Introduction
Vulvovaginal candidiasis (VVC), caused primarily by Candida albicans, remains a frequent and common disease [1]. Five to ten percent of women with a primary episode of VVC subsequently experience recurrent vulvovaginal candidiasis (RVVC) [2]. Although antifungal therapy is highly effective, it does not prevent recurrences [3]. Furthermore, there is concern that repeated treatments may induce drug resistance, and/or shift the spectrum of causative Candida species to the more intrinsically resistant non-albicans species [4–9]. Consequently, there is a clear need to identify new prophylactic and/or therapeutic strategies against VVC.
The C. albicans cell surface agglutinin-like sequence 3 protein (Als3p), plays a key role in multiple processes that are necessary for disease initiation and progression including adherence to host cells [10, 11], biofilm formation [11, 12], host cell invasion [13], and iron acquisition [14]. Functional specificity of Als3p appears to be mediated by the N-terminus (Als3p-N) [10, 11]. Thus, a recombinant engineered form of this portion of Als3p (rAls3p-N) has been developed as a candidate vaccine to protect against C. albicans infection [15, 16].
We have previously reported that vaccination with NDV-3 (rAls3p-N formulated with aluminum hydroxide adjuvant [alum]) protects mice from lethal disseminated candidiasis [17, 18]. Additionally, a formulation of rAls3p-N vaccine with CFA/IFA protected mice from mucosal candidiasis including VVC [15]. The mechanism of protection against hematogenously disseminated candidiasis in mice was primarily mediated by activation of Th1/Th17 immune responses [18, 19]. Given that immune responses to mucosal candidiasis are likely to differ from hematogenously disseminated infections [20–23], we sought to investigate the mechanisms of potential protection elicited by NDV-3 against murine VVC.
Methods
Candida strains
C. albicans SC5314 is a clinical isolate supplied by W. Fonzi (Georgetown University). C. albicans 529L is a mucosal clinical isolate kindly provided by J. Naglik (King’s College London). The organisms were serially passaged 3 times in YPD (1% yeast extract, 2% Bacto Peptone, and 2% glucose) (Difco) at room temperature prior to use.
Immunization of mice
Female inbred BALB/c or outbred ICR mice of 8–10 weeks of age were obtained from Taconic Farms (Germantown, NY). Congenic BALB/c T-cell-deficient (C.Cg/AnBomTac-Foxn1nuN20) or B-cell-deficient (C.129B6-IgH-Jhdtm1Dhu) mice were also used. We compared intramuscular (IM) or subcutaneous (SQ) immunizations of NDV-3 (rAls3p-N formulated with alum (200 μg per dose) [Alhydrogel, Brenntag Biosector], in phosphate buffered saline [PBS]). Mice were injected IM in the left hind thigh muscle with 0.1 mL of NDV-3 [24], while SQ immunization involved injection of 0.2 mL of NDV-3 at the base of the neck. Mice immunized with alum in PBS served as controls. Mice were boosted with a second dose of NDV-3 three weeks later and then were infected with C. albicans two weeks after the boost.
Vaginal infection
Infection was induced as previously described [15, 16]. Briefly, vaccinated mice were injected SQ with β-estradiol (14–16 μg/kg of mouse weight, Sigma-Aldrich, St. Louis, MO) in 100 μL of sesame oil (Sigma-Aldrich) 3 days prior to inoculation and then every 2 days throughout the study period. For inoculation, the mice were anaesthetized by intraperitoneal (i.p) injection of a mixture of ketamine (82.5 mg/kg) and xylazine (6 mg/kg). Next, 20 μL of PBS containing 1 × 106 C. albicans blastospores was injected into the vaginal lumen. Vaginas and ~1 cm of each uterine horn was dissected, homogenized, and quantitatively cultured. Half of the vaginal homogenates were used for measuring the myeloperoxidase (MPO) using the mouse ELISA kit (Hycult Biotech Inc.) [18].
Induction of neutropenia
Neutrophil depletion was carried out by using anti-Ly6G mouse mAb (Bio X Cell, NH) by intraperitoneal (i.p.) injection at a dose of 0.25 or 0.5 mg on day −1, +1, and +3 relative to infection [25]. Isotype matching antibody (2A3) was given to control mice. Alternatively, mice were vaccinated as above with the exception of giving them cyclophosphamide (200 mg/kg) on day −2 and +3 relative to infection via i.p. injection [26].
Passive transfer experiments
Mice were vaccinated with alum or NDV-3 (3 μg dose) as above then bled by cardiac puncture. Separated sera from alum-sham or NDV-3 vaccinated mice were pooled and the rAls3p-N antibody titer determined using ELISA plates coated with rAls3p-N [16]. Serum was given to naïve mice via i.p. injection two hours prior to infecting them intravaginally with 106 of C. albicans 529L strain. Vaginal fungal burden was determined as above.
