Abstract
Background
Brain neurodamage from chronic binge ethanol exposure is linked to neuroinflammation and associated oxidative stress. Using rat organotypic hippocampal-entorhinal cortical (HEC) slice cultures of developing brain age, we reported that binge ethanol promotes release of a neuroinflammatory instigator, arachidonic acid (AA), concomitant with neurodegeneration, and that mepacrine, a global inhibitor of phospholipase A2 (PLA2) enzymes mobilizing AA from phospholipids, is neuroprotective. Here we sought with binge ethanol-treated HEC cultures to establish that PLA2 activity is responsible in part for significant oxidative stress, and to ascertain the PLA2 families responsible for AA release and neurodegeneration.
Methods
HEC slices, prepared from one wk-old rats and cultured 2–2½ wks, were exposed to 100 mM ethanol over 6 successive days, with 4 daytime “withdrawals” (no ethanol). Brain 3-nitrotyrosinated (3-NT) and 4-hydroxynonenal (4-HNE)-adducted proteins, oxidative stress footprints, were immunoassayed on days 3 through 6, and mepacrine’s effect was determined on day 6. The effects of specific PLA2 inhibitors on neurodegeneration (propidium iodide staining) and AA release (ELISA levels in media) in the cultures were then determined. Also, the effect of JZL184, an inhibitor of monoacylglycerol lipase (MAGL) which is reported to mobilize AA from endocannabinoids during neuroinflammatory insults, was examined.
Results
3-NT- and 4-HNE-adducted proteins were significantly increased by the binge ethanol exposure, consistent with oxidative stress, and mepacrine prevented the increases. The PLA2 inhibitor results implicated secretory PLA2 (GII sPLA2) and to some extent Ca+2-independent PLA2 (GVI iPLA2) in binge ethanol-induced neurotoxicity and in AA release, but surprisingly, Ca+2-dependent PLA2 (GIV cPLA2) did not appear important. Furthermore, unlike PLA2 inhibition, MAGL inhibition failed to prevent the neurodegeneration.
Conclusions
In these developing HEC slice cultures, pro-oxidative signaling via sPLA2 and iPLA2, but not necessarily cPLA2 or MAGL, is involved in ethanol neurotoxicity. This study provides further insights into neuroinflammatory phospholipase signaling and oxidative stress underlying binge ethanol-induced neurodegeneration in developing (adolescent-age) brain in vitro.
Keywords: alcohol, arachidonic acid, organotypic, neuroinflammation, endocannabinoid, MAGL
Introduction
Acquired brain damage and varying degrees of dementia are often consequences of chronic ethanol dependence and alcoholism (Fein et al., 2002; Gupta and Warner, 2008). Animal and human studies now indicate that neuroinflammatory processes underlie aspects of brain damage (neurodegeneration) from chronic high ethanol levels, particularly in binge exposures (Alfonso-Loeches et al., 2010; Alikunju et al., 2011; Qin et al., 2008; Sullivan and Zahr, 2008; Tajuddin et al., 2013). A binge drinking pattern in ethanol-dependent individuals increases the risk of brain damage (Hunt, 1993); brain trauma (from falls, etc.), concomitant malnutrition, and perhaps severe stress might be important contributing factors in some cases.
Our experiments using repetitive binge ethanol treatments have focused on phospholipid-dependent neuroinflammatory pathways potentially triggered by brain edema and neurodamaging oxidative stress. Brain edema’s involvement is indicated by the facts that significant brain water elevations occur in chronic binge ethanol-intoxicated adult rats, and that a diuretic (furosemide), in preventing the edema, reduces neurodegeneration in hippocampal and entorhinal cortical regions (Collins et al., 1998). Antagonism of glutamate receptors affords negligible neuroprotection (Collins et al., 1998; Hamelink et al., 2005), indicating that excitotoxicity is not a key mechanism. Oxidative stress, a potential outcome of brain edema (Jayakumar et al., 2008), is implicated in the above rat binge intoxication model based on evidence that selected antioxidants provide neuroprotection (Crews et al., 2006; Hamelink et al., 2005). Similarly, in rat organotypic slice cultures comprising the above two vulnerable brain regions, chronic binge ethanol exposure causes significant edema and neuronal damage that are reduced by furosemide or unrelated diuretics such as acetazolamide (Collins et al., 1998; Sripathirathan et al., 2009).
When mobilized excessively from brain membrane phospholipids by stressors or insults, the essential omega-6 polyunsaturated fatty acid, arachidonic acid (AA), can promote oxidative stress and neurodegeneration through enzymatic and nonenzymatic routes (Sun et al., 2012). Physiological levels of free brain AA, typically less than 10 µM, increase ~50-fold in response to pathophysiological insult, e.g., severe ischemia (Rehncrona et al., 1982). A key AA-mobilizing enzyme activity, phospholipase A2 (PLA2), can be stimulated by cellular deformation, edema, and/or swelling (Basavappa et al., 1998; Lambert et al., 2006). In our experiments with organotypic hippocampal-entorhinal cortical (HEC) slice cultures in which chronic binge ethanol exposure causes edema, PLA2 blockade with mepacrine, a broad spectrum inhibitor, significantly antagonizes ethanol-induced neurodegeneration (Brown et al., 2009).
PLA2 gene products are composed of at least three families—notably, Ca+2-dependent cytosolic cPLA2 (cPLA2), Ca+2-independent cytosolic PLA2 (iPLA2), and secretory (also Ca+2-dependent) PLA2 (sPLA2)—that are expressed in brain (Sun et al., 2012). Multiple PLA2 isoforms or groups within these three families are implicated to varying extents in causal brain damage mechanisms distinct from ethanol, with the cPLA2 family frequently linked to neurodegeneration from insults such as ischemia or excitotoxicity. Also, the major brain endocannabinoid, monoarachidonoylglycerol, is a recently appreciated potential source of neuroinflammation-liberated AA via monoacylglycerol lipase (MAGL) (Nomura et al., 2011). We considered it tenable that MAGL activity might also contribute to binge ethanol-induced neurodegeneration.
As with earlier studies, these experiments utilized organotypic HEC slice cultures which retain the cytoarchitecture of intact (albeit developing, ~3–4 wks age) brain, and thus have unique advantages over mixed primary brain cultures. Moreover, unlike much more frequently employed slices of solely hippocampus, HEC slice cultures encompass two regions that are very susceptible to binge ethanol neurotoxicity (Collins et al., 1996), and retain functional perforant pathways (Del Turco and Deller, 2007) that might be important in hippocampal/cortical neuroinflammation. With these slice cultures we sought to confirm with inhibitors whether PLA2 is critical for oxidative stress due to binge ethanol exposure, and to determine the enzyme sources of AA involved in neuronal damage in the HEC complex.
Materials and Methods
Chemicals and supplies
PLA2 inhibitors were purchased from Sigma-Aldrich Company (St. Louis MO), with the exception of manoalide, which was from Biomol International (Plymouth Meeting PA), and JZL184 (4-nitrophenyl 4-(dibenzo[d] [1,3] dioxol-5-yl (hydroxy)methyl)piperidine-1-carboxylate) which was obtained from Cayman Chemicals (Ann Arbor MI). Anti-3-NT protein antibody was purchased from Abcam Company (Cambridge MA), anti-4-HNE adduct protein was from Millipore Corporation (Temecula CA), anti-GAPDH was from Santa Cruz Biotechnology (Santa Cruz CA), and IgG was from R & D systems (Minneapolis MN). An ELISA kit for AA was from MyBioSource (San Diego CA). Modified Eagles’ medium (MEM) media, Hanks’ buffer and horse serum were from Gibco Company (Gaitherburg MD). Tissue culture inserts and plastic ware were from Fisher Scientific Company (Pittsburgh PA).
