and underestimate their quantification, mainly in survival assays [19].
Molecular methods have now overcome, at a great extent, the problem of lack of sensibility and specificity of conventional culture-dependent techniques, and the Quantitative Real-Time PCR (qPCR) is, currently, the most used method for direct quantification of microorganisms in complex samples [20]. However, its main problem is the inability to differentiate live and dead microorganisms cells, since DNA can be amplified even if the cells are dead [21]. In order to solve this pitfall, the use of DNA intercalating agents that inhibit its amplification has been studied, among which are the ethidium monoazide (EMA) and propidium monoazide (PMA) [22]. The main criterion for distinguishing between viable and irreversibly damaged cells is the membrane activity, and since these intercalating agents can only penetrate cells with damaged membranes [21], [23], their use in combination with qPCR method allows a quantitative and differential detection of viable versus non-viable microorganisms in a given microbial community. Furthermore, PMA is activated upon light exposure and was shown to exert no toxic effect on viable cells [24].
The aim of this study was to evaluate the survival of the probiotic strains L. acidophilus La-5 and B. animalis subsp. lactis Bb-12 and of the bacteriocinogenic and potentially probiotic strain L. sakei subsp. sakei 2a in synbiotic and probiotic petit-suisse cheeses throughout their shelf-lives challenged by an in vitro assay simulating gastrointestinal conditions. The suitability of three different enumeration methods for a reliable determination of the bacterial population under the stressful conditions tested was also evaluated, including plate count, species-specific qPCR alone and combined with the use of the PMA, for which the results obtained were compared and fully discussed.
Materials and Methods
Bacterial Strains and Culture Conditions
Commercial DVS culture, ABT-4, containing the probiotic strains L. acidophilus La-5 and B. animalis subsp. lactis Bb-12 and the starter culture Streptococcus thermophilus was obtained from Christian Hansen laboratories (Hoersholm, Denmark). ABT-4 culture was added directly to commercial pasteurized skimmed milk (Salute, Descalvado, Brazil) at 1% (v/v) in order to achieve ca. 8.0 log CFU/g of each strain in the final product. The strain L. sakei subsp. sakei 2a, belonging to the collection of the Laboratory of Food Microbiology (Faculty of Pharmaceutical Sciences -University of São Paulo; FCF-USP), was cultured overnight at 30°C in de Man, Rogosa and Sharpe (MRS) (Oxoid, Basingstoke, UK) broth and harvested by centrifugation at 9000 g for 5 min at 4°C. L. sakei 2a was added to the product in order to reach a final concentration of ca. 8.0 log CFU/g.
Probiotic Petit-Suisse Cheeses Production
Two pilot-scale petit-suisse cheeses formulations, denoted F1 (synbiotic) and F2 (probiotic), were manufactured. Two batches of both F1 and F2 were produced, independently, at different days, as described by Cardarelli et al. [25]. Briefly, cultures were added to pre-heated (37°C) commercial pasteurized skimmed milk for the manufacture of the cheese-base (quark cheese); next, it was incubated until pH 6.3–6.5 (approximately 1 h) before the addition of rennet Ha-la (Christian Hansen, Valinhos, Brazil, 0.03 g/L). After curd formation, it was cut and drained in sterilized cotton cheesecloth. The quark cheese was allowed to drain at 15°C for 15 h before homogenization and mixture of the remaining ingredients (Table 1). Portions of 50 g of the petit-suisse cheeses were packaged in polypropylene plastic pots for food products (68 mm diameter, 32 mm high, 55 mL total volume, Tries Aditivos Plásticos, São Paulo, Brazil), sealed with an aluminum cover, stored at 4±1°C, and sampled on days 1, 14, and 28.
Table 1. Ingredients and respective proportions used in the preparation of the petit-suisse cheeses.
| Ingredient | Proportion added (g/100 g) | |
| Synbiotic cheese (F1) | Probiotic cheese (F2) | |
| Quark cheese | 57.50 | 61.970 |
| Sterilized milk cream (25% fat, Nestlé, Araçatuba, Brazil) | 12.00 | 13.720 |
| Pasteurized strawberry whole pulp (Ice-Fruit-Maisa, Mossoró, Brazil) | 10.00 | 11.500 |
| Sucrose (União, Limeira, Brazil) | 9.00 | 11.000 |
| Guar gum (Grindsted® Guar 250 Danisco, Cotia, Brazil) | 0.20 | 0.300 |
| Xanthan gum (Grindsted® Xanthan 80, Danisco) | 0.15 | 0.187 |
| Carrageenan gum (Grindsted® Carrageenan CY 500, Danisco) | 0.15 | 0.187 |
| Natural coloring and flavor agent (7134, Germinal, Cabreúva, Brazil) | 1.00 | 1.120 |
| Inulin (Beneo GR, Orafti, Oreye, Belgium) | 7.50 | - |
| FOS (Fructooligosacchrides, Beneo™ P95, Orafti) | 2.50 | - |
Probiotic and Synbiotic Petit-Suisse Cheeses and Gastrointestinal Tract Transit Simulation
The in vitro assay simulating the gastrointestinal conditions (gastric, enteric I, and enteric II phases) was carried out according the protocol of Liserre et al. [26] modified by Buriti et al. [27]. Some adjustments were made and are described as follows. Firstly, at each sampling day, 25 g of three different samples belonging to each batch from the same formulation (F1 and F2, n = 6) of petit-suisse cheeses were homogenized with 225 mL of sterilized sodium chloride (NaCl) solution (0.9%, w/v), using a BagMixer® 400P (Interscience, St. Nom, France) during 3 min. Aliquots of all samples were transferred in triplicates to three sterilized flasks (9 flasks in total) and incubated in a water bath at 37°C with orbital agitation of 150 rpm (Metabolic Water Bath Dubnoff MA-095, Marconi, Piracicaba, Brazil). The whole process lasted a total of six hours during which the pH of the samples was adjusted and various gastrointestinal enzymes were added according to the gastrointestinal phase simulated (Table 2). Aliquots were collected at time-points zero and after 2 h (gastric phase), 4 h (enteric phase I), and 6 h (enteric phase II) for analysis.
Table 2. Description of phases employed in the in vitro simulation of gastrointestinal transit.
| Phase | Description |
| 1. Gastric | pH adjustment to pH 2.3–2.6 with 1N HCl (Merck). |
| Addition of pepsin1 and lipase1 to homogenate, 2 h incubation | |
| 2. Enteric I | pH adjustment to pH 5.4-5.7 with alkaline solution2 |
| Addition of pancreatin1 and bile1 to homogenate, 2 h incubation | |
| 3. Enteric II | pH adjustment to pH 6.8-7.2 with alkaline solution2 |
| Addition of pancreatin and bile to homogenate, 2 h incubation |
Footnote:
Enzymes: 3.0 g/L pepsine; 0.9 mg/L lipase; 10 g/L bile; 1.0 g/L pancreatin (all from Sigma – Aldrich, St. Louis, USA).
Alkaline solution: 150 mL of 1N NaOH (Synth, Diadema, Brazil) and 14 g of PO4H2Na. 2H2O (Synth) (distilled water up to 1L).
To better assess the survival rate (R) of the strains under stress, the percentage of bacterial recovery was calculated as follows:
where N is the log of CFU of bacteria assessed after a particular in vitro phase and N0 is the initial log of CFU of bacteria determined at the same phase.
Assay Samples Analysis
Enumeration of Bacteria by the Plate Count Method
Standard pour-plate method was used for viable microbial enumeration. The culture media included: MRS agar supplemented with both sodium propionate (3.0 g/L) (Sigma-Aldrich, St. Louis, MO, USA) and lithium chloride (2.0 g/L) (Merck, Darmstadt, Germany) (MRS-LP) for quantification of B. animalis Bb-12 as described by Vinderola and Reinheimer [28]; MRS agar modified by the replacement of glucose by maltose (Difco, Le Pont de Claix, France) according to the International Dairy Federation [29] for the enumeration of La-5 and MRS agar (Oxoid) for quantification of L. sakei 2a. Bb-12 was incubated under anaerobic conditions using the Anaerobic System Anaerogen (Oxoid) at 37°C for 72 h, whereas La-5 and L. sakei 2a were incubated aerobically at 37°C and 30°C, respectively, for 48 h.