Immunohistochemistry
Mouse vaginae were excised and fixed in 4% paraformaldehyde and processed as described [27] using rat anti-mouse lysozyme, CD68 or CEA (10 μg/mL; R&D Systems) as primary antibodies and peroxidase/anti-peroxidase complex (1:1000) as secondary antibody.
C. albicans neutrophil-mediated killing ex vivo
Neutrophils were isolated from mouse blood by Ficoll-Hypaque [28]. Neutrophils were primed with different concentrations of IFN-γ or IL-17 for 1 hour prior to co-culturing with C. albicans that has been incubated with serum (5% v/v) from alum-sham or NDV-3-vaccinated mice for 1 hour. Co-culturing was conducted in a 24-well tissue culture plate at an effector:target ratio of 10:1. At the end of incubation period YPD agar was added to each well, the plate incubated at 37°C and the CFU counted. The data was expressed as % killing of the control wells (i.e. C. albicans incubated for the same period of time without neutrophils).
Statistical analysis
Data among different groups were compared by the Mann-Whitney U test for unpaired comparisons. P values of <0.05 were considered significant.
Results
rAls3p-N induces strong serum antibody response
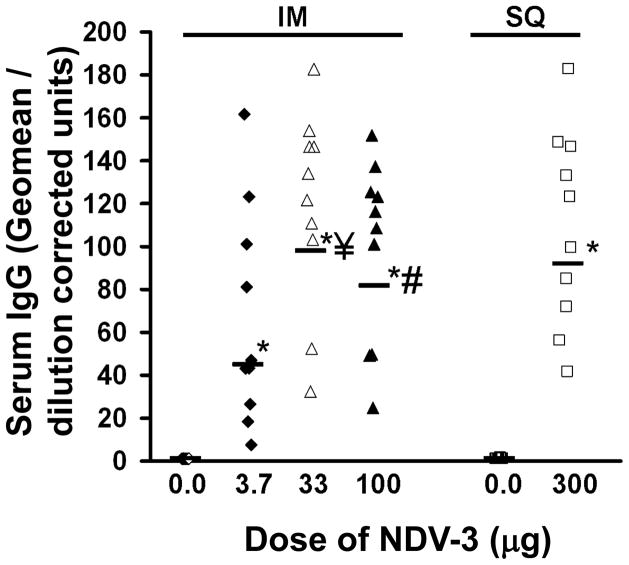
We used the FDA-approved alum adjuvant to test the immunogenicity of rAls3p-N in mice. BALB/c mice were vaccinated with NDV-3, or alum adjuvant alone as control, via IM or SQ injection. Both IM and SQ vaccination with NDV-3 induced significantly higher anti-rAls3p-N IgG titers, compared to control, regardless of the dose used (Figure 1).
Figure 1. NDV-3 induces strong serum antibody response through intramuscular or subcutaneous vaccination.
BALB/c female mice (n=10/group) were vaccinated through intramuscular (IM) or subcutaneous (SQ) injection with indicated dose of NDV-3 on Day 0. Mice receiving alum alone were used as controls. Mice were boosted with the same dose on Day 21. Serum samples were collected on Day 33 and anti-rAls3p-N IgG were evaluated with ELISA plates coated with rAls3p-N. Data are displayed as IgG geomean dilution corrected OD for each mouse [32]. *P < 0.0001 vs. 0.0 dose (alum without rAls3p-N). ¥P=0.03 vs. 3.7 μg dose. #P=0.09 vs. 3.7 μg dose.
An intermediate dose of the rAls3p-N vaccine protects mice from VVC
To determine the most effective dose of NDV-3, a 3 × 103-fold vaccine dose range was evaluated at a 3-fold dilution. BALB/c mice were immunized with NDV-3 or adjuvant alone via IM or SQ injection. For both routes of vaccination, the efficacy of the vaccine followed a sine shape curve with different doses of the vaccine demonstrating statistically significant reduction in vaginal fungal burden when compared to controls. For example, in the SQ vaccination, the 1–30 μg dose range of NDV-3 significantly reduced vaginal fungal burden by approximately 10–1000 fold when compared to counts obtained from mice vaccinated with adjuvant alone (P = 0.02 by the Mann-Whitney U test, Figure 2A). In contrast, between 0.3–10 μg administered IM resulted in approximately 10-fold reduction in vaginal fungal burden compared to alum sham-vaccinated control mice (Figure 2B). Other doses via both routes of administration had no substantial effect on vaginal fungal burden. Because SQ route demonstrated a relatively higher efficacy and the SQ dose of 3 μg showed more consistent results than any other dose, subsequent vaccination studies utilized a dose of 3 μg administered SQ.
Figure 2. Intermediate doses of the NDV-3 vaccine protect mice against VVC.
