Abstract
TiO2 photocatalysis can be used to kill surface adherent bacteria on biomaterials, and is particularly interesting for use with percutaneous implants and devices. Its efficiency and safety, however, depend on the activation energy required. This in vitro study investigates synergetic effects against the clinically relevant strains S. epidermidis and S. mutans when combining photocatalytic surfaces with H2O2. After 20 min exposure to 0.1 wt% H2O2 and UV light on TiO2 surfaces, viabilities of S. epidermidis and S. mutans were reduced by 99.7% and 98.9%, respectively. Without H2O2 the corresponding viability reduction was 86% for S. epidermidis and 65% for S. mutans. This study indicates that low concentrations of H2O2 can enhance the efficiency of photocatalytic TiO2 surfaces, which could potentially improve current techniques used for decontamination and debridement of TiO2 coated biomedical implants and devices.
Keywords: antibacterial, photocatalysis, photolysis, synergy, titanium dioxide, hydrogen peroxide, UV light
Introduction
Implant-associated infections remain one of the biggest challenges facing patients and clinicians post-surgery. Planktonic bacteria that adhere to implanted devices can colonize the surface and develop a resistant biofilm, in turn leading to a chronic infection that is resistant to host defense mechanisms and antimicrobial therapies.1,2 Dental implants and other percutaneous devices are particularly susceptible to such infections, as the implant exit sites become gateways for pathogens in cases where bonding between implant and hard or soft tissue fails.3 Along with the emergence of multiple antibiotic resistant strains, surface functionalization of biomaterials and utilization of non-antibiotic treatments are becoming more important to avoid or beat severe infections.4 Among several approaches to achieve antibacterial activity on biomaterials, photocatalysis on TiO2 coatings is a viable alternative. It presents an on-demand, self-sterilizing capability under UV light irradiation, which has been proven against a host of pathogens,5,6 and is complementary to the clinically proven track record of biocompatibility and bioactivity of TiO2.7 When irradiated with near-UV light, electron excitation occurs and reactive oxygen species (ROS) are generated at the TiO2 photocatalyst surface. Hydroxyl radicals (•OH) and superoxide anions (O2-) are particularly oxidative and can act on the cell wall of nearby bacteria. After cell wall damage, oxidative stress is exerted on the cytoplasmic membrane and the increased permeability eventually leads to cell death.8,9
A number of techniques are available to obtain photocatalytically active TiO2 coatings, including sol-gel,10 anodic oxidation,11 and various physical vapor deposition methods.12,13 These methods, however, have limitations when dealing with multifaceted devices, and often require advanced vacuum technology. Direct chemical treatments of Ti-based substrates, on the other hand, have the advantage of being simple and flexible, yet allowing homogeneous coating of complex geometries.14 In terms of antibacterial properties, chemical oxidation of Ti in H2O2 has the potential benefit of not only producing a photocatalytically active TiO2 layer,15,16 but also impregnating the surface with radical species available for photoactivation.17,18
Chemical disinfection with H2O2 itself has similarities with the photocatalytic system, except the •OH are produced via a Fenton like reaction.19 By incorporating H2O2 in the photocatalytic reaction, synergetic effects against bacteria can be achieved; H2O2 can act as an electron acceptor, reducing electron-hole recombination while producing additional •OH.20 Its presence, either by addition or production through photocatalytic reactions, has also been shown to enhance long-range bactericidal effects.21 Direct photolysis of H2O2, producing two •OH, has further been reported to occur under both UV and visible light irradiation.22-25
The gram-positive genus of Staphylococcus has been identified as responsible for orthopedic implant-associated infections in roughly 80% of cases where revision surgery has been required, with the strains S. aureus and S. epidermidis alone representing two thirds of the whole.4 Both S. aureus and S. epidermidis are persistent bacteria, prone to form biofilms that increase their antibiotic resistance.2 Considering dental implants, the prevalence of infections leading to peri-implantitis have been reported as high as 14%.26 Periodontal disease, as well as peri-implantitis, is often triggered or enhanced by plaque,27 the diverse and complex biofilm developing on the surface of both natural teeth and restorative materials. The bacteria in plaque and other biofilms are surrounded by an extracellular polysaccharide (EPS) matrix, providing a physical and chemical barrier against host defenses, antibiotics and antimicrobial agents.28 Common in plaque, and responsible for dental caries formation due to metabolic acid production from carbohydrates, is Streptococcus mutans.29 The Streptococcus bacteria, contrary to Staphylococcus, are catalase negative, i.e., missing the enzyme responsible for H2O2 decomposition, and are therefore more sensitive to its exposure.