Evaluation of Bacterial Survival by Quantitative PCR
In order to determine the survival of the studied microorganisms throughout the in vitro simulated gastrointestinal tract conditions, total DNA isolation from the in vitro samples proceeded as follows. Aliquots of 3 mL, obtained as described above, were homogenized with 27 mL of trisodium citrate dehydrate solution (2%, w/v) and incubated at 45°C for 30 min. The suspensions obtained were centrifuged at 9000 g for 10 min at 4°C. The supernatant was discarded and the pellet washed, resuspended in 500 µL of Tris EDTA (10 mM Tris-HCl, 1 mM EDTA, [pH 8]) buffer and transferred to a 2 mL screw-cap tube containing 0.3 g of 0.1 mm zirconia beads (Biospec, Bartlesville, OK, USA) plus 150 µL of buffer-saturated phenol (Invitrogen, Carlsbad, CA, USA). The samples were lyzed using a FastPrep®-24 bead-beater (MP Biomedical, Solon, OH, USA) and the initial procedure was repeated three consecutive times at a speed of 5.0 m/s for 30 s, interspaced by breaks of 1 min, during which samples were kept on ice. Total DNA was isolated by successive phenol-chloroform∶isoamyl alcohol extractions, until a clear interface was obtained, and used for purification through ethanol precipitation. DNA was collected by centrifugation and resuspended in 50 µL of TE buffer. DNA quality and concentrations were determined using a Nanodrop ND-1000 (Thermo Scientific, Waltham, USA).
The PMA (phenanthridium, 3-amino-8-azido-5-[3-(diethylmethylammonio)propyl]-6- phenyl dichloride) treatment was carried out as described by Nocker et al. [21], with slight modifications. The samples were prepared as described in item 2.3.2.1 up to the step in which the pellet was resuspended in 500 µL of buffer TE (pH 8.0). Following, the suspension was transferred to 1.5 mL light-transparent microcentrifuge tubes containing 1.25 µL of a 20 mM stock solution of PMA (Biotium, Inc., Hayward, CA, USA) (previously prepared in 20% dimethyl sulfoxide) to reach a final concentration of 50 µM and finally mixed. After a dark incubation during 5 min, the samples were light-exposed for 15 min by use of a 650-W halogen light device (DWE, 650 W, 120 V, GE Lighting, East Cleveland, Ohio, USA). The tubes containing the samples were laid horizontally on ice, to avoid excessive heating, and placed about 20 cm away from the light source. After photo-induced cross-linking, cells were pelleted at 10000 g, for 10 min at 4°C. The supernatant obtained was discarded and the pellet washed with PBS buffer in order to remove the inactivated PMA. The cells were collected by centrifugation and resuspended in 500 µL of buffer TE (pH 8.0) prior to DNA isolation procedure, as described above.
For viable microbial quantification, all quantitative PCR (qPCR) reactions were performed by using the ABI-PRISM 7500 sequencing detection system (Applied Biosystems, Bridgewater, NJ, USA). The final volume for all reactions was 25 µL, among them, 20 µL consisted of the PCR Master Mix and 5 µl of DNA extracted with and without use of PMA treatment.
For the La-5 assay, 1× Power SYBR Green PCR Master Mix (Applied Biosystems) and 400 nM of each primer was used. The primers and the amplification program used were the same ones previously described by Tabasco et al. [30]. The melting curve analysis was performed after amplification reaction to distinguish the target from the non-targeted PCR products. In order to quantify Bb-12 populations, the PCR reaction mix contained 1× TaqMan Master Mix (Applied Biosystems), 300 nM of BAL-23S-F forward and BAL-23S-R reverse primers, and 250 nM of BAL-23S-P probe, as described by Taipale et al. [31]. Finally, for the enumeration of L. sakei 2a, the PCR reaction mix consisted of 1× TaqMan Master Mix (Applied Biosystems), 900 nM of each SakF forward and SakR reverse primers, and 200 nM of SakS minor groove binder (MGB) probe [32]. The amplification profile program used for enumeration of Bb-12 and L. sakei was: 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 30 s.
For the determination of the sensitivity and the amplification efficiency of the qPCR reactions, a standard curve was constructed for each microorganism. The standard curves for L. sakei 2a and Bb-12 consisted of purified genomic DNA isolated from pure cultures serially diluted with sterilized nuclease-free water for obtaining a range of DNA concentrations equivalent to approximately 5×107 to 5 genome copies of each microorganism per amplification reaction mixture. The number of Bb-12 and L. sakei 2a genome equivalents was estimated by considering the previously known genome size of Bb-12 [33] and L. sakei 23K [34] as reference microorganisms. Consequently, the equivalent of one genome of Bb-12 and L. sakei 2a weighs, approximately 2.13 and 2 fg, respectively. The quantification of La-5 was done against a 10-fold dilution series of 16S rRNA gene fragment equivalent to 5×107 to 5 copy number of the gene. The approximately 1500-bp 16S rRNA gene fragment was obtained by PCR amplification of genomic DNA from pure culture of La-5 using universal primers 27F and 1492R and conditions described by Felske and Weller [35]. The amplified fragment was checked by using agarose gel electrophoresis and purified with the QIAquik PCR Purification kit (QIAGEN, Hilden, Germany), according to the manufacturer's instructions. Considering the genome of L. acidophilus NCFM as reference strain, the copy number of 16S rRNA gene in La-5 genome was estimated at 4 [36].
The coefficients of efficiency of the PCR amplifications, calculated as 10(−1/slope) -1, were: 95.6%; 100%, and 99%, respectively, for Bb-12, L. sakei 2a, and La-5. The correlation coefficients (R2) of the standard curves obtained were between 0.995 and 0.998 for the initial copy numbers of standards within the range covered per reaction. Non-template controls (NTC) samples were included in all PCR runs and tested negative. The experiment was repeated two independent times, with triplicates within each assay.
Scanning Electron Microscopy (SEM)
Samples obtained as described above were centrifuged at 5000 g for 10 min, and the supernatant was discarded. The cell pellets were resuspended in NaCl solution (0.9%, w/v) at a final concentration of ca. 5 log CFU/ mL. Aliquots of 1 mL of the cell suspensions were placed onto 0.2 µm-pore size membrane filter (Isopore membrane filters, Millipore, Billerica, MA, USA) and fixed for 2 h in 2% (w/v) glutaraldehyde solution. Subsequently, the membranes were washed three times with Milli-Q water (Millipore), dehydrated sequentially with 25, 50, 75, 90, and 95% ethanol solutions and finally with 100% ethanol (three times), and critical- point dried by the CO2 method. The dried membranes were placed on aluminum stubs, sputter coated with gold and analyzed by using a field emission scanning electron microscope (JEOL JSM-7401F; JEOL, Tokyo, Japan) at 2.5 kV at the facilities of the Chemistry Institute (IQ), at the University of São Paulo, Brazil.
Statistical Analysis
The CT values obtained by the qPCR method and used to quantify the viable cells (CFU/g) were automatically generated by the ABI-PRISM 7500 software. One-way analysis of variance (ANOVA) with post-hoc Tukey test was used to compare the results obtained by qPCR with and without the use of PMA treatment, and by the plate counts from the three phases of the in vitro assay, at every sampled day. The XLSTAT 2013 (Addinsoft, SARL, New York, USA) and MINITAB 14 (Minitab Inc., State College, USA) software were used to determine the statistical significance of the results obtained.