Organotypic hippocampal-entorhinal cortical (HEC) slice cultures
Adult rats and rat pups were cared for according to guidelines published in the NIH Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23) and principles in the “Guidelines for the Use of Animals in Neuroscience Research” by the Society for Neuroscience. All protocols were approved by the LUMC Institutional Animal Care and Use Committee. Sprague-Dawley rat pups (7–8 days old; maternal source, Harlan, Indianapolis IN) were used to prepare static HEC slice cultures (Collins et al., 1998). Pups were cold-anesthetized and quickly decapitated. The HEC complex was removed and transverse slices (~350 µ), cut with a McIlwain tissue chopper, was placed on Millipore 0.4 µ Millicel tissue culture inserts in 6-well Falcon plates. They were cultured under covers in an atmosphere of 95%O2/5%CO2 at 37°C on MEM media containing 25% horse serum, 25% Hanks buffer, 20 mM HEPES and 6.5 mg/ml glucose. Each treatment group contained ≥6 wells initially containing 3–4 slices/well; media was changed every 2–3 days. Slices were periodically examined and those appearing unhealthy under the phase contrast microscope (darkened appearance) were discarded.
Binge ethanol and inhibitor treatments
After 2–2½ weeks of culture and maturation, healthy HEC slices were treated with ethanol at 100 mM—a neurotoxic level reported in chronic ethanol abusers and alcoholics (Adachi et al., 1991; Minion et al., 1989); controls received an equal volume of media. Ethanol-containing plates and control plates in respective covered Tupperware brand containers were placed in separate incubators. Slices were subjected to an initial 3-day ethanol (or control media) incubation exposure and a complete media change, followed by a 9 hr daytime withdrawal (no ethanol), and then three 15-hr ethanol (or control) overnight incubation exposures and three more daytime withdrawals, for a total of 4 ethanol exposures and 4 withdrawal episodes over 6 successive days (Brown et al., 2009; Sripathirathan et al., 2009). Containers with ethanol-treated slice cultures held a small open dish of 1.8% v/v ethanol/water to partially counter ethanol loss from media in culture wells; control containers had a similar dish with water only. Nonetheless, since initial media ethanol concentrations dropped ~15% (unpublished data) during each incubation period, concentrations of 100 mM were re-established at the start of each subsequent ethanol incubation. For inhibition experiments, PLA2 inhibitors (manoalide, 5 uM; ATA, 10 uM; MAFP,20 uM; AACOCF3, 10 uM; BEL, 5 uM) were each added at the same time as ethanol (co-treatment) and were continued throughout the 6 day ethanol + withdrawal period. Inhibitors were dissolved in dimethylsulfoxide if necessary and diluted appropriately into all incubation and withdrawal media throughout 6 days; control slices received changes with media only ± relevant dimethylsulfoxide dilutions. The media is expected to be hyperosmolar to varying degrees during the ethanol exposure, consistent with the plasma of alcoholics during intoxication (Purssell et al., 2001), but iso-osmolar during withdrawal periods.
Immunoblot assays for oxidative stress
At indicated times, HEC slices were rinsed twice with phosphate-buffered saline (PBS), pooled (6–9 slices), and lysed in an isotonic buffer (50mM Tris, 1mM EDTA, protease and phosphatase inhibitor cocktail, pH 7,4). After centrifugation, protein concentrations were acquired with a bicinchoninic acid–based method (Pierce Biotech., Rockford, IL); Aliquots (~10 µg protein) of supernatants were separated in the conventional manner on 12% SDS-PAGE, transferred to PVDF-immobilon membranes, and subjected to immunoblot analysis by using anti-3-NT or 4-HNE adduct protein antibodies. The intensities of immunoblots were normalized to GAPDH immunoblots, obtained on the same gels following stripping and reprobing, after scanning images with LABwork 4.5 image acquisition and analysis software from Ultra Violet Products (Upland CA).
Neurodegeneration in HEC slices using propidium iodide (PI)
Degenerating neurons were detected in HEC slices with the vital dye PI, added during the last hour of withdrawal incubation (5 µg PI/ml media). The use of PI to quantitatively stain dying neurons in organotypic brain slice cultures is well-documented (Prendergast et al., 2004; Sripathirathan et al., 2009). PI uptake in each slice was assessed by capturing 8–12 sec exposures with a DS-5M Nikon color camera attached to an Eclipse TS100 inverted microscope with an Epi-fluorescence attachment. Using Image J version 1.36 (NIH, Bethesda MD), the entire slice was outlined, and the percentage of the slice area that displayed red PI fluorescence was recorded and used in overall statistical analyses.
AA release
The AA ELISA kit is based on a monoclonal antibody to AA that has negligible cross reactivity or interference with AA metabolites. Media from control and ethanol-treated cultures with and without inhibitors from “withdrawal” days (days 3–6) were pooled, and aliquots were taken for the ELISA. Values were determined based on an appropriate standard curve and expressed as picograms/ml media.
Statistical analyses
Experiments were replicated 2–3 times and data were expressed as means ± SEM. The results were analyzed by ANOVA followed by multiple comparisons using the “R” open source statistical software (http://www.r-project.org) and its multcomp procedure to do individual pairwise comparisons giving multiplicity-adjusted p-values (Hothorn et al., 2008).