In the present study, bactericidal activities of H2O2, TiO2 photocatalysis, and their potential synergetic effects against planktonic S. epidermidis and S. mutans were investigated, with the aim of strengthening current debridement and decontamination techniques used on Ti-based implants and medical devices. A direct contact test (DCT) method for planktonic bacteria was also developed, aimed at increasing the accuracy when quantifying short-range, UV-activated antibacterial properties on TiO2 surfaces.
Results
Figure 1 shows the viability of S. mutans after the DCT for up to 60 min, illustrating that the timeframe during which antibacterial tests can be performed without significant viability loss due to desiccation was 20 min. A high degree of variation in viability was observed after 40 min of the DCT, and at 60 min virtually no metabolically active cells were detected.
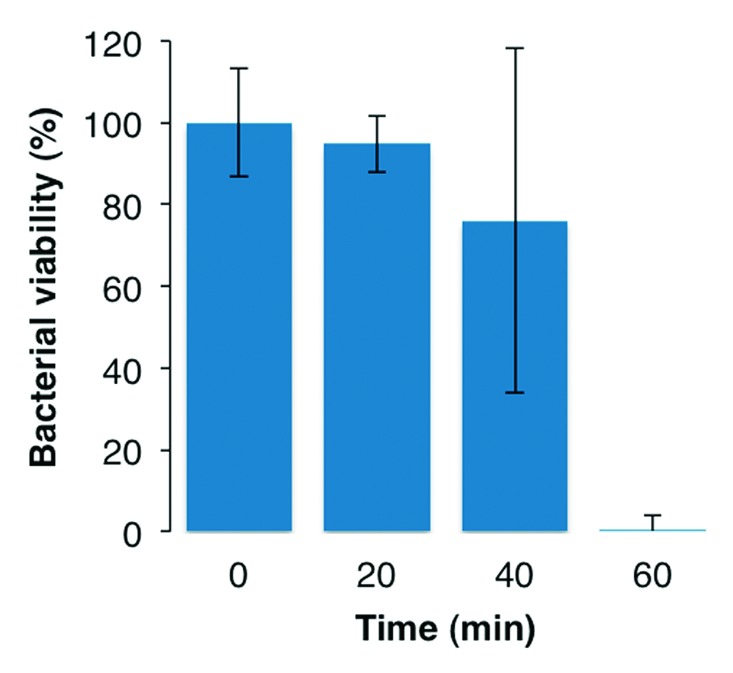
Figure 1. Viability of S. mutans after DCT for up to 60 min. 100% viability corresponds to 5.4 × 105 CFU/mL.
The effect of 15 min exposure to H2O2 on S. epidermidis and S. mutans is shown in Figure 2, with a clear depreciation of viability with increasing H2O2 concentration. An addition of 0.1 wt% H2O2 resulted in a 24% and 42% reduction in viability of S. epidermidis and S. mutans, respectively. This amount was deemed appropriate for further testing with TiO2 discs and UV light, as lower or higher end concentrations resulted in either uncertain or too high bactericidal effects due to H2O2.
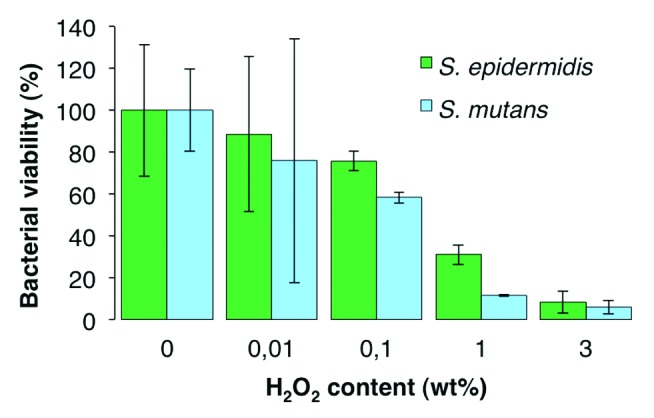
Figure 2. Effect of varying H2O2 concentrations on bacterial viability, performed on Ti discs and analyzed after 15 min incubation at 37 °C. 100% viability corresponds to 7.8 × 105 and 7.3 × 105 CFU/mL of S. epidermidis and S. mutans, respectively.
In Figure 3, individual and synergetic effects of TiO2 substrates, 0.1 wt% H2O2 and UV irradiation against the bacteria are shown when subjected to a 10 and 20 min DCT. Compared with the DCT with Ti control discs (i.e., without UV or added H2O2), bacterial viability after direct contact with the TiO2 discs was significantly lower in all cases except after 20 min with S. epidermidis, indicating a presence and effect of radical species after the H2O2 surface treatment used to prepare the TiO2 discs. When the TiO2 discs were irradiated with UV light to generate hydroxyl radicals via photocatalysis (TiO2/UV in Fig. 3), the increased bactericidal effect was statistically significant in tests with S. epidermidis, but not with S. mutans. Similar values of viability reduction were obtained with DCT of TiO2 discs and the addition of 0.1 wt% H2O2 (TiO2/H2O2 in Fig. 3) as in the DCT of TiO2 discs with the addition of UV, except against S. mutans after 20 min where the effect of TiO2 with H2O2 was markedly enhanced. Finally, the synergetic effects of H2O2 photolysis and photocatalysis on TiO2 substrates (TiO2/H2O2/UV in Fig. 3) was most substantial after 20 min in which the viability on average was reduced by 99.7% for S. epidermidis, and by 98.9% for S. mutans.