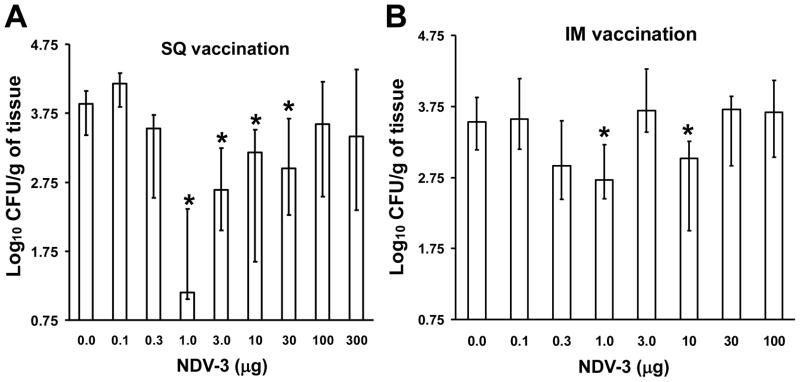
BALB/c female mice were vaccinated with indicated dose of NDV-3 on Day 0. Mice receiving alum alone were used as control. Mice were boosted with the same dose of NDV-3 on Day 21 and then challenged on day 35 with 1×106 CFU of C. albicans strain SC5314. Vaginas were harvested on day 5 post infection for fungal burden determination. (A) Mice were vaccinated subcutaneously. *P <0.03 vs. mice receiving alum alone (0). n=20 mice/group except for 300 μg dose in which n=10. (B) Mice were vaccinated intramuscularly. n= 20 mice/group except for 1, 10, and 100 μg in which n=10 mice per group. *P <0.02 vs. mice receiving alum alone (0).
NDV-3 efficacy in the VVC model requires B and T cells
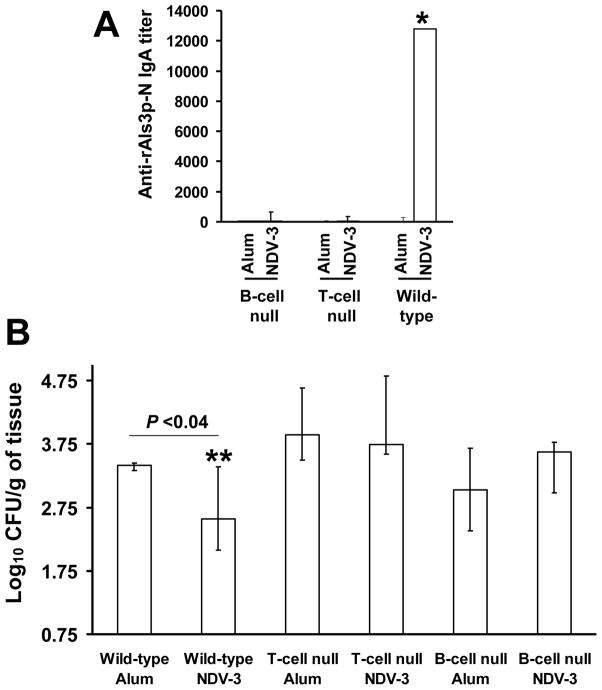
To define the role(s) of antibody and T cells in vaccine-mediated protection, B-cell-deficient, T-cell-deficient nude, or congenic BALB/c wild-type control mice were SQ immunized with 3 μg of NDV-3. Control mice from each of the three types of mice received alum alone. Only wild-type mice developed vaginal anti-rAls3-N IgA antibody titers of 1:12,000 (Figure 3A). Next, we examined the efficacy of the NDV-3 vaccine in protecting these mice from VVC. Interestingly, the susceptibility of B-cell and T-cell deficient mice to VVC was similar to the wild-type mice. Further, only congenic wild-type mice, and neither B-cell nor T-cell deficient mice, were protected by the vaccine (Figure 3B). As expected there was no difference in MPO activity among vagina from wild-type or lymphocyte deficient mice (data not shown).
Figure 3. NDV-3 efficacy requires B and T lymphocytes cells.
B-cell-deficient, T-cell-deficient nude, or congenic BALB/c wild-type control mice were immunized with 3 μg of NDV-3 vaccine or given alum alone as control on study days 0 and 21. Eleven days post boost (day 32), mice were treated with β-estradiol. (A) Two days after estradiol administration (day 34), 20 μL of PBS was used to collect vaginal lavage to determine the titers of IgA by ELISA plates coated with rAls3p-N. (B) Vaccinated mice were challenged with 1×106 CFU C. albicans SC5314 on day 35 then vaginas were harvested on day 5 post infection for fungal burden. Data are displayed as medians ± interquartile ranges of 10 mice per group. *P<0.001 compared to all other arms. **P <0.04 vs. wild-type alum-vaccinated mice.