Results
Quantification of Viable Microorganisms in the Petit-Suisse Cheeses Stored at 4±1°C, Throughout 28 Days Under Digestive Stress Conditions
The quantification of viable cells was evaluated before and during the different stages of the simulated digestive stress and throughout the storage time evaluated (28 days) of the petit-suisse cheeses using three different methodologies (culture, qPCR, and PMA-qPCR). In this study, the specificity of the three sets of primers used in the molecular methods were checked and no cross reactions were observed in the petit-suisse cheese formulations, with any of the non-target microorganism tested. On the contrary, four different selective media were employed and up to 72 h were required for enumeration of microorganisms. Bb-12 grew slowly and formed small colonies in MRS-LP agar, whereas the modified MRS agar, employed for La-5 enumeration, allowed the growth of few lenticular colonies of L. sakei 2a, different from the typical star shape colonies of La-5.
As can be seen in Tables 3 and 4, the viability results of the microbial species in the cheeses were influenced by the method employed and the values obtained were statistically different (P<0.05). Furthermore, higher numbers of viable cells were always detected by the qPCR method, in comparison with the values obtained using the plate counts and the PMA-qPCR techniques, regardless the strain or the in vitro phase analyzed. The strains in the cheese matrixes showed different patterns of survival during each phase of simulated digestive stress. Although the sensitivity to low pH and digestive enzymes was observed to be strain-specific, the bacterial survival ratios obtained by plate counting and PMA-qPCR methods were lower than those obtained by qPCR (P<0.05) (Fig. 1). The survival rates obtained for La-5, Bb-12, and L. sakei 2a were always above 90%, when the qPCR method was used, showing its inability to distinguish between live and dead cells, particularly with higher background of dead bacteria, as observed at the end of the 6th h assay. Since the accurate enumeration of viable cells was the main objective of this study, the qPCR method was withdrawn from the subsequent discussion performed in here.
Table 3. Populations of B. animalis Bb-12, L. acidophilus La-5, and L. sakei 2a assessed in the synbiotic petit-suisse cheese (F1) throughout storage (1, 14, and 28 days) at 4°C and during the in vitro assay simulating the gastrointestinal conditions [time-point zero, and after 2 h (gastric phase), 4 h (enteric phase I), and 6 h (enteric phase II)].
| Method of analysis | |||||
| Species | Storage period (days) | In vitro phase | Plate Count | qPCR | PMA-qPCR |
| B. animalis Bb-12 | 1 | 0 | 8.70±0.06C a | 9.84±0.02A | 9.10±0.01B a |
| gastric | 8.03±0.09C a | 9.29±0.03A | 8.31±0.04B a | ||
| enteric I | 6.95±0.16C a | 9.31±0.01A | 7.83±0.03B a | ||
| enteric II | 6.89±0.13C a | 8.60±0.02A | 7.95±0.06B a | ||
| 14 | 0 | 8.39±0.06C b | 9.81±0.02A | 9.03±0.05B b | |
| gastric | 7.48±0.13C b | 9.07±0.02A | 7.74±0.02B b | ||
| enteric I | 5.81±0.16C b | 9.44±0.01A | 6.79±0.12B b | ||
| enteric II | 6.14±0.09C b | 9.30±0.02A | 6.81±0.04B b | ||
| 28 | 0 | 8.27±0.05C b | 9.99±0.02A | 9.16±0.03B a | |
| gastric | 6.94±0.22B c | 9.11±0.01A | 7.19±0.08B c | ||
| enteric I | 5.05±0.41C c | 9.63±0.02A | 6.68±0.02B b | ||
| enteric II | 5.52±0.32C c | 9.59±0.02A | 6.76±0.01B b | ||
| L. acidophilus La-5 | 1 | 0 | 8.40±0.07B a | 8.75±0.04A | 7.97±0.01C a |
| gastric | 5.47±0.19B a | 7.16±0.02A | 5.08±0.01C a | ||
| enteric I | 3.96±0.66C a | 7.84±0.01A | 4.99±0.04B a | ||
| enteric II | 4.11±0.28C a | 7.00±0.01A | 5.06±0.02B a | ||
| 14 | 0 | 8.15±0.04B b | 8.54±0.04A | 7.81±0.01C b | |
| gastric | 3.44±0.30C b | 7.55±0.03A | 4.96±0.05B ab | ||
| enteric I | 3.00±0.35C b | 8.06±0.01A | 4.93±0.03B a | ||
| enteric II | 3.67±0.38C b | 7.98±0.03A | 4.94±0.02B a | ||
| 28 | 0 | 8.09±0.05B b | 8.75±0.03A | 7.79±0.04C b | |
| gastric | 3.98±0.39C b | 7.68±0.01A | 4.82±0.07B b | ||
| enteric I | 2.91±0.29C b | 8.24±0.01A | 4.93±0.02B a | ||
| enteric II | 2.98±0.28C c | 8.18±0.02A | 4.89±0.01B a | ||
| L. sakei 2a | 1 | 0 | 7.78±0.07C a | 9.32±0.03A | 8.00±0.03B a |
| gastric | 1.25±0.19C c | 8.05±0.03A | 6.01±0.02B a | ||
| enteric I | 1.97±0.32C a | 8.39±0.02A | 6.34±0.05B a | ||
| enteric II | 2.96±0.46C a | 7.52±0.08A | 6.01±0.03B a | ||
| 14 | 0 | 7.69±0.12C a | 8.69±0.03A | 7.97±0.03B a | |
| gastric | 2.25±0.29C a | 8.64±0.06A | 5.93±0.10B a | ||
| enteric I | 2.05±0.11C a | 8.97±0.01A | 6.35±0.09B a | ||
| enteric II | 3.30±0.46C a | 8.50±0.11A | 5.99±0.03B a | ||
| 28 | 0 | 7.47±0.10C b | 9.00±0.04A | 7.97±0.02B a | |
| gastric | 1.70±0.66C b | 8.49±0.02A | 5.48±0.06B b | ||
| enteric I | 2.19±0.18C a | 8.84±0.01A | 6.21±0.10B a | ||
| enteric II | 2.63±0.42C a | 8.73±0.02A | 5.86±0.12B a | ||
Populations of B. animalis Bb-12, L. acidophilus La-5, and L. sakei 2a assessed in the synbiotic petit-suisse cheese (F1) throughout storage (1, 14, and 28 days) at 4°C and during the in vitro assay simulating the gastrointestinal conditions [time-point zero, and after 2 h (gastric phase), 4 h (enteric phase I), and 6 h (enteric phase II)]. The samples were analyzed using three methods: plate count, Real-time PCR (qPCR) and Real-time PCR combined with propidium monoazide (PMA-qPCR).
Footnote:
Values are expressed as mean log CFU/g ± standard deviation (SD) obtained by plate count method and as log CFU equivalents/g as calculated from Ct values for qPCR and PMA-qPCR;
Different superscript capital letters in a row denote significant differences between methods (P<0.05).
Different superscript lowercase letters in the same column for each phase denote significant differences between storage days (P<0.05).
Table 4. Populations of B. animalis Bb-12, L. acidophilus La-5, and L. sakei 2a assessed in the probiotic petit-suisse cheese (F2) throughout storage (1, 14, and 28 days) at 4°C and during the in vitro assay simulating the gastrointestinal conditions [time-point zero, and after 2 h (gastric phase), 4 h (enteric phase I), and 6 h (enteric phase II)].