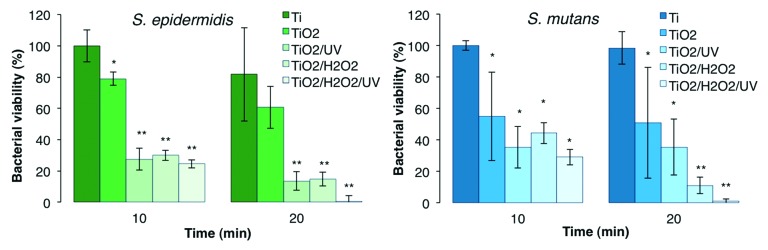
Figure 3. Comparative viability of S. epidermidis and S. mutans after 10 and 20 min exposure to individual and synergetic effects of TiO2 discs, UV light and 0.1 wt% H2O2. 100% viability corresponds to 1.04 × 106 and 1.28 × 106 CFU/mL for S. epidermidis and S. mutans, respectively. Asterisk (*) indicates significant difference from the Ti DCT, and double asterisk (**) indicates significant difference from both Ti and TiO2 DCTs, both at P < 0.05.
Discussion
Infections following surgical placement of dental or orthopedic implants are difficult to treat, and can lead to failure in establishing or maintaining proper osseointegration.30 This can lead to, for example, loss of supporting bone around a dental implant, which is a determining factor for peri-implantitis. However, this diagnosis is often preceded by other, reversible peri-implant diseases developing in the surrounding soft tissue.30,31 Systemic or local administration of antibiotics in response to infection should generally be limited due to the growing number of resistant bacteria,32 which makes development of non-antibiotic methods that preemptively kill bacteria to limit biofilm formation, or function as efficient debridement techniques in early stages of infection, increasingly important for securing implant integrity.4 In this study, individual and synergetic effects of TiO2, H2O2 and UV light against planktonic S. epidermidis and S. mutans were evaluated by means of a DCT. As a prophylactic measure or as a treatment option in the event of biofilm formation, a TiO2 coated surface could be treated with a low concentration H2O2 solution and irradiated with UV light to generate ROS, effectively inactivating nearby bacteria.
The dual phase (α + β) Ti-6Al-4V alloy was used as substrate material in the present study. Compared with commercially pure Ti, Ti-6Al-4V presents superior mechanical properties and is often the biomaterial of choice in dental and orthopedic applications.7 The TiO2 coatings obtained after H2O2-oxidation of Ti-6Al-4V has been shown to mainly consist of poorly crystalline anatase, essentially free from alloying element oxides Al2O3 and V2O5,33 and present bioactive34 as well as photocatalytic properties.16 Photocatalysis on TiO2 surfaces and its bactericidal effect has been researched extensively in the last two decades for specific biomedical purposes5 as well as for other disinfection applications.35 Low concentration H2O2 is already widely used as a disinfectant36 and photolysis of H2O2 has been evaluated for degradation of pharmaceuticals23 and oral bacteria.25,37 However, most research performed on the combined/synergetic effects of TiO2 photocatalysis and H2O2 photolysis have focused on the degradation of organic contaminants in wastewater.38-40 In the biomedical field, there lies a potential benefit in applying these combined effects when bacterial colonization occurs on TiO2 coated devices, implants, abutments, and/or crown and bridgeworks.
As demonstrated in this work, both photocatalysis and presence of H2O2 show bactericidal effects, but the effect is markedly enhanced when the two are combined. The role of H2O2 in supporting TiO2 photocatalysis lies mainly in acting as an efficient electron acceptor, inhibiting electron-hole recombination while producing additional •OH that can oxidize organic matter.20,41 A prerequisite for UV induced decontamination is that the septic site is available for irradiation, and that the incoming photon energy is sufficient to promote an electron from the valence band to the conduction band. For anatase TiO2 this occurs with wavelengths below 385 nm, which corresponds to the band gap energy of 3.2 eV. On the other hand, photolysis of H2O2 is possible with longer wavelength light in the visible region, although with a relatively higher power density than that required at shorter wavelengths.25 However, to achieve synergetic effects the use of UV light is required to enable electron excitation in the TiO2 crystal.38 Although extension of TiO2 photoactivity into the visible region is currently being widely researched and has been shown to be possible via doping,42 the activation energy corresponding to the band gap needs to be overcome for the treatment to be effective.