NDV-3 is protective against another strain of C. albicans in ICR mice
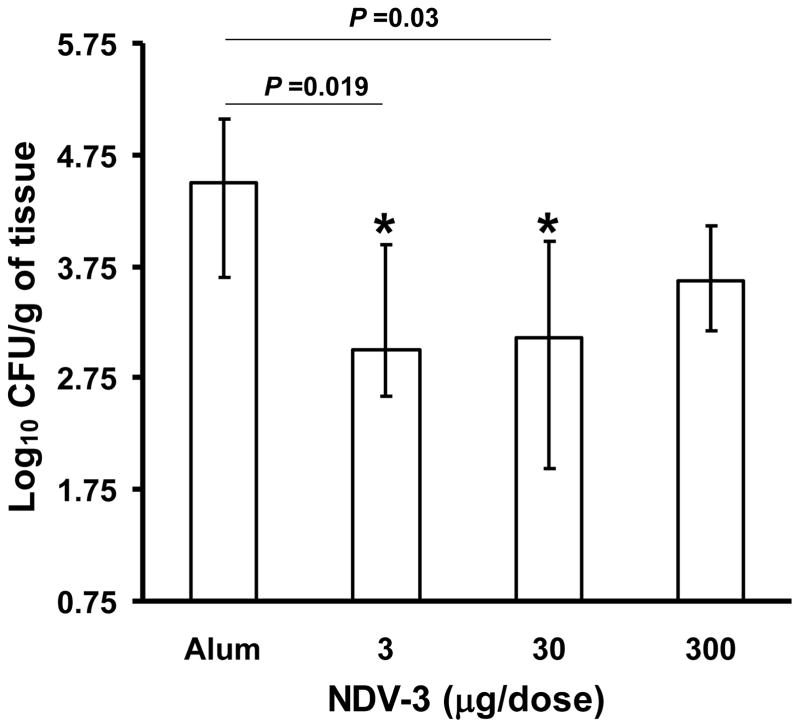
To examine for the breadth of NDV-3 protection against VVC, we vaccinated the outbred ICR mice with different doses of NDV-3, ranging from 3–300 μg. Mice were infected intravaginally with the mucosal C. albicans clinical isolate 529L [29]. This isolate consistently demonstrated higher colonization of the vagina vs. SC5314 (compare alum only control mice in Figure 2 vs. alum only control mice in Figure 4). Further, vaccination of ICR mice with 3 or 30 μg of NDV-3 resulted in 15-fold reduction in C. albicans vaginal count vs. that of alum sham-vaccinated mice (Figure 4). Although vaccination of ICR mice with the higher dose of 300 μg also reduced the vaginal fungal burden, this decrease did not achieve statistical significance (Figure 4). These results demonstrate that the NDV-3 vaccine protection is not mouse or C. albicans strain dependent.
Figure 4. NDV-3 vaccination protects ICR mice against a high colonizer oral isolate of C. albicans.
ICR female mice (n=10) were vaccinated with the indicated dose of NDV-3 on day 0, boosted with the same dose on day 21, and then infected with 1×106 CFU C. albicans strain 529L on day 35. Mice that received alum alone were used as controls. Vaginas were harvested on day 5 post infection for fungal burden. Data are displayed as medians ± interquartile ranges. *P≤0.03 vs. alum.
The NDV-3 vaccine recruits functional phagocytes to the vagina
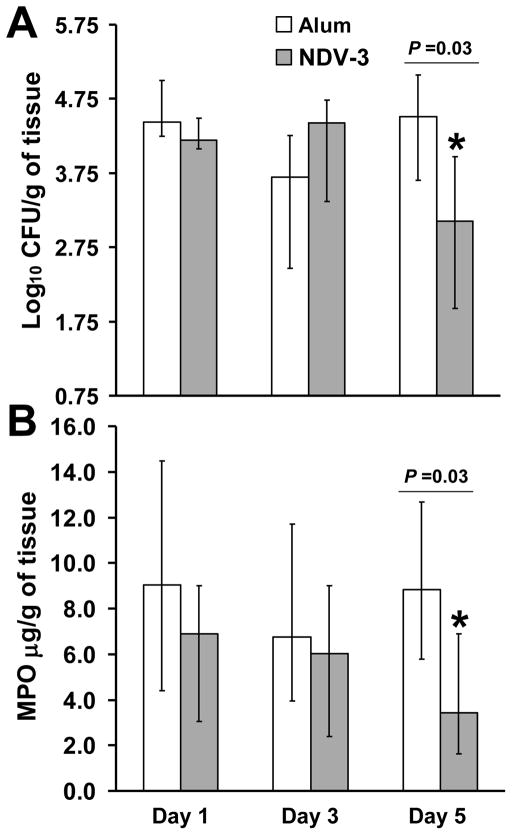
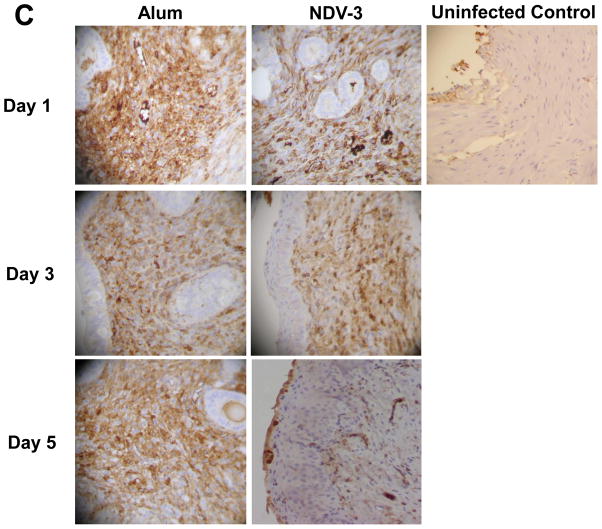
To investigate the potential role of phagocytes in protecting mice vaccinated with NDV-3 against VVC, we vaccinated ICR mice as above and then infected them with C. albicans 529L. MPO is constitutively expressed in neutrophils and has been extensively used in previous studies to quantify neutrophil influx into tissues during infection and inflammation [18, 30, 31]. In alum injected mice, the level of C. albicans colonization was constant over time (Figure 5A). In contrast, vaccination with NDV-3 resulted in approximately 15-fold reduction in vaginal fungal burden vs. alum controls at day 5 but not at day 1 or 3 post infection (Figure 5A). Additionally, the vaginal MPO levels were reduced only in NDV-3 vaccinated mice only at day 5 post infection when compared to alum sham-vaccinated mice (Figure 5B). These results were further confirmed by immunohistopathology. Sections of vagina from alum or NDV-3 vaccinated mice were stained with CEA stain to