Results
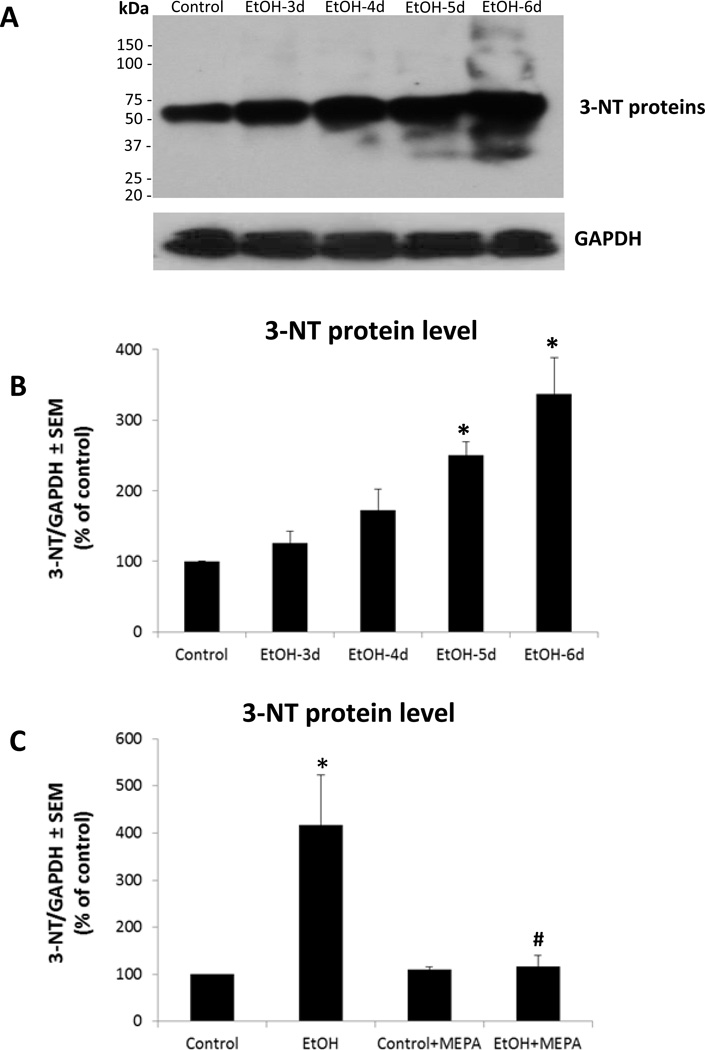
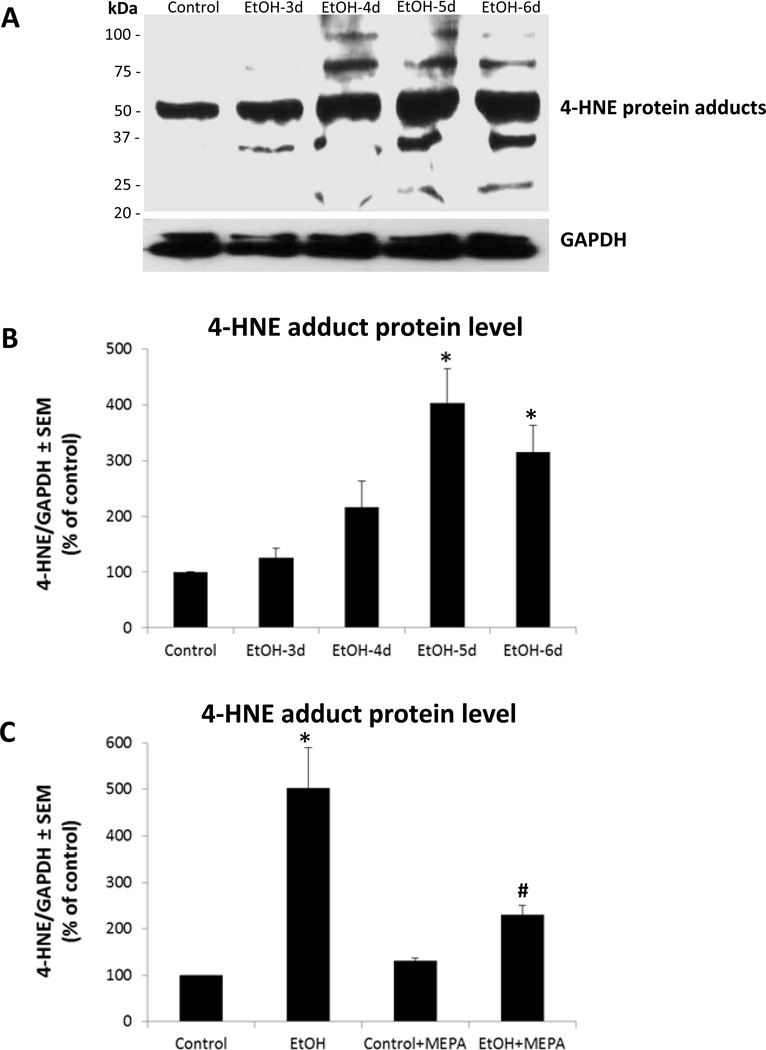
As reported (Brown et al., 2009), co-treatment of developing HEC slice cultures with a broad spectrum PLA2 inhibitor, mepacrine, prevented chronic binge ethanol-induced AA release relatively early and suppressed later degeneration of hippocampal dentate granule and pyramidal cortical neurons. To verify that tissue oxidative stress is substantial and is linked to PLA2 activity during the binge ethanol exposure timecourse, neuroinflammatory oxidative stress indicators of 3-nitrotyrosinated (3-NT) and 4-hydroxynonenal (4-HNE)-adducted proteins were determined in HEC slices with and without mepacrine. In Figure 1A are representative immunoblots of 3-NT proteins between 3 to 6 days of binge ethanol exposure, a treatment causing neurodamage (Brown et al., 2009). Control slices showed 3NT-proteins, reflecting basal oxidative stress. Quantitation of 3-NT protein blots (Figure 1B) confirmed ethanol-dependent increases above controls at the 5- and 6-day timepoints in proteins from 30 to 160 kDa, but particularly those in the range of 55 kDa, which likely constitutes a number of tyrosine-nitrosylated proteins based on 2-D gel studies (Moon et al., 2006). Mepacrine co-treatment did not alter control 3-NT protein levels, suggesting that basal oxidative stress in vitro was not PLA2 activity-derived, but it abolished binge ethanol-induced 6-day increases (Figure 1C; EtOH + MEPA). In Figure 2A are representative immunoblots for 4-HNE-adducted proteins, and controls contained basal levels of adducts (Figure 2B). The 4-HNE adducts in ethanol-treated cultures, ranging between 35 kDa and 95 kDa and concentrated at ~50 kDa, were significantly elevated by 5–6 days of binge exposure, as with 3-NT proteins. Mepacrine co-treatment blocked ethanol’s potentiation of 4-HNE-adducted proteins while not affecting control levels (Figure 2C; EtOH + MEPA), again indicating that basal oxidative stress did not arise from PLA2 activity.
Figure 1. Binge ethanol exposure significantly increases 3-nitrotyrosinated (3-NT)-protein levels in organotypic HEC slice cultures, and PLA2 inhibition by mepacrine prevents the increase.
A. Representative immunoblots of 3-NT-proteins and GAPDH in HEC slice cultures during the daytime withdrawal periods following overnight binge treatment with ethanol (100 mM) for 3, 4, 5 or 6 successive days. B. 3-NT-proteins as represented in immunoblots in A are significantly increased after binge ethanol exposure (100 mM) for 5 or 6 days (n=3; 6–9 slices/grp). *p<0.05 vs. Control. C. Mepacrine (MEPA, 1 µM) co-treatment abolishes the increase in 3-NT-proteins after 6 days of binge treatment with 100 mM ethanol (n=3; 6–9 slices/grp). *p<0.05 vs. Control.
Figure 2. Binge ethanol exposure significantly increases 4-hydroxy-2-nonenal (HNE)-protein adduct levels in organotypic HEC slice cultures, and PLA2 inhibitor mepacrine abolishes the increase.
A. Representative immunoblots of 4-HNE-proteins and GAPDH in HEC slice cultures during the daytime withdrawal periods following overnight binge ethanol exposure (100 mM) for 3, 4, 5 and 6 successive days. B. 4-HNE-proteins as represented in immunoblots in A were significantly increased after binge ethanol exposure (100 mM) for 5 and 6 days (n=3; 6–9 slices/grp). *p<0.05 vs. Control. C. Mepacrine (MEPA, 1 µM) co-treatment abolished the increase in 4-HNE-adducted proteins after 6 days of binge treatment with 100 mM ethanol (n=3; 6–9 slices/grp). *p<0.05 vs. Control.