This study found that after 20 min TiO2/H2O2/UV treatment, a respective 99.7% and 98.9% viability reduction occurred for S. epidermidis and S. mutans, whereas the corresponding viability reduction with TiO2/UV was approximately 86% and 65% for S. epidermidis and S. mutans, respectively. Removing the UV altogether (TiO2 in Fig. 3) resulted in corresponding 40% and 50% viability reductions after 20 min. This indicates that photocatalysis did occur, and that the effect was enhanced due to synergy when H2O2 was added. However, it also indicates that the TiO2 discs had inherent bactericidal properties after surface modification with H2O2. This is attributed to the slow degradation and release of surface bound superoxide and peroxides known to occur in Ti-peroxide systems, resulting in a bactericidal effect.43,44 Such inherent bactericidal action is not expected to occur on other TiO2 surfaces produced by alternate means such as physical vapor deposition, sol-gel or anodic oxidation unless specifically loaded with antibiotics or other antimicrobial agents such as silver.45 A gradual decline in activity, which indicates the release and effect of surface bound peroxides from H2O2-oxidized Ti-based substrates, accelerated under UV irradiation, was reported in a previous study.46 Interestingly, the same study showed that addition of 3% H2O2 during the reaction was sufficient to maintain a high photocatalytic activity through repeated UV activation, which would be key in an application setting to keep persistent infections at bay.
The results show that the two bacteria strains used in this study have different susceptibilities to the ROS attack. For example, in Figure 3 it can be seen that the viability of S. mutans remained essentially unchanged between 10 and 20 min TiO2/UV treatment, whereas it dropped significantly between 10 and 20 min of TiO2/H2O2 treatment. This is likely due to the inability of S. mutans to degrade H2O2 as it lacks the catalase enzyme. This is further supported by Figure 2, where it can be observed that for H2O2 concentrations greater than 0.01 wt%, S. mutans suffered higher viability losses than S. epidermidis.
The current study shows that a DCT method for antibacterial testing could be adapted for use on photocatalytic surfaces, also in combination with H2O2, and that metabolic activity is a sensitive indicator for bacterial viability assessment after the DCT. There are, however, certain limitations to the method, namely the time window for UV light application and incubation time prior to complete desiccation. The occasionally observed large standard deviations indicate not only the sensitivity of bacteria to local conditions, e.g., radical formation and uneven drying, but also the sensitivity of the metabolic activity indicator. Additionally, the H2O2-oxidation technique employed in this study is known to increase surface roughness and surface area of the substrate, and consequently also photocatalytic activity.16 Although demonstrated to generate a relatively homogeneous surface,16,46 some irregularities that shelter or expose bacteria may still exist, which could account for some variability in the data. It is also recognized that UV exposure has certain bactericidal effects in its own, and that such effects could be enhanced in the current model due to an increase in temperature and accelerated drying. Nevertheless, the developed method offers a fairly simple means of quantifying antibacterial properties at the surface, also in presence of an added oxidant for synergetic effects. Longer exposure times would most certainly lead to further decimation of viability, as also supported in the literature,47 but a short treatment time is desirable for both practical applications and safety reasons in vivo. UV exposure to unprotected eyes, skin and other soft tissue should generally be limited according wavelength and intensity, and applying the Threshold Limit Value (TLV®) issued by the American Conference of Governmental Industrial Hygienists (ACGIH) to conditions used in this study (λ = 365 ± 10 nm, E = 1.5 mW/cm2), the exposure time should not exceed 16 min. Even within this timeframe, a considerable reduction of viable bacteria was achieved. Further reduction would also be possible with a device allowing higher UV dose directed to the infected site of the implant, abutment or device while shielding sensitive host tissue.
Materials and Methods
Electron beam melted (EBM) rods of Ti-6Al-4V (Arcam AB, Sweden) were cut into sample discs measuring 9 mm in diameter and 1 mm in thickness. The discs were then washed in acetone, ethanol and distilled water for 15 min each in an ultrasonic bath. Surface modification was conducted by placing each disc in a 50 mL falcon tube containing 10 mL 30 wt% H2O2, held at 80 °C for a duration of 24 h. Discs were then transferred to individual 50 mL falcon tubes containing 10 mL H2O, and held at 80 °C for 72 h. This treatment has previously been shown to produce a nano-porous coating of poorly crystalline TiO2 with photocatalytic properties.16 Such samples are denoted as TiO2 discs. Additional discs of Ti-6Al-4V (Elos A/S, Denmark) were cut and machined to the same dimensions from wrought stock, and served as control substrates, denoted as Ti discs. Prior to experimentation with bacteria, all discs were washed twice in ethanol and twice in distilled water for 10 min in ultrasound, followed by 10 min irradiation in a UV-Ozone photoreactor (Model PR-100, UVP, USA). This treatment serves to remove contaminants and increase the hydrophilicity for closer bacterial contact in following tests.