detect neutrophil influx or with anti-CD68 antibody to detect macrophages. When compared to uninfected control mice which had no inflammatory immune response, infected mice had an inflammatory infiltrate which was predominantly neutrophilic (Figure 5C) with scattered foci of macrophages (data not shown). Only at day 5 post infection neutrophils in vagina harvested from mice vaccinated with NDV-3 demonstrated less neutrophilic presence vs. vagina from alum sham-vaccinated mice (Figure 5C). Collectively, these results indicate that vaginal phagocyte accumulation resulted ultimately in reduced fungal burden followed by reduction in inflammation.
Figure 5. NDV-3 vaccination results in fewer phagocytes being recruited to the vagina in the later time points.
ICR female mice (n = 9 or 10 per group) were vaccinated SQ with NDV-3 (3 μg) or alum alone. Two weeks after the boost, mice were infected intravaginally with 1×106 of C. albicans 529L. At the indicated time point, individually marked vagina was harvested, homogenized, and quantitatively cultured (A) and the MPO levels in the organ homogenates were measured by ELISA (B) Data are displayed as medians ± interquartile ranges. * P=0.03 vs. alum. Immunohistochemistry was performed on fixed sections of vagina using rat anti-mouse CEA antibody (neutrophils are stained in brown) (C) Magnification is 200x.
Anti-rAls3p-N antibodies enhance neutrophil-mediated opsonophagocytosis killing of C. albicans when primed with IFN-γ
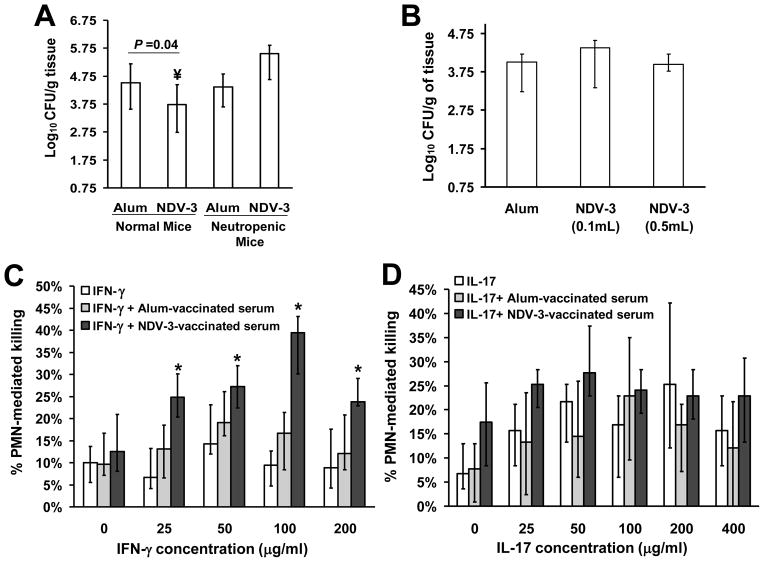
We have shown that NDV-3 induces a potent immune response characterized by high antibody titer and by stimulation of PBMC-production of IFN-γ and IL-17 [18, 32]. To decipher the role of anti-rAls3p-N antibodies, neutrophils, IFN-γ and IL-17 in conferring protection against VVC, we evaluated the efficacy of the vaccine in neutropenic mice and conducted passive transfer experiments. Additionally, we evaluated the ability of anti-rAls3p-N antibodies to promote opsonophagocytosis killing of C. albicans using neutrophils that have been primed with either IFN-γ or IL-17 or both. Mice were given anti-Ly6G mouse mAb which depleted neutrophil function. However, in preliminary data this antibody protected mice from VVC (data not shown), thereby making it impossible to draw any conclusion from these studies. Therefore, we used cyclophosphamide to induce neutropenia. The NDV-3 vaccine lost its ability to protect neutropenic mice from VVC (Figure 6A) indicating the requirement of neutrophils for the efficacy of the vaccine.