| Method of analysis | |||||
| Species | Storage period (days) | In vitro phase | Plate Count | qPCR | PMA-qPCR |
| B. animalis Bb-12 | 1 | 0 | 8.82±0.08C a | 9.94±0.02A | 9.00±0.05B a |
| gastric | 8.69±0.07B a | 9.28±0.03A | 7.55±0.05C b | ||
| enteric I | 8.09±0.06B a | 9.44±0.01A | 6.79±0.05B a | ||
| enteric II | 7.87±0.05B a | 9.62±0.03A | 6.80±0.04B a | ||
| 14 | 0 | 8.83±0.06C a | 9.95±0.04A | 8.98±0.03B a | |
| gastric | 8.35±0.14B a | 9.25±0.02A | 7.92±0.05C a | ||
| enteric I | 7.63±0.13B b | 9.14±0.02A | 6.72±0.08B a | ||
| enteric II | 7.46±0.08B b | 9.41±0.03A | 6.67±0.05B b | ||
| 28 | 0 | 8.80±0.05C a | 9.86±0.02A | 8.97±0.05B a | |
| gastric | 7.99±0.08B b | 9.06±0.02A | 7.39±0.10C b | ||
| enteric I | 6.17±0.31C c | 8.97±0.03A | 6.68±0.05B a | ||
| enteric II | 6.31±0.10C c | 9.01±0.04A | 6.64±0.02B b | ||
| L. acidophilus La-5 | 1 | 0 | 8.52±0.05B a | 8.86±0.01A | 7.86±0.03C a |
| gastric | 5.66±0.22B a | 7.86±0.02A | 4.39±0.02C a | ||
| enteric I | 3.71±0.10C a | 8.26±0.04A | 4.90±0.03B a | ||
| enteric II | 3.59±0.28C a | 8.30±0.01A | 4.84±0.02B a | ||
| 14 | 0 | 8.60±0.07A a | 8.63±0.08A | 7.70±0.04B b | |
| gastric | 5.14±0.16B b | 7.66±0.02A | 4.51±0.05C a | ||
| enteric I | 3.41±0.10C b | 7.77±0.03A | 4.65±0.03B b | ||
| enteric II | 3.66±0.20C a | 8.37±0.03A | 4.62±0.04B b | ||
| 28 | 0 | 8.26±0.04B b | 8.75±0.01A | 7.65±0.04C b | |
| gastric | 3.91±0.32B c | 8.00±0.01A | 4.49±0.05B a | ||
| enteric I | 3.27±0.43C b | 8.06±0.01A | 4.42±0.05B c | ||
| enteric II | 3.19±0.34C b | 8.03±0.08A | 4.41±0.04B c | ||
| L. sakei 2a | 1 | 0 | 8.09±0.02C a | 9.13±0.04A | 8.24±0.02B c |
| gastric | 3.00±0.23C c | 8.38±0.03A | 6.17±0.01B a | ||
| enteric I | 2.58±0.13C b | 8.66±0.02A | 6.26±0.09B a | ||
| enteric II | 2.36±0.14C b | 8.74±0.03A | 6.34±0.05B a | ||
| 14 | 0 | 8.27±0.03C a | 9.26±0.03A | 8.44±0.01B a | |
| gastric | 4.82±0.32C a | 8.55±0.04A | 6.23±0.02B a | ||
| enteric I | 3.36±0.31C a | 8.68±0.01A | 6.35±0.06B a | ||
| enteric II | 3.65±0.22C a | 8.71±0.02A | 6.38±0.02B a | ||
| 28 | 0 | 8.11±0.04C a | 9.15±0.01A | 8.36±0.03B b | |
| gastric | 4.01±0.32C b | 8.42±0.05A | 6.20±0.03B a | ||
| enteric I | 2.91±0.33C a | 8.41±0.01A | 6.30±0.02B a | ||
| enteric II | 3.11±0.33C a | 8.42±0.05A | 6.31±0.04B a | ||
Populations of B. animalis Bb-12, L. acidophilus La-5, and L. sakei 2a assessed in the probiotic petit-suisse cheese (F2) throughout storage (1, 14, and 28 days) at 4°C and during the in vitro assay simulating the gastrointestinal conditions [time-point zero, and after 2 h (gastric phase), 4 h (enteric phase I), and 6 h (enteric phase II)]. The samples were analyzed using three methods: plate counts, Real-time PCR (qPCR) and Real-time PCR combined with propidium monoazide (PMA-qPCR).
Footnote:
Values are expressed as mean log CFU/g ± standard deviation (SD) obtained by plate count method and as log CFU equivalents/g as calculated from Ct values for qPCR and PMA-qPCR;
Different superscript capital letters in a row denote significant differences between methods (P<0.05).
Different superscript lowercase letters in the same column for each phase denote significant differences between storage days (P<0.05).
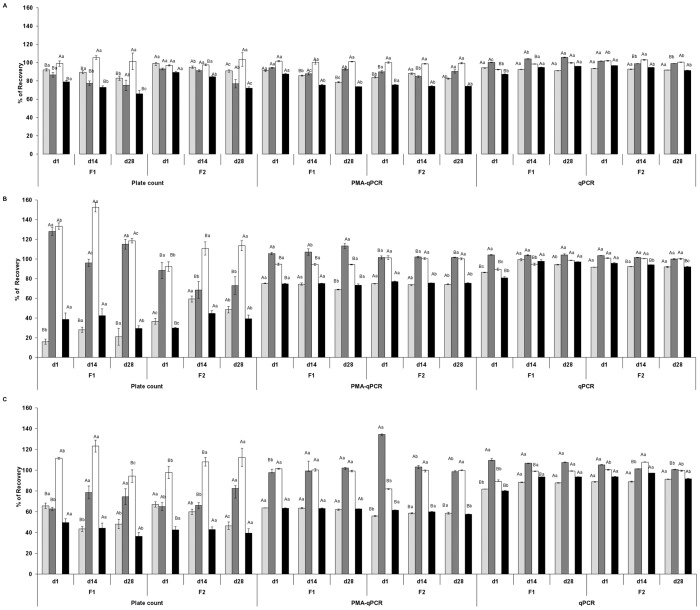
Figure 1. Bacterial recovery rates throughout the in vitro assay simulating the gastrointestinal tract conditions.
Recovery (%) expressed as the ratio of live cells of B. animalis Bb-12 (A), L. acidophilus La-5 (B), and L. sakei 2a (C) before and after every step throughout the in vitro assay simulating the gastrointestinal tract conditions, for each storage time and both formulations (F1 - synbiotic; F2 - prebiotic). (light gray columns) after 2 h of gastric phase (pH 2.3–2.6 in the presence of pepsin and lipase); (dark gray columns) after 2 h of enteric phase I (pH 5.4–5.7 in the presence of pancreatin and bile); (white columns) after 2 h of enteric phase II (pH 6.8–7.2 in the presence of pancreatin and bile) and (black columns) total recovery after 6 hours relative to the initial inocula of cheeses. Footnote: A,B,C Different superscript capital letters denote significant differences between formulations for the same storage period and assay phase (P<0.05). a,b,c Different superscript lowercase letters denote significant differences between storage days for the same phase (P<0.05).
The three strains studied lost their viability after going through the simulated gastric digestion. However, Bb-12 exhibited a significant better survival capacity than the two Lactobacillus strains tested. Bb-12 viable cell loss ranged from less than 0.8 log units in both formulations (day 1) to 1.33 and 1.97 log units, respectively, for F1 and F2 (day 28). In F1, the results of Bb-12 enumeration obtained by plate count were 0.3 log unit lower than those obtained by PMA-qPCR, whereas they were 0.8 log units higher in F2 (P<0.05). The decline in La-5 viability was more noticeable, dropping about 2.9 log units for both formulations, as observed on day 1. La-5 viability loss in cheeses F1 and F2 were, respectively, 4.7 and 3.5 log units as shown on day 14 and of 4.1 and 4.4 log units after 28 days of storage, considering the plate count method. On the other hand, with bacterial counts significantly higher, using PMA-qPCR (P<0.05), the mean La-5 viability loss was of 3.9 and 3.3 log units for F1 and F2, respectively. The major discrepancy between both methods was observed for L. sakei 2a quantification. According to the plate count method, L. sakei 2a showed a very poor survival capacity, as shown by its low recovery rates after 2 h under gastric conditions, observed for F1 - 16.0%, 28.0%, and 21.0% and F2 - 36.7%, 59.3%, and 48.5%, respectively on days 1, 14, and 28 (Fig. 1). In contrast, the survival ratio was superior to 74% in all time-points evaluated during the food products storage [except for F1 on day 28 (68.8%)] by the use of PMA-qPCR method, suggesting a survival rate even better than the one assessed for La-5.