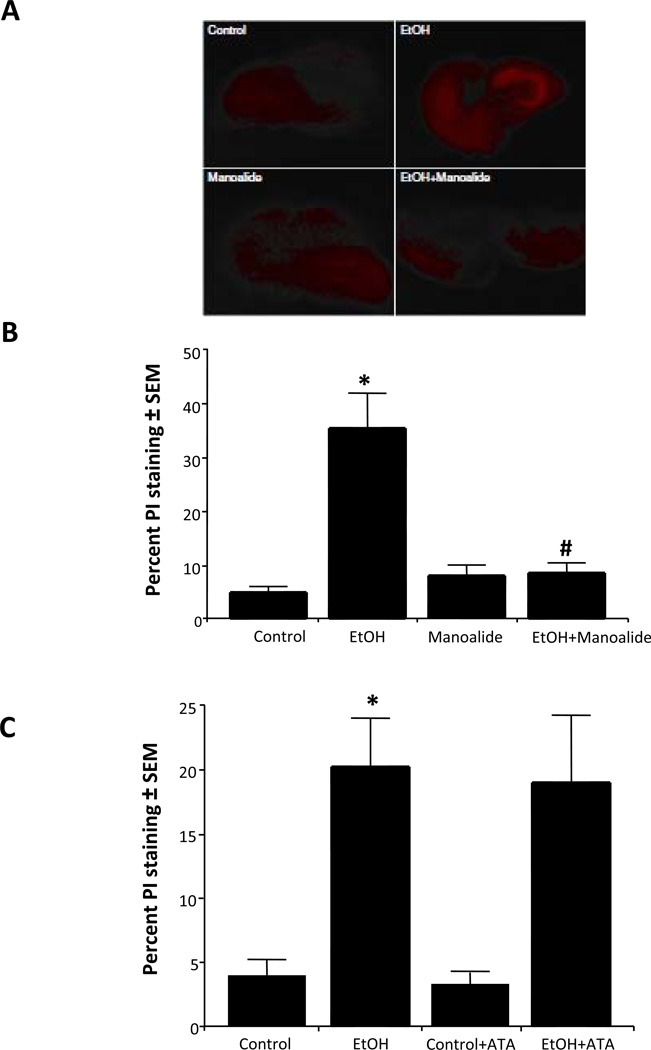
To address what phospholipase enzyme activities have roles in binge ethanol-induced neurodegeneration in these developing HEC cultures, relatively specific inhibitors were used. Whether sPLA2 activity is involved was examined with manoalide, which irreversibly inhibits most sPLA2 isoforms (Lombardo and Dennis, 1985). Representative photomicrograph images of PI staining of HEC slices (Figure 3A) indicated extensive neurodegeneration due to binge exposure at 100 mM ethanol (EtOH) in entorhinal cortical and hippocampal regions relative to control slice regions. Co-treatment with 5 µM manoalide (EtOH + Manoalide) largely suppressed the ethanol-induced PI fluorescence. Quantitation of slice fluorescence in Figure 3B showed that robust neurodegeneration due to binge ethanol was almost completely abolished by manoalide (suppressed relative to Mano alone), indicating that sPLA2 activity has an important role. However, as shown in Figure 3C, aristolochic acid (ATA, 10 µM), an inhibitor of groups I+IIA-B sPLA2 isoforms (Moreno, 1993), was ineffective, suggesting that sPLA2 activities in groups other than I and IIA-B are responsible in these developing brain slice cultures.
Figure 3. Effect of co-treatment with manoalide or aristolochic acid (ATA) on binge ethanol-induced neurodegeneration in rat developing HEC slice cultures.
A. Representative photomicrographs of PI staining in representative HEC slices indicating neuroprotection by manoalide: control, binge ethanol (EtOH, 100 mM), manoalide (5 µM)-treated, and EtOH+Manoalide. B. Quantitation of PI staining shows that chronic binge ethanol exposure (EtOH) caused increased neurodegeneration that was completely prevented by Manoalide (EtOH + Manoalide, 5 µM). n=6, *p<0.05 vs. Control. #p<0.05 vs. EtOH. C. Chronic binge ethanol exposure caused increased PI staining (EtOH) that was not reduced by the co-presence of 10 µM ATA (EtOH + ATA). n=6, *p<0.05 vs. Control or ATA.
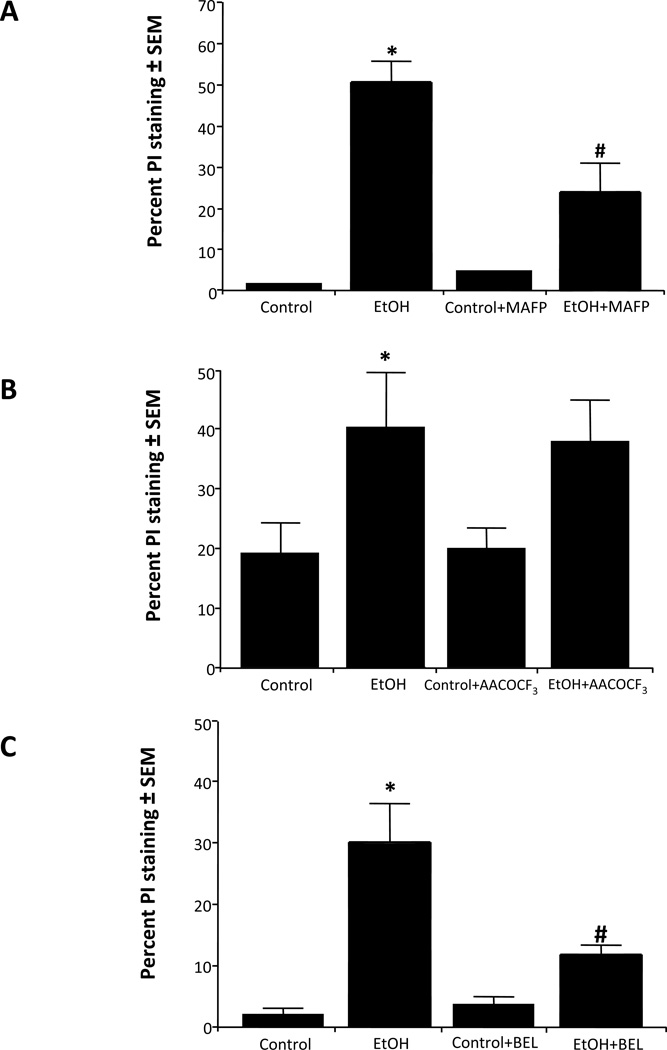
In Figure 4A, the percent PI-labeled neurons was determined in binge ethanol-treated HEC slice cultures co-treated with methyl arachidonyl fluorophosphonate (MAFP), an irreversible inhibitor of iPLA2 and cPLA2 families (Lio et al., 1996). Quantitation of PI staining confirmed increased neurodegeneration due to binge ethanol, which was reduced ~60% by MAFP (20 µM); a lower MAFP concentration (1 µM) was ineffective (not shown). To examine more specifically the involvement of IVA/B (85 kD) cPLA2 enzymes, HEC slice cultures with and without ethanol exposure were treated with the selective cPLA2 inhibitor, arachidonyl trifluoromethylketone (AACOCF3) (Street et al., 1993). Figure 4B shows that AACOCF3 (10 µM) failed to inhibit or suppress neurodegeneration; also, a lower AACOCF3 concentration (1 µM) was not protective (not shown). Group VI iPLA2 was then examined with (±) bromoenol lactone (BEL, 5 µM), an irreversible suicide inhibitor of this enzyme class (Ackermann et al., 1995). As shown (Figure 4C), (±) BEL reduced neurodegeneration by ~50%, indicating that the iPLA2 family is likely to be important, in contrast to cPLA2, in binge ethanol’s neuronal damaging mechanism in these adolescent-age HEC slices.
Figure 4. Effect of co-treatment with methyl arachidonyl fluorophosphonate (MAFP), arachidonyl-trifluoromethylketone (AACOCF3) or bromoenol lactone (± BEL) on binge ethanol-induced neurodegeneration in rat developing HEC slice cultures.