Figure 6. NDV-3 protects mice from VVC by enhancing IFN-γ primed neutrophil killing via opsonophagocytosis.
To study the role of neutrophils in the efficacy of NDV-3, ICR female mice (n=10 per arm) were vaccinated SQ with NDV-3 (3 μg) or alum alone on day 0, boosted with another dose on day 21 made neutropenic by cyclophosphamide-treatment on day −2 and +3 relative to infection. Mice were infected intravaginally with 1×106 of C. albicans 529L. Normal mice (n ≥ 18) were used as a control (A). ¥P =0.04 vs. alum sham-vaccinated mice. To study the effect to anti-rAls3p-N antibodies, serum from mice vaccinated with alum (n=25) or NDV-3 (n=25) were pooled and adoptively transferred to naïve mice (n=10 per arm) at a low concentration (0.1 mL) or high concentration (0.5 mL) 2 hours prior to infecting with C. albicans 529L (B). To investigate the effect of IFN-γ (C) and IL-17 (D) priming of neutrophil on the anti-rAls3p-N antibodies opsonophagocytosis killing of C. albicans, mouse neutrophils were primed with different concentrations of IFN-γ or IL-17 for 1 hour prior to co-culturing with C. albicans that has been incubated with serum (5% v/v) from alum- or NDV-3-vaccinated mice for 1 hour. N=12 from three independent experiments. *P < 0.05 vs. IFN-γ alone or IFN-γ + alum-vaccinated serum. Data in all experiments are expressed as the median ± interquartile range.
For the passive immunization transfer experiments, naïve mice receiving a low (0.1 mL) or high (0.5 mL) dose of the NDV-3-vaccinated sera which had an anti-rAls3p-N antibody titer of ~ 1:256,000 did not show any difference in vaginal fungal burden when compared to counts from mice receiving sera from alum-sham vaccinated mice with anti-rAl3p-N antibody titer of 1:400 (Figure 6B). These studies indicated that anti-rAls3p-N antibodies by themselves do not confer protection against VVC.
Since our MPO and immunohistopathology studies suggest that NDV-3 stimulates neutrophils to act as effecter cells and since anti-rAls3p-N antibodies by themselves did not confer protection against VVC despite the lack of protection of the vaccine in B cell-deficient mice, we hypothesized that antibodies exert their function by enhancing opsonophagocytosis killing of C. albicans only when neutrophils are primed by IFN-γ or IL-17, two cytokines that are up-regulated by NDV-3 [18, 32]. Hence, we primed mouse neutrophils with IFN-γ, IL-17 and then determined the effect of anti-rAls3p-N antibodies on the ability of these primed neutrophils to kill C. albicans ex vivo. Consistent with the lack of protection in the passive transfer experiment, anti-rAls3p-N pooled sera did not enhance unprimed neutrophil-mediated killing of C. albicans when compared to sera collected from alum-sham vaccinated mice or to no added sera (Figure 6C, D). In contrast, neutrophils that have been primed with different concentrations of IFN-γ (Figure 6C), but not with IL-17 (Figure 6D), killed significantly more C. albicans cells that had been opsonized with anti-rAls3p-N antibodies vs. C. albicans that had been opsonized with sera collected from alum-sham vaccinated mice. Importantly, IFN-γ primed neutrophils did not demonstrate enhanced killing activity when C. albicans was opsonized with alum-sham vaccinated serum vs. killing activity of the same neutrophils of unopsonized C. albicans. Collectively, these data indicate that anti-rAls3p-N antibodies are required to opsonize C. albicans prior to killing with IFN-γ-primed neutrophils.