Bacterial strains of S. mutans (UA159) and S. epidermidis (CCUG 18000A) were used for all experiments. Brain Heart Infusion and Mueller Hinton Broth (Sigma-Aldrich, Steinheim, Germany) were used to inoculate S. mutans and S. epidermidis, respectively. The cultures were incubated at 37 °C overnight, after which the bacteria were centrifuged, collected and re-suspended in 500 μL phosphate-buffered saline (PBS, Dulbecco, Sigma-Aldrich). The concentration of bacteria was adjusted to OD600 = 1.0 using a UV spectrophotometer (Model UV-1800, Shimadzu), which corresponds to approximately 109 colony forming units (CFU)/mL.
The bacterial viability test employed in this study originates from a DCT developed by Weiss et al.48 for evaluating antibacterial properties of non-soluble materials. In short, a small amount of bacterial suspension, typically 5–10 μL, is spread over the surface of a substrate. The entity is then incubated at 37 °C for up to 1 h, allowing evaporation of the suspension media and intimate contact between substrate and remaining bacteria. Growth medium is then added and proliferation is monitored under further incubation, either continuously or after a certain time point. With materials and antibacterial mechanisms in this study being different from previous studies,48-50 the critical parameter in the DCT was to establish a close contact between bacteria and substrate. This would enable a more precise evaluation of antibacterial properties at the surface, rather than in any volume above it. In order to determine the optimum time window for analyzing bacterial viability (i.e., maximizing the contact time prior to desiccation), 5 μL of S. mutans bacteria suspension was spread on Ti discs placed in wells of a sterile 24-well plate (Nunclon) and incubated at 37 °C for 0, 20, 40, and 60 min before checking viability. Three replicates for each time point were used.
The direct bactericidal effect of H2O2 on bacterial viability was evaluated by incorporating total concentrations of 0.01, 0.1, 1, and 3 wt% H2O2 in suspensions of S. mutans and S. epidermidis. Immediately after introduction of H2O2 to the bacterial suspensions, 5 μL of the suspensions were spread on Ti discs in replicates of three and incubated at 37 °C for 15 min before evaluation. H2O2 concentrations up to 3 wt% have been considered safe for human use and are routinely used as an antiseptic in the oral cavity.24,36 Short-term disinfection by hydroxyl radicals produced via H2O2 photolysis have also been deemed to present little or no risk of carcinogenicity.51
Photocatalytic activity against bacteria was evaluated on TiO2 discs under UV irradiation at a wavelength of 365 ± 10 nm, with light intensity at the disc surface adjusted to 1.5 mW/cm2 (light meter UV-340, Lutron, Taiwan). By comparison, ambient UVA on a clear summer day in the United Kingdom can reach 4.5 mW/cm2.52 Bacteria were spread on surfaces as previously described and incubated at 37 °C under UV light for 10 and 20 min. For equal distribution of light during UV tests, discs were placed together in a sterile petri dish and later transferred to individual wells of a 24-well plate for viability analysis. Similar experiments were conducted with total a concentration of 0.1 wt% H2O2 present in the bacterial solutions, with and without UV light to investigate synergetic effects. These tests were performed in replicates of four.
Quantification of bacterial viability subsequent to the DCT was performed using a metabolic activity assay, with resazurin as an indicator. In the assay, blue non-fluorescent resazurin is reduced to pink, fluorescent resorufin by metabolic intermediates, resulting in the fluorescence being a sensitive indicator of viable bacteria.53 After each test, the discs were washed in their individual wells with 500 μL PBS, turned upside down and sonicated for 20–30 s to detach bacteria from the surface and to re-suspend the cells in solution. Then, 100 μL of each solution was extracted and placed along with 100 μL of premixed culture medium/resazurin in wells of a 96-well plate. The plate was then incubated at 37 °C for 6 h using a shaking incubator, and fluorescence readings of the wells were made every 2 h using a microplate reader (Tecon Infinite M200), set to 530 nm excitation and 590 nm emission. To correlate the fluorescence readings with known amounts of bacteria, a standard curve was prepared and analyzed along with every experiment. The viable amount of bacteria detected after each test was then expressed as a percentage of a control for each experiment that represented the amount of viable bacteria in a similar DCT test without bactericidal action. Statistically significant differences (P < 0.05) were identified by one-way ANOVA followed by a post-hoc Tukey’s multiple comparisons test, using IBM SPSS v19.0 statistics software.