A. Chronic binge ethanol exposure (EtOH, 100 mM) for 6 days caused increased PI staining that was significantly reduced by 20 µM MAFP (EtOH + MAFP). n=6, *p<0.05 vs. Control or MAFP. #p<0.05 vs. EtOH. B. Chronic binge ethanol exposure (EtOH, 100 mM) caused increased PI staining that was not reduced by 10 µM AACOCF3 (AACOCF3 + EtOH). n=6, *p<0.05 vs. Control or AACOCF3. C. Chronic binge ethanol exposure (EtOH, 100 mM) caused increased PI staining that was significantly reduced by 5 µM BEL (EtOH + BEL). n=6, *p<0.05 vs. Control or BEL. # p<0.05 vs. EtOH.
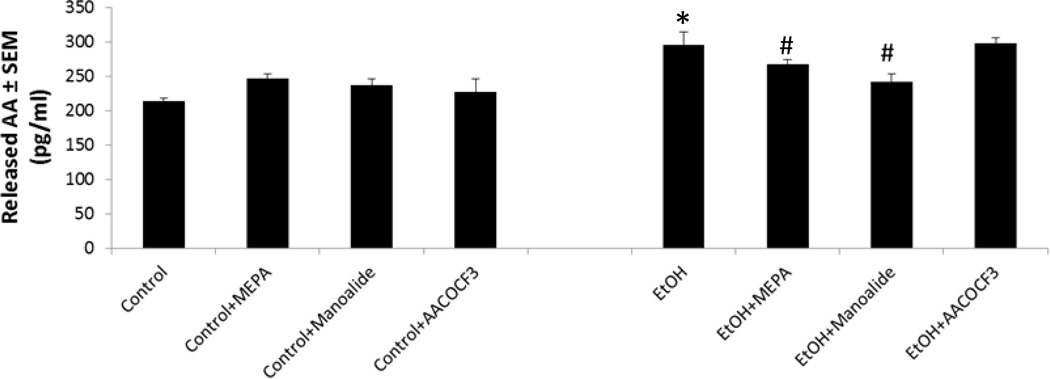
To better understand why inhibitors of PLA2 generally and sPLA2 specifically are neuroprotective while cPLA2 inhibition is not, the effects of mepacrine, manoalide, or AACOCF3 on levels of AA in withdrawal media during binge ethanol exposure, which indicates AA release, were examined (Figure 5). As shown, the above inhibitors alone did not affect AA levels in withdrawal media. Concurring with our previous experiments using 3H-AA release (Brown et al., 2009), binge ethanol+withdrawal significantly increased media AA levels above control values. Mepacrine and manoalide co-treatments modestly but significantly reduced ethanol-dependent media AA levels, reflecting inhibition of release—which corresponds with their respective neuroprotective effects. However, AACOCF3 did not significantly change ethanol-induced AA release, consistent with the inhibitor’s lack of neuroprotection, and further implying that cPLA2 is not critical to AA mobilization and neurodegeneration in the ethanol-exposed developing HEC slices.
Fig. 5. Effects of selected PLA2 inhibitors on media levels (release) of arachidonic acid (AA) in binge ethanol-exposed adolescent-age HEC slice cultures.
Binge ethanol exposure (100 mM) for 6 days significantly increased levels of AA in media pooled from the four withdrawal episodes (EtOH, *p<0.05 vs. Control; n = 3). Mepacrine (1 µM) significantly reduced EtOH withdrawal media AA levels (EtOH+MEPA vs. EtOH, #p<0.05). Manoalide (5 µM) significantly lowered EtOH withdrawal media AA levels (EtOH+Manoalide vs. EtOH, #p<0.05). AACOCF3 (10 µM) had no effect on withdrawal media AA levels (EtOH+AACOCF3 vs. EtOH, n.s.).
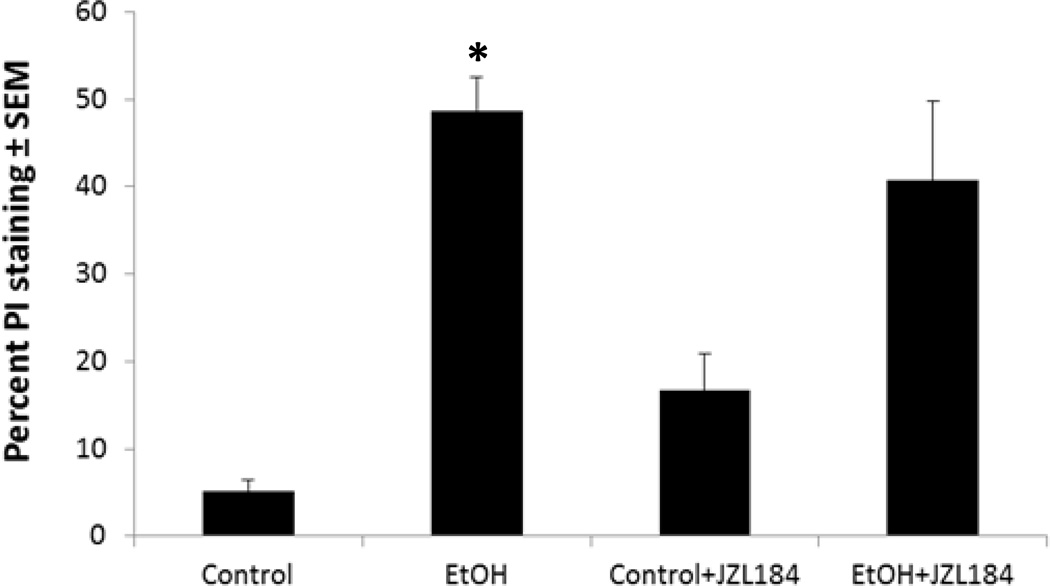
The possibility that an alternate (non-PLA2) AA-mobilizing pathway, the augmented activity of MAGL, is involved in binge ethanol-induced neurodegeneration was examined with the specific MAGL inhibitor, JZL184. The experiments (Figure 6) showed that the extent of PI labeling after binge ethanol treatment was not significantly reduced by co-treatment with 50 nM (not shown) or 1 µM JZL184 (concentration range that prevents endotoxin-induced AA-derived eicosanoid production (Nomura et al., 2011)). The results thus argue against MAGL activity as a neuroinflammatory source of AA in this binge ethanol-treated organotypic brain slice model.
Fig. 6. Effect of monoacylglycerol lipase (MAGL) inhibitor JZL184 on binge ethanol-induced neurodegeneration in adolescent-age HEC slice cultures.
Chronic binge ethanol exposure (EtOH, 100 mM) for 6 days caused increased PI staining that was not significantly reduced by 1 µM JZL184 (EtOH + JZL184). n=6, *p<0.05 vs. Control or JZL184.
Discussion
These experiments were predicated on the idea that neuroinflammatory AA mobilization plays a critical role in oxidative stress and neurodegeneration caused by repetitive binge ethanol exposure. Previously we reported that global inhibition of PLA2 activities with mepacrine during binge ethanol treatment of adolescent-age HEC slice cultures prevented neurodegeneration (Brown et al., 2009). From experiments with added antioxidants, oxidative stress has been linked in vivo to binge ethanol-induced neurodegeneration (Collins and Neafsey, 2012); however, it is not clear whether PLA2 contributes significantly to increased oxidative stress in the organotypic slice model. In dispersed brain cultures 3-NT and 4-HNE protein adducts are indicative of ethanol-dependent, oxidative/peroxidative stress-induced damage (“inflammatory footprints”) to proteins and ω-6 polyunsaturated membrane lipids (e.g., AA) (Alikunju et al., 2011). Interestingly, assays of these fingerprint proteins in organotypic slice cultures appear to be lacking, with the exception of 3-NT protein assays in a parkinsonian neurotoxin model (Larsen et al., 2008).