Discussion
We recently reported that the NDV-3 vaccine was safe, well-tolerated, and induced a robust immune response characterized by high anti-rAls3p-N IgG and IgA1 titers as well as IFN-γ and IL-17A producing peripheral blood mononuclear cells in healthy human volunteers [32]. These results warrant further development of NDV-3 vaccine in Phase 2 clinical trials to investigate the role of humoral and/or cellular responses in prevention of Candida infections such as those seen in VVC.
The present data demonstrate that NDV-3 immunization induces a robust immune response characterized by high serum and vaginal antibody titers and was effective in protecting inbred BALB/c and outbred ICR mice from VVC caused by different clinical strains of C. albicans, which are responsible for 80–90% of all VVC cases [33]. Interestingly, the NDV-3 vaccine efficacy was characterized by a sinusoidal profile as a function of the administered dose with moderate doses being the most effective in reducing vaginal fungal burden vs. lower or higher doses. These results are concordant with our previous results showing that a dose of 20 μg rAls3p-N formulated with CFA/IFA adjuvants was protective against hematogenously disseminated candidiasis while a dose of 200 μg was not [16]. The sinusoidal efficacy profile may be explained in-part by the studies of Parish and of Parish & Liew, who first described the anergic threshold in which an inverse relationship can be observed between the induction of humoral and cell-mediated immunity by a given dose of an antigen [34–36].
We have also previously shown that NDV-3 vaccine protects mice from hematogenously disseminated candidiasis via a mixed Th1/Th17 adaptive immune response resulting in recruitment and activation of phagocytes at sites of infection with IFN-γ and IL-17 playing a major role in the inflammatory immune response [18], [17]. In the hematogenously disseminated model, antibodies produced in response to NDV-3 had little effect on the protective activity of the vaccine [16]. Because the relative contributions of innate and adaptive immunity appear to be tissue- and compartment-specific [20–23], the current studies sought to potentially dissect the role of T and B lymphocytes in the efficacy of NDV-3 against VVC. Unlike the limited role of B lymphocytes in hematogenously disseminated model, we found that B lymphocytes were required for NDV-3-mediated protection against VVC since the efficacy of the vaccine was abrogated in B lymphocyte deficient mice. In this regard previous reports demonstrated that antibodies targeting secreted aspartyl proteinases (SAP) or mannoproteins were protective in experimental VVC [37, 38]. Additionally, the lack of activity of NDV-3 vaccine in the B-cell deficient mice is also concordant with the lack of activity of the vaccine in the T lymphocyte deficient animals since antibody production (e.g. IgG1 subclass) is T cell-dependent [39, 40]. However, passive transfer of anti-rAls3p-N antibodies to naïve mice did not protect against VVC. This finding suggests that the anti-rAls3p-N antibodies do not protect against VVC via prevention of C. albicans adhesion/invasion of host tissues but rather they function in a coordinated fashion with the adaptive immune arm consistent with the lack of activity of the vaccine in both B-cell and T-cell deficient mice.
Exacerbated neutrophil influx that is characterized by inconsistency and inefficiency in clearing the infection has been reported in experimental vaginal candidiasis [41] and in women with VVC [42]. These studies led to the hypothesis that in women with RVVC, vaginal epithelial cells are hyper-sensitive to the presence of C. albicans and respond by secreting the chemoattractant molecules S100 alarmins. These alarmins recruit neutrophils that lack the ability to clear the infection but instead cause vaginal inflammation. In contrast, women with infrequent histories of VVC have vaginal epithelial cells that are less sensitive to the presence of Candida and therefore do not secrete S100 alarmins [23]. Our results are concordant with this hypothesis since the predominant phagocytes present at the site of infection during murine VVC are neutrophils. Only NDV-3-vaccinated mice were able to mount a neutrophilic immune response that was capable of decreasing C. albicans counts followed by reduced influx of neutrophil to the vagina (Figure 5). Since the NDV-3 elicits a robust immune response characterized by high antibody titers and the pro-inflammatory cytokines IFN-γ and IL-17 [18, 32], we hypothesized that antibodies function as opsonins to enhance neutrophil function in the milieu of IFN-γ or IL-17. Indeed, neutrophils primed with IFN-γ ex vivo had higher ability to kill C. albicans when the fungus is opsonized with serum collected from mice vaccinated with NDV-3 (Figure 6C). In contrast and despite the established role of IL-17 in protection against the mucosal infection of oropharyngeal candidiasis [43, 44], anti-rAls3p-N antibodies did not enhance the C. albicans killing activity of neutrophils primed with IL-17. These results are consistent with the findings showing that IL-23p19(−/−), IL-17RA(−/−) and IL-22(−/−) knockout mice had similar C. albicans vaginal count, neutrophil infiltration and S100 alarmins and cytokine expression to wild-type mice [45].