Conclusions
Combining the photocatalytic properties of TiO2 with an addition of H2O2 generates an efficient surface disinfection system due to synergetic effects that increase the production of ROS. The combined effects were tested in vitro against bacterial species S. epidermidis and S. mutans using a DCT method, and a respective 99.7% and 98.9% viability reduction was achieved after 20 min. As an on-demand, site-specific decontamination technique, the TiO2/H2O2/UV system could be applied as a primary or complementary defense against biomedical implant or medical device infection.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research was funded by the Swedish Foundation for Strategic Research (SSF), through the ProViking program.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/26727
References
- 1.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–8. doi: 10.1016/S0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 3.Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237:1588–95. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 4.Campoccia D, Montanaro L, Arciola CR. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials. 2006;27:2331–9. doi: 10.1016/j.biomaterials.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 5.Visai L, De Nardo L, Punta C, Melone L, Cigada A, Imbriani M, Arciola CR. Titanium oxide antibacterial surfaces in biomedical devices. Int J Artif Organs. 2011;34:929–46. doi: 10.5301/ijao.5000050. [DOI] [PubMed] [Google Scholar]
- 6.Foster HA, Ditta IB, Varghese S, Steele A. Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activity. Appl Microbiol Biotechnol. 2011;90:1847–68. doi: 10.1007/s00253-011-3213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Chu P, Ding C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater Sci Eng Rep. 2004;47:49–121. doi: 10.1016/j.mser.2004.11.001. [DOI] [Google Scholar]
- 8.Maness PC, Smolinski S, Blake DM, Huang Z, Wolfrum EJ, Jacoby WA. Bactericidal activity of photocatalytic TiO(2) reaction: toward an understanding of its killing mechanism. Appl Environ Microbiol. 1999;65:4094–8. doi: 10.1128/aem.65.9.4094-4098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Z, Maness PC, Blake DM, Wolfrum EJ, Smolinski SL, Jacoby WA. Bactericidal mode of titanium dioxide photocatalysis. J Photoch Photobio A. 2000;130:163–70. doi: 10.1016/S1010-6030(99)00205-1. [DOI] [Google Scholar]
- 10.Fu G, Vary PS, Lin C-T. Anatase TiO2 nanocomposites for antimicrobial coatings. J Phys Chem B. 2005;109:8889–98. doi: 10.1021/jp0502196. [DOI] [PubMed] [Google Scholar]
- 11.Sreekantan S, Hazan R, Lockman Z. Photoactivity of anatase–rutile TiO2 nanotubes formed by anodization method. Thin Solid Films. 2009;518:16–21. doi: 10.1016/j.tsf.2009.06.002. [DOI] [Google Scholar]
- 12.Rupp F, Haupt M, Klostermann H, Kim HS, Eichler M, Peetsch A, Scheideler L, Doering C, Oehr C, Wendel HP, et al. Multifunctional nature of UV-irradiated nanocrystalline anatase thin films for biomedical applications. Acta Biomater. 2010;6:4566–77. doi: 10.1016/j.actbio.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Suketa N, Sawase T, Kitaura H, Naito M, Baba K, Nakayama K, Wennerberg A, Atsuta M. An antibacterial surface on dental implants, based on the photocatalytic bactericidal effect. Clin Implant Dent Relat Res. 2005;7:105–11. doi: 10.1111/j.1708-8208.2005.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Chu PK, Ding C. Surface nano-functionalization of biomaterials. Mater Sci Eng Rep. 2010;70:275–302. doi: 10.1016/j.mser.2010.06.013. [DOI] [Google Scholar]
- 15.Wu J-M. Photodegradation of rhodamine B in water assisted by titania nanorod thin films subjected to various thermal treatments. Environ Sci Technol. 2007;41:1723–8. doi: 10.1021/es0623562. [DOI] [PubMed] [Google Scholar]
- 16.Unosson E, Persson C, Welch K, Engqvist H. Photocatalytic activity of low temperature oxidized Ti-6Al-4V. J Mater Sci Mater Med. 2012;23:1173–80. doi: 10.1007/s10856-012-4602-x. [DOI] [PubMed] [Google Scholar]