Binge ethanol treatment significantly increased the oxidative protein footprints concomitant with HEC slice neurodeneration (5–6 days). Results with mepacrine verified that PLA2 activity was responsible, at least in part, for binge ethanol-induced generation of oxidative stress and resulting protein/lipid oxidation. However, it should be emphasized that other ROS-generating pathways stimulated by ethanol, e.g., NADPH oxidase, are likely to have roles, as others have found (Alikunju et al., 2011; Qin and Crews, 2012b).
The inhibitory results by PLA2 inhibitors indicated that an atypical combination of sPLA2 and iPLA2 is upstream of neurodegeneration in developing HEC slices repetitively binge-exposed to ethanol. These activities can give rise to AA and its eicosanoid products—notably, prostaglandins and leukotrienes that might be deleterious 2nd (or 3rd) messengers (Sun et al., 2012). Cyclooxygenase (COX) and its prostaglandin products receive much attention in the neuroinflammatory literature, and inducible COX-2 is increased in chronic ethanol-binged mice (Qin et al., 2008). However, the intermediacy of leukotrienes via multiple lipoxygenase (LOX) activities is unexplored with regard to ethanol’s neuropathological effects. Indeed, LOX activity is the most prominent source of oxidative stress in neuronal cultures under apoptotic conditions such as high KCl (Bobba et al., 2008). In addition, when directly added to neuronal cultures, AA exerts pro-cytotoxic, apoptotic, necrotic and/or excitatory effects (Chen and Chang, 2009). Mechanistically, AA may induce neuronal apoptosis via cytosolic cationic overload (Fang et al., 2008). In hepatoma or liver cells, AA causes apoptosis via cytochrome P450-mediated metabolism (Caro and Cederbaum, 2006), and loss of mitochondrial transmembrane potential (MTP) (Scorrano et al., 2001); in fibroblasts, the omega-6 fatty acid was the most damaging among unsaturated fatty acids tested with regard to MTP (Maia et al., 2006).
The most effective neuroprotection was observed with manoalide. While this supports the involvement of sPLA2, neuroprotection could also involve suppression of NADPH oxidase activity already mentioned, because such an effect was reported in stressed non-neuronal cultures to be downstream of manoalide/PLA2 inhibition (de Carvalho et al., 2008). Nevertheless, the fact that manoalide significantly counters ethanol-induced AA media elevations is consistent with a key role for sPLA2. The observed partial protection with MAFP, a dual iPLA2/cPLA2 inhibitor, we consider to be mainly iPLA2-related, since BEL, a specific suicide inhibitor of iPLA2, was similarly effective, while the selective cPLA2 inhibitor, AACOCF3, provided no protection. Reports suggest that the iPLA2 family has a homeostatic or housekeeping role in membrane phospholipid turnover (Winstead et al., 2000), but iPLA2 activation in brain mitochondria has been linked to pro-apoptotic signaling (Brustovetsky et al., 2005) including that initiated by Fas receptors (Atsumi et al., 1998). In contrast, whereas cPLA2 family isoforms play important roles in AA mobilization and downstream neuroinflammation in neurodegenerative conditions other than ethanol (Sun et al., 2012), in these ethanol-treated developing rat brain slices the cPLA2 family does not appear important for neurodegeneration. This conclusion is further supported by the failure of the cPLA2 inhibitor AACOCF3 to suppress binge ethanol’s augmentation of AA media levels (Figure 5) in the HEC slices.
Concerning sPLA2 family members, evaluations of ischemic or excitotoxic neurodegeneration mechanisms have frequently implicated these small phospholipases in brain necrosis and/or apoptosis. In certain circumstances sPLA2 groups I and II are particularly involved (Kolko et al., 2003), but in our binge ethanol model, an inhibitor of group I/IIA-B, ATA, was ineffective. However, at least six sPLA2 groups are expressed in the brain, and sPLA2 group III (DeCoster, 2003) and perhaps others such as sPLA2 groups IIF and V, which are induced in rodent brain by inflammatory endotoxin (Hamaguchi et al., 2003), could be responding to chronic binge ethanol exposure with upregulation/secretion. In view of evidence that ethanol can alter brain levels of endotoxin-linked toll-like receptors and their endogenous brain protein agonists (Alfonso-Loeches et al., 2010; Qin and Crews, 2012a), downstream activation of these sPLA2’s via neuroimmune pathways is an attractive possibility.
A recent finding on AA mobilization is through a non-PLA2 route during exposure to neuroinflammatory stressors such as endotoxin—i.e., endogenous cannabinoid hydrolysis involving MAGL activity on brain 2-monoarachidonoylglycerol (Nomura et al., 2011). We used MAGL inhibitor JZL184 to test whether binge ethanol-induced neurodegeneration involved this AA route, in addition to PLA2 pathways. Although relatively specific for MAGL, this inhibitor displays some cross-reactivity with fatty acid amide hydrolase (Chang et al., 2012), the enzyme releasing AA from the second key brain endocannabinoid, anandamide. Interestingly, if significant suppression of neurodegeneration with JZL184 had occurred, it could also have been explained by neuroprotective elevations in the two above-mentioned endocannabinoids, and not just by reduced AA mobilization. At any rate, results with JZL inhibitor provided no evidence that MAGL activity was involved in binge ethanol-induced neurodegeneration in this developing brain slice model.
Reiterating, these inhibition studies indicate that excessive liberation of AA by sPLA2 and iPLA2 is important in neuropathological signaling induced by chronic binge ethanol treatment of developing brain in vitro, whereas cPLA2 has little or no role. The findings are unusual, because as previously mentioned, in other degenerative or inflammatory insults, cPLA2 often is a critical enzymatic activity mobilizing AA, either alone or in combination with sPLA2 family members. Also, the finding is unexpected because brain edema, which is triggered by repeated binges of ethanol and is potentially upstream of PLA2 activation, is viewed as largely astrocytic—based on histochemical indications, on the increased expression of the astroglial-enriched water channel, aquaporin-4 (Sripathirathan et al., 2009), and on evidence that furosemide largely blocks astrocytic swelling (Hochman et al., 1995). Indeed, group IV cPLA2 isoforms are known to be activated as a consequence of brain cell swelling (Basavappa et al., 1998), and chronic ethanol treatment in mice is reported to increase brain cPLA2 activity (Basavarajappa et al., 1998); in addition, cPLA2 is reported to be increased in chronic ethanol-treated astrocytes (Floreani et al., 2010).
Furthermore, the PLA2 inhibitor findings in these developing HEC slices—possible neurotoxic role for iPLA2 but none for cPLA2 resulting from binge ethanol exposure—are inconsistent with our quantitative immunoblot results from binge ethanol-exposed adult rats (Tajuddin et al., 2013). In that study, binge-pattern intoxicated adult rats showed depleted iPLA2 levels in concert with significant elevations in cPLA2 and (activated) phospho-cPLA2 in hippocampal and entorhinal cortical tissues. It is perhaps relevant that reduction of iPLA2 levels agrees with studies that have found brain iPLA2 depletion/inactivation, as opposed to elevations, during neurotoxic insults in adult rodents (Wilkins and Barbour, 2008).