During the simulated enteric phases (I and II), the three strains were more tolerant to the enteric conditions than to the previous gastric phase (P<0.05) (data not shown). At the end of the enteric phase I, Bb-12 counts had decreased 1 log unit on day 1, reaching a ca. 2 log units reduction on the 28th day of storage, as verified using the plate count method. Using PMA-qPCR technique, Bb-12 populations showed a maximum average reduction of 1 log unit throughout the product shelf-life. On the other hand, at the end of enteric phase II, Bb-12 viability did not change significantly (P>0.05) (Tables 3 and 4). The total percentage recovery of Bb-12, relative to the initial levels inoculated into the cheeses, was affected by the storage time, showing a decreasing trend (P<0.05) for both formulations regardless the method employed for counting (Figure 1). Considering the PMA-qPCR method, no significant differences on the total percentage recovery for Bb-12 between F1 and F2 were observed, for the three storage periods evaluated, except for day 1, in which recovery was higher in the synbiotic cheese (F1) (P<0.05). However, by use of the classic method, F1 exhibited significant lower percentage recovery rates (P<0.05).
L. sakei 2a showed a higher capacity to resist against the presence of bile and pancreatic juices, when compared to Bb-12, considering the recovery rates obtained (Fig. 1). Similarly to that observed in the gastric phase, higher counts (P<0.05) were obtained for L. sakei 2a by the use of the PMA-qPCR method, compared to the plate count technique (4.4, 4.3, and 3.9 log units higher for F1 and 3.7, 3.0, and 3.4 log units higher for F2, assessed on days 1, 14, and 28, respectively). In addition, after 2 h of exposure to pH 5.4–5.7 plus bile and pancreatic juices, a recovery effect was observed in F1, relatively to the population of cells surviving the previous gastric phase. Subsequent recovery effects were observed by pH adjustment to 6.8–7.2 during the next two hours (enteric phase II) for both formulations. At the end of the 6 h-assay, L. sakei 2a populations were reduced by approximately 62% (plate count) and 25% (PMA-qPCR) of its initial counts. The storage time did not influence the total recovery observed in the F1 (P>0.05), whereas a significant decreasing trend was observed in F2 (P<0.05).
Similarly, the results obtained for the survival of La-5 were higher (P<0.05) when assessed by the use of the molecular method in comparison to the traditional plate count technique. Based on PMA-qPCR, the viability observed for La-5 at the end of the enteric phase I in F1 remained relatively constant (ca. 4.9 CFU/g) throughout the storage of the food product and did not differ from the mean populations verified at the end of enteric phase II (P>0.05) (data not shown). On the other hand, at the end of both enteric phases, a decrease in the La-5 viability was observed in F2 during its shelf-life but, although statistically significant (P<0.05), they were assessed as less than 0.5 log unit. Differently, the classical method revealed a clear decrease in La-5 viability after enteric phase I (approximately 1.9 log unit at day 1 in both formulations). At the end of the enteric phase II for synbiotic cheese (F1), a small, although statistically significant (P<0.05) recovery effect was demonstrated throughout the storage time. This recovery was not observed for F2. The total average reduction in La-5 viability at the end of the in vitro assay, assessed in F1 and F2 was respectively 63-59.7% (PMA-qPCR method) and 43.2-41.6% (plate count technique). Overall, considering the three strains studied, Bb-12 showed the highest survival rates, in the range or above 6 log CFU/g, throughout the in vitro assay.
Morphological Changes During the In Vitro Assay Simulating the GIT Conditions
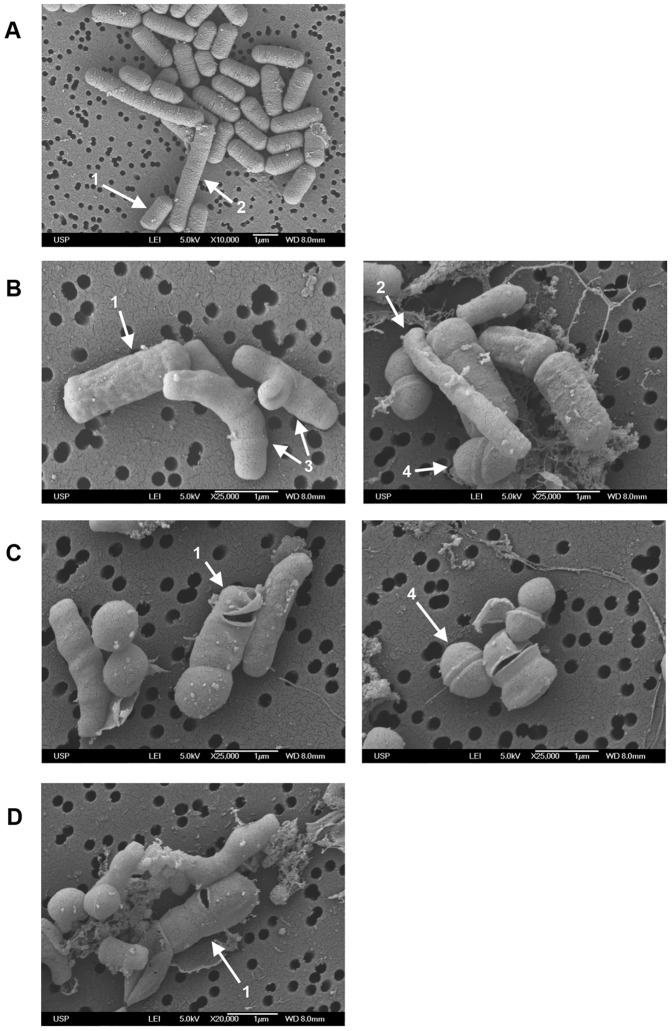
Scanning electron micrographs revealed morphological changes in S. thermophilus, L. acidophilus La-5, B. animalis Bb-12, and L. sakei 2a, tested under simulated GIT stress conditions (Fig. 2). Untreated cells exhibited the characteristic rod shape of Lactobacillus and a smooth and spherical appearance of S. thermophilus (Fig. 2A). However, drastic changes were observed in the cell surface and shape throughout the course of the assay. After 2 h of incubation at pH 2.3–2.6, in the presence of pepsin (3 g/L) and lipase (0.9 mg/L) (gastric phase) the surface of the bacteria clearly seemed to be damaged and the presence of dimples was also observed (Fig. 2B). After exposure to the gastric phase and enteric phase I, the cells demonstrated a shrunken and empty appearance (Fig. 2C). At these incubation times several cells had surface ruptures. Between enteric phases I and II, no further noticeable changes were observed in cells' morphology. At the end of the 6 h-assay, numerous lysed cells and cell debris were verified (Fig. 2D).
Figure 2. Morphological changes in S. thermophilus, La-5, Bb-12, and L. sakei 2a during simulated digestive stress.
Morphological changes, observed through scanning electron microscopy, in S. thermophilus, L. acidophilus La-5, B. animalis Bb-12, and L. sakei 2a throughout the different phases of the in vitro assay simulating the gastrointestinal conditions. Over the entire experiment, some representative photographs were obtained at time-point zero (untreated cells) (A); after 2 h, gastric phase (pH 2.3–2.6 in the presence of pepsin and lipase, 2 h) (B); after 4 h, enteric phase I (pH 5.4–5.7 in the presence of pancreatin and bile, 2 h) (C), and after 6 h, enteric phase II (pH 6.8–7.2 in the presence of pancreatin and bile (D) are shown. (1) L. sakei 2a; (2) L. acidophilus La-5; (3) B. animalis Bb-12, and (4) S. thermophilus.
Discussion
Even though inactivated, dead or cellular components of probiotic bacteria are also thought to mediate beneficial effects on the host [37], the current consensus is that probiotics should be alive to exert their beneficial effect(s). In addition to the safety and functionality criteria, the probiotic candidates are chosen for their resistance to passage throughout the GIT and their capacity to transiently colonize the gut. The choice of the food matrix also influences the activity and survival of the probiotic strain until the end of the food product shelf-life and during its passage throughout the GIT [38], [39], [40].