- 17.Tengvall P, Wälivaara B, Westerling J, Lundström I. Stable titanium superoxide radicals in aqueous Ti-peroxy gels and Ti-peroxide solutions. J Colloid Interface Sci. 1991;143:589–92. doi: 10.1016/0021-9797(91)90291-F. [DOI] [Google Scholar]
- 18.Takemoto S, Yamamoto T, Tsuru K, Hayakawa S, Osaka A, Takashima S. Platelet adhesion on titanium oxide gels: effect of surface oxidation. Biomaterials. 2004;25:3485–92. doi: 10.1016/j.biomaterials.2003.10.070. [DOI] [PubMed] [Google Scholar]
- 19.Flores MJ, Brandi RJ, Cassano AE, Labas MD. Chemical disinfection with H2O2 - The proposal of a reaction kinetic model. Chem Eng J. 2012;198-199:388–96. doi: 10.1016/j.cej.2012.05.107. [DOI] [Google Scholar]
- 20.Kositzi M, Poulios I, Malato S, Caceres J, Campos A. Solar photocatalytic treatment of synthetic municipal wastewater. Water Res. 2004;38:1147–54. doi: 10.1016/j.watres.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi Y, Sunada K, Iyoda T, Hashimoto K, Fujishima A. Photocatalytic bactericidal effect of TiO2 thin films: dynamic view of the active oxygen species responsible for the effect. J Photoch Photobio A. 1997;106:51–6. doi: 10.1016/S1010-6030(97)00038-5. [DOI] [Google Scholar]
- 22.Li X, Chen C, Zhao J. Mechanism of Photodecomposition of H2O2 on TiO2 Surfaces under Visible Light Irradiation. Langmuir. 2001;17:4118–22. doi: 10.1021/la010035s. [DOI] [Google Scholar]
- 23.Lopez A, Bozzi A, Mascolo G, Kiwi J. Kinetic investigation on UV and UV/H2O2 degradations of pharmaceutical intermediates in aqueous solution. J Photoch Photobio A. 2003;156:121–6. doi: 10.1016/S1010-6030(02)00435-5. [DOI] [Google Scholar]
- 24.Hayashi E, Mokudai T, Yamada Y, Nakamura K, Kanno T, Sasaki K, Niwano Y. In vitro and in vivo anti-Staphylococcus aureus activities of a new disinfection system utilizing photolysis of hydrogen peroxide. J Biosci Bioeng. 2012;114:193–7. doi: 10.1016/j.jbiosc.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Ikai H, Nakamura K, Shirato M, Kanno T, Iwasawa A, Sasaki K, Niwano Y, Kohno M. Photolysis of hydrogen peroxide, an effective disinfection system via hydroxyl radical formation. Antimicrob Agents Chemother. 2010;54:5086–91. doi: 10.1128/AAC.00751-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norowski PA, Jr., Bumgardner JD. Biomaterial and antibiotic strategies for peri-implantitis: a review. J Biomed Mater Res B Appl Biomater. 2009;88:530–43. doi: 10.1002/jbm.b.31152. [DOI] [PubMed] [Google Scholar]
- 27.Serino G, Ström C. Peri-implantitis in partially edentulous patients: association with inadequate plaque control. Clin Oral Implants Res. 2009;20:169–74. doi: 10.1111/j.1600-0501.2008.01627.x. [DOI] [PubMed] [Google Scholar]
- 28.ten Cate JM. Biofilms, a new approach to the microbiology of dental plaque. Odontology. 2006;94:1–9. doi: 10.1007/s10266-006-0063-3. [DOI] [PubMed] [Google Scholar]
- 29.van Houte J. Role of micro-organisms in caries etiology. J Dent Res. 1994;73:672–81. doi: 10.1177/00220345940730031301. [DOI] [PubMed] [Google Scholar]
- 30.Esposito MM, Hirsch JMJ, Lekholm UU, Thomsen PP. Biological factors contributing to failures of osseointegrated oral implants. (I). Success criteria and epidemiology. Eur J Oral Sci. 1998;106:527–51. doi: 10.1046/j.0909-8836..t01-2-.x. [DOI] [PubMed] [Google Scholar]
- 31.Pye AD, Lockhart DEA, Dawson MP, Murray CA, Smith AJ. A review of dental implants and infection. J Hosp Infect. 2009;72:104–10. doi: 10.1016/j.jhin.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Neu HC. The crisis in antibiotic resistance. Science. 1992;257:1064–73. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 33.Sun T, Wang M. A comparative study on titania layers formed on Ti, Ti-6Al-4V and NiTi shape memory alloy through a low temperature oxidation process. Surf Coat Tech. 2010;205:92–101. doi: 10.1016/j.surfcoat.2010.06.019. [DOI] [Google Scholar]
- 34.Karthega M, Rajendran N. Hydrogen peroxide treatment on Ti–6Al–4V alloy: A promising surface modification technique for orthopaedic application. Appl Surf Sci. 2010;256:2176–83. doi: 10.1016/j.apsusc.2009.09.069. [DOI] [Google Scholar]