At this juncture it appears that binge ethanol exposure in developing adolescent-age rat brain (i.e., organotypic slices in culture) stimulates neuroinflammatory PLA2 isoform activation pathways that may differ from those of PLA2 families in brain of similarly-exposed adult rats. Developmentally, rat brain levels of cPLA2 are unchanged between neonatal and adult ages (Yoshihara et al., 1992), so further research on ethanol-induced signaling differences among PLA2 families is needed to understand the respective mechanisms. Integrating in vitro results with adult in vivo studies should permit elucidation of the PLA2 families and groups that are important in neuroinflammatory oxidative signaling mechanisms triggered by chronic binge ethanol abuse.
Acknowledgements
This research was supported by the National Institutes of Health National Institute of Alcohol Abuse and Alcoholism [Grants U01 AA018279, T32 AA13527, R21 AA011543]; and the Loyola University Potts Foundation.
References
- Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of macrophage Ca(2+)-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J Biol Chem. 1995;270:445–450. doi: 10.1074/jbc.270.1.445. [DOI] [PubMed] [Google Scholar]
- Adachi J, Mizoi Y, Fukunaga T, Ogawa Y, Ueno Y, Imamichi H. Degrees of alcohol intoxication in 117 hospitalized cases. J Stud Alcohol. 1991;52:448–453. doi: 10.15288/jsa.1991.52.448. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alikunju S, Abdul Muneer PM, Zhang Y, Szlachetka AM, Haorah J. The inflammatory footprints of alcohol-induced oxidative damage in neurovascular components. Brain Behav Immun. 2011;25:S129–S136. doi: 10.1016/j.bbi.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsumi G, Tajima M, Hadano A, Nakatani Y, Murakami M, Kudo I. Fas-induced arachidonic acid release is mediated by Ca2+-independent phospholipase A2 but not cytosolic phospholipase A2, which undergoes proteolytic inactivation. J Biol Chem. 1998;273:13870–13877. doi: 10.1074/jbc.273.22.13870. [DOI] [PubMed] [Google Scholar]
- Basavappa S, Pedersen SF, Jorgensen NK, Ellory JC, Hoffmann EK. Swelling-induced arachidonic acid release via the 85-kDa cPLA2 in human neuroblastoma cells. J Neurophysiol. 1998;79:1441–1449. doi: 10.1152/jn.1998.79.3.1441. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Cooper TB, Hungund BL. Effect of chronic ethanol exposure on mouse brain arachidonic acid specific phospholipase A2. Biochem Pharmacol. 1998;55:515–521. doi: 10.1016/s0006-2952(97)00501-7. [DOI] [PubMed] [Google Scholar]
- Bobba A, Atlante A, Petragallo VA, Marra E. Different sources of reactive oxygen species contribute to low potassium-induced apoptosis in cerebellar granule cells. Int J Mol Med. 2008;21:737–745. [PubMed] [Google Scholar]
- Brown J, 3rd, Achille N, Neafsey EJ, Collins MA. Binge ethanol-induced neurodegeneration in rat organotypic brain slice cultures: effects of PLA2 inhibitor mepacrine and docosahexaenoic acid (DHA) Neurochem Res. 2009;34:260–267. doi: 10.1007/s11064-008-9765-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustovetsky T, Antonsson B, Jemmerson R, Dubinsky JM, Brustovetsky N. Activation of calcium-independent phospholipase A (iPLA) in brain mitochondria and release of apoptogenic factors by BAX and truncated BID. J Neurochem. 2005;94:980–994. doi: 10.1111/j.1471-4159.2005.03248.x. [DOI] [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. Role of cytochrome P450 in phospholipase A2- and arachidonic acid-mediated cytotoxicity. Free Radic Biol Med. 2006;40:364–375. doi: 10.1016/j.freeradbiomed.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Chang JW, Niphakis MJ, Lum KM, Cognetta AB3rd, Wang C, Matthews ML, Niessen S, Buczynski MW, Parsons LH, Cravatt BF. Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates. Chem Biol. 2012;19:579–588. doi: 10.1016/j.chembiol.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KC, Chang LS. Arachidonic acid-induced apoptosis of human neuroblastoma SK-N-SH cells is mediated through mitochondrial alteration elicited by ROS and Ca(2+)-evoked activation of p38alpha MAPK and JNK1. Toxicology. 2009;262:199–206. doi: 10.1016/j.tox.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Collins MA, Corso TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcohol Clin Exp Res. 1996;20:284–292. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ. Ethanol and adult CNS neurodamage: oxidative stress, but possibly not excitotoxicity. Front Biosci (Elite Ed) 2012;4:1358–1367. doi: 10.2741/465. [DOI] [PubMed] [Google Scholar]
- Collins MA, Zou JY, Neafsey EJ. Brain damage due to episodic alcohol exposure in vivo and in vitro: furosemide neuroprotection implicates edema-based mechanism. FASEB J. 1998;12:221–230. doi: 10.1096/fasebj.12.2.221. [DOI] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, Zou J. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcohol Clin Exp Res. 2006;30:1938–1949. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- de Carvalho DD, Sadok A, Bourgarel-Rey V, Gattacceca F, Penel C, Lehmann M, Kovacic H. Nox1 downstream of 12-lipoxygenase controls cell proliferation but not cell spreading of colon cancer cells. Int J Cancer. 2008;122:1757–1764. doi: 10.1002/ijc.23300. [DOI] [PubMed] [Google Scholar]
- DeCoster MA. Group III secreted phospholipase A2 causes apoptosis in rat primary cortical neuronal cultures. Brain Res. 2003;988:20–28. doi: 10.1016/s0006-8993(03)03326-2. [DOI] [PubMed] [Google Scholar]
- Del Turco D, Deller T. Organotypic entorhino-hippocampal slice cultures--a tool to study the molecular and cellular regulation of axonal regeneration and collateral sprouting in vitro. Methods Mol Biol. 2007;399:55–66. doi: 10.1007/978-1-59745-504-6_5. [DOI] [PubMed] [Google Scholar]
- Fang KM, Chang WL, Wang SM, Su MJ, Wu ML. Arachidonic acid induces both Na+ and Ca2+ entry resulting in apoptosis. J Neurochem. 2008;104:1177–1189. doi: 10.1111/j.1471-4159.2007.05022.x. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- Floreani NA, Rump TJ, Abdul Muneer PM, Alikunju S, Morsey BM, Brodie MR, Persidsky Y, Haorah J. Alcohol-induced interactive phosphorylation of Src and toll-like receptor regulates the secretion of inflammatory mediators by human astrocytes. J Neuroimmune Pharmacol. 2010;5:533–545. doi: 10.1007/s11481-010-9213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Warner J. Alcohol-related dementia: a 21st-century silent epidemic? Br J Psychiatry. 2008;193:351–353. doi: 10.1192/bjp.bp.108.051425. [DOI] [PubMed] [Google Scholar]