Comparison of the survival data from literature is somehow difficult due to the diversity of types of assays and conditions employed. However, the same protocol and probiotic strains (La-5 and Bb-12) tested in this study were also used by Bedani et al. [41]. The authors showed that fermented soy food matrix improved the survival capacity of the probiotic strains, particularly for Bb-12, when compared to a freshly prepared culture tested under the same stress conditions. The present study (for comparative purposes, only the plate count values are being considered at this point) indicated that petit-suisse cheese also improved La-5 and Bb-12 survival against simulated GIT conditions, in comparison with the pure culture values reported by Bedani et al. [41]. However, the survival of La-5 and Bb-12 verified by those authors in the fermented soy matrix was better than the one observed in our petit-suisse cheese throughout all the storage period tested.
According to the results obtained by both PMA-qPCR and culture methods, our petit-suisse cheeses (F1 and F2) supported the probiotics survival and could be a good alternative as a food matrix to deliver live probiotic microorganisms. The differences in viability loss among Bb-12, La-5, and L. sakei 2a strains observed in here further support the idea of the strain-specificity survival capacity [42], [43] and corroborate other studies, suggesting that Bb-12 is one of the most resistant strains to GIT conditions [42].
Contrary to our expectations, the prebiotic mix of inulin and FOS, used in this study, did not show any additional protection for the strains tested in the simulated gastrointestinal environment (Tables 3 and 4). According to the literature, there is some evidence that the introduction of a prebiotic ingredient in a product formulation may improve the viability of probiotic strains during manufacture, storage, and also during in vitro conditions of the GIT, even though the underlying mechanisms remain unclear [44].
Possibly, the strains viability would benefit from an assay in which the changes in pH and gastric juice flow are dynamically simulated, as in most advanced digestion systems described in the literature [43], instead of the static model employed in this study. However, the viability rates determined here are within the range of fecal recovery (as intestinal survival indicator) previously reported in in vivo interventions with healthy humans [40], [45].
The second question raised in the research here described was to examine the suitability of three different enumeration methods for a reliable determination of bacteria viability in petit-suisse cheeses during storage and under the stressful conditions of in vitro GIT simulation. The results of plate counts, the “gold standard” for viability counts [46], were compared with those obtained by the culture-independent methods, qPCR and PMA-qPCR. Prior studies have shown that the plate counts differed significantly from the results of other viability assays, including qPCR, LIVE/DEAD bacterial viability kit, fluorescent in situ hybridization (FISH), and multiparameter flow cytometry [47], [48], [49].
The presence of multiple and phylogenetically highly related strains in our petit-suisse cheeses made the differential enumeration of those microorganisms, by the use of plate count technique, a difficult and laborious task due to the similarity in growth requirements and the time of incubation needed to yield the results. Additionally, the differentiation based on morphology of the colonies was necessary to avoid an overestimation of La-5 counts. Similarly, several authors have shown that differential enumeration of bacteria in probiotic foods is often compromised due to the presence of multiple and close related species [30], [50]–[52]. Besides, the distinction based on differences in morphology of the colonies is highly subjective and may lead to an inaccurate estimation of the population of probiotic bacteria in commercial products [30], [53]. Moreover, culture-dependent techniques reflect only the culturable fraction of the bacterial population [19], [20] and it is now known that many Gram-positive and Gram-negative bacteria enter in the so called viable but not culturable state (VBNC) in response to various environmental factors or as a result of sub-lethal injury [54].
In this study, the species-specific primers used allowed an unequivocal detection among the closely related species studied and improved the assay discriminatory power, compared to the use of plate count technique. Another advantage offered by the qPCR method was its speed, since in our work the bacterial quantification was performed in about 4 h, compared to the 72 h required by the use of selective media.
In this study, viable counts obtained by qPCR were slightly higher, but similar to those obtained by PMA-qPCR and plate count at the initial time of the assay, confirming the presence of mainly viable and culturable cells. However, with the intensification of the stress induced throughout the various stages of the assay, the differences between qPCR and the other two methods increased. A decrease in the counts obtained by plate count and PMA-qPCR was observed, while qPCR count values remained fairly similar (Tables 3 and 4). The SEM results confirmed, at the morphological level, the effect of the challenge posed by low-pH and gastric juice for these microorganisms (Fig. 2). The observed decline in viability assessed by plate count and PMA-qPCR methods coincided with the gradual increase in severity of changes in morphology and loss of cell integrity, resulting in the leakage of intracellular contents. Therefore the bacterial populations are overestimated by qPCR, showing the unreliability of the method for the quantification of intact bacteria mixed with large numbers of injured and dead cells. Similar results were observed elsewhere [20], [49], [55] and demonstrated the inability of qPCR method to differentiate between live and dead cells, as its main drawback.
Moreover, the difference among count values obtained by PMA-qPCR and plate methods increased with the intensification of stress during gastric and enteric phases (Tables 3 and 4). The accumulation of injured, VBNC or dead cells unable to form colonies, within the stressed cells, throughout the assay may explain the lower plate count values determined. The existence of probiotic bacteria in the dormant or active, but unculturable state, has been reported before in probiotic products and dairy starters [48], [56]–[59]. On the other hand, in the current study, the PMA treatment efficiently suppressed the amplification of DNA from dead cells, in agreement with Nocker et al. [21], [60], Pan and Breidt [24], and Wuertz and Bae [22]. It may be assumed that part of the sub-lethal injured and VBNC subpopulations maintained its integrity preventing the PMA diffusion. Thus, they could be quantified by the PMA-qPCR method, but not by plate counts technique. In fact, the viability recovery observed by the use of the plate count method, particularly for La-5 and L. sakei 2a, after the enteric phases I and II, could be attributed to injured cells needing time and optimal conditions for reparation and recovery. Similar recovery effect was observed for La-5 [27], [41] and for Bb-12 [41], [61], after exposure to the enteric phase of simulated GIT. According to our study, for certain conditions, the use of plate count technique may also not provide a reliable tool to monitor bacterial populations. Therefore, caution must be taken when interpreting/extrapolating viability studies results based on plate counts, as they may not reflex the real physiological bacterial state.
In contrast to the discrepancy observed in our study, a good correlation between PMA-qPCR and the plate count methods was observed elsewhere [23], [62]. A possible explanation for the differences observed among the latter studies and our study might be the drastic conditions used in the present work, which led to an accumulation of injured, VBNC or dead cells, leading to lower population levels detected by plate count technique. Our findings are in line with those published in other studies [48], [49], in which discrepancies in results assessed by plate count and qPCR methods, regarding the application of stress conditions, were observed, whereas in no stress or low-stress conditions a very good correlation between them was obtained.
In conclusion, the petit-suisse cheeses supported probiotic survival against in vitro simulated gastrointestinal stress conditions and, therefore, are a good alternative food matrixes for the delivery of alive probiotics. Our findings demonstrated that the in vitro approach tested, although not an accurate reproduction of the in vivo events, allows the standardization of testing conditions and the direct comparison among probiotic strains and different food matrices and ingredients, contributing for the selection of the most appropriate matrix for the delivery of specific probiotic strains. This study has shown that the enumeration method deeply influences the analysis results. Therefore, it should be carefully considered depending on the physiological state of the bacterial population and the main objectives of the study. The plate count method is not a reliable enumeration technique for high-stress conditions, such as those verified in in vitro assays, which simulate the gastrointestinal environment. The use of PMA overcomes the inability of the qPCR method to differentiate between alive and dead cells. The combination of PMA and species-specific qPCR in this study allowed a quick and unequivocal way of enumeration of viable closely related species under stress in the probiotic and synbiotic petit-suisse cheeses. The bacteriocin production by L. sakei 2a in the probiotic (F2) and synbiotic (F1) petit-suisse cheeses and its possible role in food safety will be addressed in future studies.
Acknowledgments
The authors acknowledge Orafti, Salute, Germinal, and Danisco for providing part of the material resources employed and Dr. Vanessa Biscola for her useful suggestions during the manuscript review.