- 35.Gamage J, Zhang Z. Applications of Photocatalytic Disinfection . Int J Photoenergy. 2010;2010:1–11. doi: 10.1155/2010/764870. [DOI] [Google Scholar]
- 36.Linley E, Denyer SP, McDonnell G, Simons C, Maillard JY. Use of hydrogen peroxide as a biocide: new consideration of its mechanisms of biocidal action. J Antimicrob Chemother. 2012;67:1589–96. doi: 10.1093/jac/dks129. [DOI] [PubMed] [Google Scholar]
- 37.Feuerstein O, Moreinos D, Steinberg D. Synergic antibacterial effect between visible light and hydrogen peroxide on Streptococcus mutans. J Antimicrob Chemother. 2006;57:872–6. doi: 10.1093/jac/dkl070. [DOI] [PubMed] [Google Scholar]
- 38.Dionysiou DD, Suidan MT, Baudin I, Laîné J-M. Effect of hydrogen peroxide on the destruction of organic contaminants-synergism and inhibition in a continuous-mode photocatalytic reactor. Appl Catal B. 2004;50:259–69. doi: 10.1016/j.apcatb.2004.01.022. [DOI] [Google Scholar]
- 39.Elmolla ES, Chaudhuri M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination. 2010;252:46–52. doi: 10.1016/j.desal.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Lanao M, Ormad MP, Mosteo R, Ovelleiro JL. Inactivation of Enterococcus sp. by photolysis and TiO2 photocatalysis with H2O2 in natural water. Sol Energy. 2012;86:619–25. doi: 10.1016/j.solener.2011.11.007. [DOI] [Google Scholar]
- 41.Carp O, Huisman C, Reller A. Photoinduced reactivity of titanium dioxide. Prog Solid state Ch. 2004;32:33–177. doi: 10.1016/j.progsolidstchem.2004.08.001. [DOI] [Google Scholar]
- 42.Nah Y-C, Paramasivam I, Schmuki P. Doped TiO2 and TiO2 nanotubes: synthesis and applications. Chemphyschem. 2010;11:2698–713. doi: 10.1002/cphc.201000276. [DOI] [PubMed] [Google Scholar]
- 43.Tengvall P, Hörnsten EG, Elwing H, Lundström I. Bactericidal properties of a titanium-peroxy gel obtained from metallic titanium and hydrogen peroxide. J Biomed Mater Res. 1990;24:319–30. doi: 10.1002/jbm.820240305. [DOI] [PubMed] [Google Scholar]
- 44.Tengvall P, Bertilsson L, Liedberg B, Elwing H, Lundström I. Degradation of dried Ti-peroxy gels made from metallic titanium and hydrogen peroxide. J Colloid Interface Sci. 1990;139:575–80. doi: 10.1016/0021-9797(90)90131-7. [DOI] [Google Scholar]
- 45.Zhao L, Chu PK, Zhang Y, Wu Z. Antibacterial coatings on titanium implants. J Biomed Mater Res B Appl Biomater. 2009;91:470–80. doi: 10.1002/jbm.b.31463. [DOI] [PubMed] [Google Scholar]
- 46.Unosson E, Welch K, Persson C, Engqvist H. Stability and prospect of UV/H2O2 activated titania films for biomedical use. Appl Surf Sci. 2013 doi: 10.1016/j.apsusc.2013.08.057. In press. [DOI] [Google Scholar]
- 47.Sunada K. Studies on photokilling of bacteria on TiO2 thin film. J Photoch Photobio A. 2003;156:227–33. doi: 10.1016/S1010-6030(02)00434-3. [DOI] [Google Scholar]
- 48.Weiss EI, Shalhav M, Fuss Z. Assessment of antibacterial activity of endodontic sealers by a direct contact test. Endod Dent Traumatol. 1996;12:179–84. doi: 10.1111/j.1600-9657.1996.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 49.Beyth N, Domb AJ, Weiss EI. An in vitro quantitative antibacterial analysis of amalgam and composite resins. J Dent. 2007;35:201–6. doi: 10.1016/j.jdent.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 50.Unosson E, Cai Y, Jiang X, Lööf J, Welch K, Engqvist H. Antibacterial properties of dental luting agents: potential to hinder the development of secondary caries. Int J Dent. 2012;2012:529495. doi: 10.1155/2012/529495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanno T, Nakamura K, Ikai H, Kikuchi K, Sasaki K, Niwano Y. Literature review of the role of hydroxyl radicals in chemically-induced mutagenicity and carcinogenicity for the risk assessment of a disinfection system utilizing photolysis of hydrogen peroxide. J Clin Biochem Nutr. 2012;51:9–14. doi: 10.3164/jcbn.11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diffey BL. Sources and measurement of ultraviolet radiation. Methods. 2002;28:4–13. doi: 10.1016/S1046-2023(02)00204-9. [DOI] [PubMed] [Google Scholar]
- 53.Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42:321–4. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