- Hamaguchi K, Kuwata H, Yoshihara K, Masuda S, Shimbara S, Oh-ishi S, Murakami M, Kudo I. Induction of distinct sets of secretory phospholipase A(2) in rodents during inflammation. Biochimica Biophys Acta. 2003;1635:37–47. doi: 10.1016/j.bbalip.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J Pharmacol Exp Ther. 2005;314:780–788. doi: 10.1124/jpet.105.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman DW, Baraban SC, Owens JW, Schwartzkroin PA. Dissociation of synchronization and excitability in furosemide blockade of epileptiform activity. Science. 1995;270:99–102. doi: 10.1126/science.270.5233.99. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- Hunt WA. Are binge drinkers more at risk of developing brain damage? Alcohol. 1993;10:559–561. doi: 10.1016/0741-8329(93)90083-z. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Rao KV, Panickar KS, Moriyama M, Reddy PV, Norenberg MD. Trauma-induced cell swelling in cultured astrocytes. J Neuropathol Exp Neurol. 2008;67:417–427. doi: 10.1097/NEN.0b013e31816fc9d4. [DOI] [PubMed] [Google Scholar]
- Kolko M, Rodriguez de Turco EB, Diemer NH, Bazan NG. Neuronal damage by secretory phospholipase A2: modulation by cytosolic phospholipase A2, platelet-activating factor, and cyclooxygenase-2 in neuronal cells in culture. Neurosci Lett. 2003;338:164–168. doi: 10.1016/s0304-3940(02)01385-x. [DOI] [PubMed] [Google Scholar]
- Lambert IH, Pedersen SF, Poulsen KA. Activation of PLA2 isoforms by cell swelling and ischaemia/hypoxia. Acta Physiol (Oxf) 2006;187:75–85. doi: 10.1111/j.1748-1716.2006.01557.x. [DOI] [PubMed] [Google Scholar]
- Larsen TR, Soderling AS, Caidahl K, Roepstorff P, Gramsbergen JB, Larsen TR, Soderling A-S, Caidahl K, Roepstorff P, Gramsbergen JB. Nitration of soluble proteins in organotypic culture models of Parkinson's disease. Neurochem Int. 2008;52:487–494. doi: 10.1016/j.neuint.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Lio YC, Reynolds LJ, Balsinde J, Dennis EA. Irreversible inhibition of Ca(2+)-independent phospholipase A2 by methyl arachidonyl fluorophosphonate. Biochim Biophys Acta. 1996;1302:55–60. doi: 10.1016/0005-2760(96)00002-1. [DOI] [PubMed] [Google Scholar]
- Lombardo D, Dennis EA. Cobra venom phospholipase A2 inhibition by manoalide. A novel type of phospholipase inhibitor. J Biol Chem. 1985;260:7234–7240. [PubMed] [Google Scholar]
- Maia RC, Culver CA, Laster SM, Maia RC, Culver CA, Laster SM. Evidence against calcium as a mediator of mitochondrial dysfunction during apoptosis induced by arachidonic acid and other free fatty acids. J Immunol. 2006;177:6398–6404. doi: 10.4049/jimmunol.177.9.6398. [DOI] [PubMed] [Google Scholar]
- Minion GE, Slovis CM, Boutiette L. Severe alcohol intoxication: a study of 204 consecutive patients. J Toxicol Clin Toxicol. 1989;27:375–384. doi: 10.3109/15563658909000358. [DOI] [PubMed] [Google Scholar]
- Moon KH, Hood BL, Kim BJ, Hardwick JP, Conrads TP, Veenstra TD, Song BJ. Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of rats. Hepatology. 2006;44:1218–1230. doi: 10.1002/hep.21372. [DOI] [PubMed] [Google Scholar]
- Moreno JJ. Effect of aristolochic acid on arachidonic acid cascade and in vivo models of inflammation. Immunopharmacology. 1993;26:1–9. doi: 10.1016/0162-3109(93)90061-t. [DOI] [PubMed] [Google Scholar]
- Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MC, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast MA, Harris BR, Mullholland PJ, Blanchard JA, 2nd, Gibson DA, Holley RC, Littleton JM. Hippocampal CA1 region neurodegeneration produced by ethanol withdrawal requires activation of intrinsic polysynaptic hippocampal pathways and function of N-methyl-D-aspartate receptors. Neuroscience. 2004;124:869–877. doi: 10.1016/j.neuroscience.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Purssell RA, Pudek M, Brubacher J, Abu-Laban RB. Derivation and validation of a formula to calculate the contribution of ethanol to the osmolal gap. Ann Emerg Med. 2001;38:653–659. doi: 10.1067/mem.2001.119455. [DOI] [PubMed] [Google Scholar]
- Qin L, Crews FT. Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. J Neuroinflammation. 2012a;9:130. doi: 10.1186/1742-2094-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Crews FT. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J Neuroinflammation. 2012b;9:5. doi: 10.1186/1742-2094-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehncrona S, Westerberg E, Akesson B, Siesjo BK. Brain cortical fatty acids and phospholipids during and following complete and severe incomplete ischemia. J Neurochem. 1982;38:84–93. doi: 10.1111/j.1471-4159.1982.tb10857.x. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Penzo D, Petronilli V, Pagano F, Bernardi P. Arachidonic acid causes cell death through the mitochondrial permeability transition. Implications for tumor necrosis factor-alpha apoptotic signaling. J Biol Chem. 2001;276:12035–12040. doi: 10.1074/jbc.M010603200. [DOI] [PubMed] [Google Scholar]
- Sripathirathan K, Brown J, Neafsey EJ, Collins MA. Linking binge alcohol-induced neurodamage to brain edema and potential aquaporin-4 upregulation: evidence in rat organotypic brain slice cultures and in vivo. J Neurotrauma. 2009;26:261–273. doi: 10.1089/neu.2008.0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street IP, Lin H-K, Laliberte F, Ghomashchi F, Wang Z, Perrier H, Tremblay NM, Huang Z, Weech PK, Gelb MH. Slow- and Tight-Binding Inhibitors of the 85-kDa Human Phospholipase A2. Biochemistry. 1993;32:5935–5940. doi: 10.1021/bi00074a003. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Zahr NM. Neuroinflammation as a neurotoxic mechanism in alcoholism: commentary on “Increased MCP-1 and microglia in various regions of human alcoholic brain”. Exp Neurol. 2008;213:10–17. doi: 10.1016/j.expneurol.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GY, He Y, Chuang DY, Lee JC, Gu Z, Simonyi A, Sun AY. Integrating cytosolic phospholipase A2 with oxidative/nitrosative signaling pathways in neurons: a novel therapeutic strategy for AD. Mol Neurobiol. 2012;46:85–95. doi: 10.1007/s12035-012-8261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajuddin N, Przybycien-Szymanska MM, Pak TR, Neafsey EJ, Collins MA. Effect of repetitive daily ethanol intoxication on adult rat brain: Significant changes in phospholipase A2 enzyme levels in association with increased PARP-1 indicate neuroinflammatory pathway activation. Alcohol. 2013;47:39–45. doi: 10.1016/j.alcohol.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins WP3rd, Barbour SE. Group VI phospholipases A2: homeostatic phospholipases with significant potential as targets for novel therapeutics. Curr Drug Targets. 2008;9:683–697. doi: 10.2174/138945008785132385. [DOI] [PubMed] [Google Scholar]
- Winstead MV, Balsinde J, Dennis EA. Calcium-independent phospholipase A(2): structure and function. Biochim Biophys Acta. 2000;1488:28–39. doi: 10.1016/s1388-1981(00)00107-4. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, Yamaji M, Kawasaki M, Watanabe Y. Ontogeny of cytosolic phospholipase A2 activity in rat brain. Biochem Biophys Res Commun. 1992;185:350–355. doi: 10.1016/s0006-291x(05)80992-1. [DOI] [PubMed] [Google Scholar]