Funding Statement
Fundação de Amparo á Pesquisa do Estado de São Paulo (FAPESP) Grant numbers: 2009/52600-6, 2009/52599-8, 2011/05203-1, and 2012/13535-7; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Masood MI, Qadir MI, Shirazi JH, Khan IU (2011) Beneficial effect of lactic acid bacteria on human beings. Crit Rev Microbiol 37 (1) 91–98. [DOI] [PubMed] [Google Scholar]
- 2. Carr FJ, Chill D, Maida N (2002) The Lactic Acid Bacteria: A Literature Survey. Crit Rev Microbiol 28 (4) 281–370. [DOI] [PubMed] [Google Scholar]
- 3. O'Sheal EF, Cotter PD, Ross RP, Hill C (2013) Strategies to improve the bacteriocin protection provided by lactic acid bacteria. Curr Opin Biotechnol 24: 130–134. [DOI] [PubMed] [Google Scholar]
- 4.FAO/WHO (2001) Regulatory and Clinical Aspects of Dairy Probiotics, Food and Agriculture Organization of the United Nations, World Health Organization, Cordoba. Available: http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. Accessed 17 January 2010.
- 5. Reid G, Jass J, Sebulsky MT, McCormick J K (2003) Potential uses of probiotics in clinical practice. Clin Microbiol Rev 16 (4) 658–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sakar S (2013) Probiotic as functional foods: documented health benefits. Nutrition Food Sci 43 (2) 107–115. [Google Scholar]
- 7.Chr. Hansen A/S (2011) Study summaries BB12. Available: http://cdn.chr-hansen.com/uploads/tx_tcdownloadables/Selected_summaries_BB-12.pdf. Accessed 17 May 2013.
- 8.Chr. Hansen A/S (2011) Study summaries La-5. Available: http://www.chr-hansen.com/uploads/tx_tcdownloadables/Selected_summaries_LA-5.pdf. Accessed 17 May 2013.
- 9. De Vuyst L, Leroy LF (2007) Bacteriocins from Lactic acid bacteria: production, purification, and food applications. J Mol Microbiol Biotechnol 13: 194–199. [DOI] [PubMed] [Google Scholar]
- 10. De Martins ECP, Franco BDGM (1997) Inhibition of foodborne pathogens by bacteriocin-producing Leuconostoc sp. and Lactobacillus sakei isolated from “lingüiça frescal”. Rev Microbiol 28 (4) 284–287. [Google Scholar]
- 11. Roberfroid MB (2007) Inulin-type fructans: functional food ingredients. J Nutr 137 (11) 2493–2502. [DOI] [PubMed] [Google Scholar]
- 12. Hasler CM (2002) Functional foods: benefits, concerns and challenges—A position paper from the American Council on Science and Health. J Nutr 132: 3772–3781. [DOI] [PubMed] [Google Scholar]
- 13. Karimi R, Mortazavian AM, Cruz AG (2011) Viability of probiotic microorganisms in cheese during production and storage: a review. Dairy Sci Technol 91 (3) 283–308. [Google Scholar]
- 14. Fiorentini AM, Ballus CA, Oliveira ML, Cunha MF, Klajn VM (2011) The influence of different combinations of probiotic bacteria and fermentation temperatures on the microbiological and physicochemical characteristics of fermented lactic beverages containing soybean hydrosoluble extract during refrigerated storage. Ciênc Tecnol Aliment 31 (3) 597–607. [Google Scholar]
- 15. Alves LL, Richards NSPS, Mattanna P, Andrade DF, Rezer AP, et al. (2013) Cream cheese as a symbiotic food carrier using Bifidobacterium animalis Bb-12 and Lactobacillus acidophilus La-5 and inulin. Int J Dairy Technol 86 (1) 63–69. [Google Scholar]
- 16.Buriti FCA, Souza CHB, Saad SMI (2012) Cheese as probiotic carrier: technological aspects and benefits. In: Hui YH, Evranuz EÖ, editors. Handbook of Food and Beverage Fermentation Technology. 2.ed. Boca Raton: CRC Press. pp. 749–784. [Google Scholar]
- 17. van Bokhorst-van de Veen H, van Swam I, Wels M, Bron PA, Kleerebezem M (2012) Congruent strain specific intestinal persistence of Lactobacillus plantarum in an intestine-mimicking in vitro system and in human volunteers. PLoS One 7 (9) e44588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghadimi D, Fölster-Holst R, De Vrese M, Winkler P, Heller KJ, et al. (2008) Effects of probiotic bacteria and their genomic DNA on TH 1/TH 2 cytokine production by peripheral blood mononuclear cells (PBMCs) of healthy and allergic subjects. Immunobiology 213: 677–692. [DOI] [PubMed] [Google Scholar]
- 19.Giraffa G, Carminati D (2008) Molecular techniques in food fermentation: principles and applications. In: Cocolin L, Ercolini D, editors. Molecular techniques in the microbial ecology of fermented foods. New York: Springer. pp.1–30. (Food microbiology and food safety series). [Google Scholar]
- 20. Justé A, Thomma BP, Lievens B (2008) Recent advances in molecular techniques to study microbial communities in food-associated matrices and processes. Food Microbiology 25: 745–761. [DOI] [PubMed] [Google Scholar]
- 21. Nocker A, Cheung CY, Camper AY (2006) Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J Microbiol Methods 67: 310–320. [DOI] [PubMed] [Google Scholar]
- 22. Wuertz S, Bae S (2009) Discrimination of viable and dead fecal Bacteroides bacteria by quantitative PCR with propidium monoazide. Appl Environ Microbiol 75 (9) 2940–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. García-Cayuela T, Tabasco R, Peláez C, Requena T (2009) Simultaneous detection and enumeration of viable lactic acid bacteria and bifidobacteria in fermented milk by using propidium monoazide and real-time PCR. Int Dairy J 19: 405–409. [Google Scholar]
- 24. Pan Y, Breidt F (2007) Enumeration of viable Listeria monocytogenes cells by real-time PCR with propidium monoazide and ethidium monoazide in the presence of dead cells. Appl Environ Microbiol 73: 8028–8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cardarelli HR, Buriti FCA, Castro IA, Saad SMI (2008) Inulin and oligofructose improve sensory quality and increase the probiotic viable count in potentially synbiotic petit-suisse cheese. LWT – Food Sci Technol 41 (6) 1037–1046. [Google Scholar]
- 26. Liserre AM, Ré MI, Franco BDGM (2007) Microencapsulation of Bifidobacterium animalis subsp. lactis in modified alginate-chitosan beads and evaluation of survival in simulated gastrointestinal conditions. Food Biotechnol 21: 1–16. [Google Scholar]
- 27. Buriti FCA, Castro IA, Saad SMI (2010) Viability of Lactobacillus acidophilus in synbiotic guava mousses and its survival under in vitro simulated gastrointestinal conditions. Int J Food Microbiol 137: 121–129. [DOI] [PubMed] [Google Scholar]
- 28. Vinderola CG, Reinheimer JA (2000) Enumeration of L. acidophilus, bifidobacteria and lactic starter bacteria in fermented dairy products. Int Dairy J 10: 271–275. [Google Scholar]
- 29. International Dairy Federation (1995) Fermented and non-fermented milk products: detection and enumeration of Lactobacillus acidophilus . Bulletin of the International Dairy Federation 306: 23–33. [Google Scholar]
- 30. Tabasco R, Paarup T, Janer C, Peláez C, Requena T (2007) Selective enumeration and identification of mixed cultures of Streptococcus thermophilus, Lactobacillus delbrueckii subsp. bulgaricus, L. acidophilus, L. paracasei subsp. paracasei and Bifidobacterium lactis in fermented milk. Int Dairy J 17: 1107–1114. [Google Scholar]
- 31. Taipale TJ, Pienihäkkinen K, Isolauri E, Larsen CN, Brockmann E, et al. (2011) Bifidobacterium animalis subsp. lactis Bb-12 in reducing the risk of infections in infancy. Br J Nutr 105 (3) 409–416. [DOI] [PubMed] [Google Scholar]
- 32. Martin BA, Jofre M, Garriga MP, Aymerich T (2006) Rapid quantitative detection of Lactobacillus sakei in meat and fermented sausages by real-time PCR. Appl Environ Microbiol 72 (9) 6040–6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garrigues C, Johansen E, Pedersen MB (2010) Complete genome sequence of Bifidobacterium animalis subsp. lactis BB-12, a widely consumed probiotic strain. J Bacteriol 192: 2467–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chaillou S, Champomier-Verges MC, Cornet M, Crutz-Le Coq AM, Dudez AM, et al. (2005) The complete genome sequence of the meat-borne lactic acid bacterium Lactobacillus sakei 23K. Nat Biotechnol 23: 1527–1533. [DOI] [PubMed] [Google Scholar]
- 35.Felske A, Weller R (2001) Cloning 16S rRNA genes and utilization to bacterial communities. In: Akkermans ADL, Van Elsas JD, De Bruijn FJ, editors. Molecular Microbial Ecology Manual, Dordrecht: Kluwer Academic Publishers. pp.1–16. [Google Scholar]
- 36. Altermann E, Russell WM, Azcarate-Peril MA, Barrangou R, Buck BL, et al. (2005) Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc Natl Acad Sci USA 102 (11) 3906–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taverniti V, Guglielmetti S (2011) The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr 6 (3) 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dommels YE, Kemperman RA, Zebregs YE, Draaisma RB, Jol A, et al. (2009) Survival of Lactobacillus reuteri DSM 17938 and Lactobacillus rhamnosus GG in the human gastrointestinal tract with daily consumption of a low-fat probiotic spread. Appl Environ Microbiol 75: 6198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Madureira AR, Amorim M, Gomes AM, Pintado ME, Malcata FX (2011) Protective effect of whey cheese matrix on probiotic strains exposed to simulated gastrointestinal conditions. Food Res Int 44: 465–470. [Google Scholar]
- 40. Saxelin M, Lassig A, Karjalainen H, Tynkkynen S, Surakka A, et al. (2010) Persistence of probiotic strains in the gastrointestinal tract when administered as capsules, yoghurt, or cheese. Int J Food Microbiol 144: 293–300. [DOI] [PubMed] [Google Scholar]
- 41. Bedani R, Rossi EA, Saad SMI (2013) Impact of inulin and okara on Lactobacillus acidophilus La-5 and Bifidobacterium animalis Bb-12 viability in a fermented soy product and probiotic survival under in vitro simulated gastrointestinal conditions. Food Microbiol 34 (2) 382–389. [DOI] [PubMed] [Google Scholar]
- 42. Masco L, Crockaert C, Van Hoorde K, Swings J, Huys G (2007) In vitro assessment of the gastrointestinal transit tolerance of taxonomic reference strains from human origin and probiotic product isolates of Bifidobacterium . J Dairy Sci 90 (8) 3572–3578. [DOI] [PubMed] [Google Scholar]
- 43.Venema K, Havenaar R, Minekus M (2009) Improving in vitro simulation of the stomach and intestines. In: McClements DJ, Decker EA, editors. Designing functional foods: Measuring and controlling food structure breakdown and nutrient absorption. Cambridge: Woodhead Publishing Ltd. pp. 314–339. [Google Scholar]
- 44. Saulnier DMA, Molenaar D, de Vos WM, Gibson GR, Kolida S (2007) Identification of prebiotic fructooligosaccharide metabolism in Lactobacillus plantarum WCFS1 through microarrays. Appl Environ Microbiol 73 (6) 1753–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Savard P, Lamarche B, Paradis ME, Thiboutot H, Laurin E, et al. (2011) Impact of Bifidobacterium animalis subsp. lactis BB-12 and, Lactobacillus acidophilus LA-5 containing yoghurt, on fecal bacterial counts of healthy adults. Int J Food Microbiol 149: 50–57. [DOI] [PubMed] [Google Scholar]
- 46. Champagne CP, Ross RP, Saarela M, Hansen KF, Charalampopoulos D (2011) Recommendations for the viability assessment of probiotics as concentrated cultures and in food matrices. Int J Food Microbiol 149: 185–193. [DOI] [PubMed] [Google Scholar]
- 47. Furet JP, Quénée P, Tailliez P (2004) Molecular quantification of lactic acid bacteria in fermented milk products using real-time quantitative PCR. Int J Food Microbiol 97 (2) 197–207. [DOI] [PubMed] [Google Scholar]
- 48. Lahtinen SJ, Gueimonde M, Ouwehand AC, Reinikainen JP, Salminen SJ (2006) Comparison of four methods to enumerate probiotic bifidobacteria in a fermented food product. Food Microbiol 23 (6) 571–577. [DOI] [PubMed] [Google Scholar]
- 49. Reichert-Schwillinsky F, Pin C, Dzieciol M, Wagner M, Hein I (2009) Stress- and growth rate-related differences between plate count and real-time PCR data during growth of Listeria monocytogenes . Appl Environ Microbiol 75 (7) 2132–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vinderola CG, Reinheimer JA (1999) Culture media for the enumeration of Bifidobacterium bifidum and Lactobacillus acidophilus in the presence of yoghurt bacteria. Int Dairy J 9: 497–505. [Google Scholar]
- 51. Roy D (2001) Media for the isolation and enumeration of bifidobacteria in dairy products. Int J Food Microbiol 69: 167–182. [DOI] [PubMed] [Google Scholar]
- 52. van de Casteele S, Vanheuverzwijn T, Ruyssen T, van Assche P, Swings J, et al. (2006) Evaluation of culture media for selective enumeration of probiotic strains of lactobacilli and bifidobacteria in combination with yoghurt or cheese starters. Int Dairy J 16 (12) 1470–1476. [Google Scholar]
- 53. Talwalkar A, Kailasapathy K (2004) Comparison of selective and differential media for the accurate enumeration of strains of Lactobacillus acidophilus, Bifidobacterium spp. and Lactobacillus casei complex from commercial yoghurts. Int Dairy J 14: 142–149. [Google Scholar]
- 54. Oliver JD (2005) The viable but nonculturable state in bacteria. J Microbiol 43: 93–100. [PubMed] [Google Scholar]
- 55. Postollec F, Falentin H, Pavan S, Combrisson J, Sohier D (2011) Recent advances in quantitative PCR (qPCR) applications in food microbiology. Food Microbiol 28: 848–861. [DOI] [PubMed] [Google Scholar]
- 56. Bunthof CJ, Abee T (2002) Development of a flow cytometric method to analyse subpopulations of bacteria in probiotic products and dairy starters. Appl Environ Microbiol 68: 2934–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Amor KB, Breeuwer P, Verbaarschot P, Rombouts FM, Akkermans AD, et al. (2002) Multiparametric flow cytometry and cell sorting for the assessment of viable, injured, and dead Bifidobacterium cells during bile salt stress. Appl Environ Microbiol 68: 5209–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lahtinen SJ, Gueimonde M, Ouwehand AC, Reinikainen JP, Salminen SJ (2005) Probiotic bacteria may become dormant during storage. Appl Environ Microbiol 71: 1662–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lahtinen SJ, Ahokoski H, Reinikainen JP, Gueimonde M, Nurmi J, et al. (2008) Degradation of 16S rRNA and attributes of viability of viable but nonculturable probiotic bacteria. Lett Appl Microbiol 46: 693–698. [DOI] [PubMed] [Google Scholar]
- 60. Nocker A, Sossa KE, Camper AK (2007) Molecular monitoring of disinfection efficacy using propidium monoazide in combination with quantitative PCR. J Microbiol Methods 70 (2) 252–260. [DOI] [PubMed] [Google Scholar]
- 61.Souza CHB, Gioielli LA, Saad SMI (2009) Efeito da adição de inulina, concentrado proteíco de soro e caseína sobre a viabilidade de Bifidobacterium animalis Bb-12 em margarina. Annais of the 25th Congresso Brasileiro de Microbiologia Abstract ID: 1855-1. Available: http://sbmicrobiologia.org.br/pdf/cdsbm/resumos/R1855-1.html. Accessed 29 August 2013.
- 62. Kramer M, Obermajer N, Bogovič BM, Rogelj I, Kmetec V (2009) Quantification of live and dead probiotic bacteria in lyophilized product by real-time PCR and by flow cytometry. Appl Microbiol Biotechnol 84: 1137–1147. [DOI] [PubMed] [Google Scholar]