In summary, NDV-3 elicited protection against VVC points to a model by which anti-rAls3p-N antibodies act as opsonin to enhance IFN-γ primed neutrophil function in killing C. albicans. These results provide compelling rationale for further development of NDV-3 as a candidate vaccine against VVC.
Highlights.
NDV-3 vaccine is protective against murine vulvovaginal candidiasis (VVC)
Protection requires both T and B lymphocytes for cell-mediated and humoral immunity
Reduction in vaginal fungal count is accompanied by reduced neutrophil influx
These results warrant further development of NDV-3 against VVC
Acknowledgments
We thank Dr. Mingfu Liu for technical assistance. This work was presented in part at the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Abstract #G1-768. Chicago. IL. (Winner of ICAAC Program Committee Award for outstanding Abstract Presentation in the Area of Therapy and Preventions of Microbial Disease).
Research described in this manuscript was conducted at the research facilities of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
All procedures involving mice were approved by the institutional animal care and use committee and followed the National Institutes of Health guidelines for animal housing and care.
Funding: This work was supported by Public Health Service grants R01 AI19990, R01 AI063382 from the National Institutes of Health to JEE and by a NovaDigm Therapeutics Inc. grant to ASI (through subcontract from grant 2R42 AI071554-02A1 from the National Institute of Healthy to JPH). SGF is funded by grant R01 AI054928 and R01 DE017088.
Footnotes
Author Contributions:
ASI, CSS, JPH, MRY, SGF, and JEE conceived and designed the experiments. ASI, GL, TG, HL, CSS, and SWF performed the experiments. ASI, CSS, and JPH analyzed the data. JPH and SWF contributed reagents. ASI and GL wrote the paper. CSS, JPH, MRY, SGF, and JEE revised the paper. All authors have approved the final article.
Conflict of Interest: ASI, CSS, JPH, MRY, SGF and JEE own equity in NovaDigm Therapeutics, Inc., which develops vaccine technologies. NovaDigm Therapeutics, Inc. provided partial financial support for these studies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sobel JD, Hasegawa A, Debernardis F, Adriani D, Pellegrini G, Cassone A, et al. Selected animal models: vaginal candidosis, Pneumocystis pneumonia, dermatophytosis and trichosporosis. Med Mycol. 1998;36( Suppl 1):129–36. [PubMed] [Google Scholar]
- 2.Fidel PL, Jr, Sobel JD. Immunopathogenesis of recurrent vulvovaginal candidiasis. Contracept Fertil Sex. 1996 Jan;24(1):33–40. [PubMed] [Google Scholar]
- 3.Sobel JD, Wiesenfeld HC, Martens M, Danna P, Hooton TM, Rompalo A, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004 Aug 26;351(9):876–83. doi: 10.1056/NEJMoa033114. [DOI] [PubMed] [Google Scholar]
- 4.Bauters TG, Dhont MA, Temmerman MI, Nelis HJ. Prevalence of vulvovaginal candidiasis and susceptibility to fluconazole in women. Am J Obstet Gynecol. 2002 Sep;187(3):569–74. doi: 10.1067/mob.2002.125897. [DOI] [PubMed] [Google Scholar]
- 5.Cernicka J, Subik J. Resistance mechanisms in fluconazole-resistant Candida albicans isolates from vaginal candidiasis. Int J Antimicrob Agents. 2006 May;27(5):403–8. doi: 10.1016/j.ijantimicag.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Eckert LO. Clinical practice. Acute vulvovaginitis. N Engl J Med. 2006 Sep 21;355(12):1244–52. doi: 10.1056/NEJMcp053720. [DOI] [PubMed] [Google Scholar]
- 7.Pinjon E, Jackson CJ, Kelly SL, Sanglard D, Moran G, Coleman DC, et al. Reduced azole susceptibility in genotype 3 Candida dubliniensis isolates associated with increased CdCDR1 and CdCDR2 expression. Antimicrob Agents Chemother. 2005 Apr;49(4):1312–8. doi: 10.1128/AAC.49.4.1312-1318.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richter SS, Galask RP, Messer SA, Hollis RJ, Diekema DJ, Pfaller MA. Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J Clin Microbiol. 2005 May;43(5):2155–62. doi: 10.1128/JCM.43.5.2155-2162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ventolini G, Baggish MS, Walsh PM. Vulvovaginal candidiasis from non-albicans species: retrospective study of recurrence rate after fluconazole therapy. J Reprod Med. 2006 Jun;51(6):475–8. [PubMed] [Google Scholar]
- 10.Sheppard DC, Yeaman MR, Welch WH, Phan QT, Fu Y, Ibrahim AS, et al. Functional and structural diversity in the Als protein family of Candida albicans. J Biol Chem. 2004 Jul 16;279(29):30480–9. doi: 10.1074/jbc.M401929200. [DOI] [PubMed] [Google Scholar]
- 11.Zhao X, Oh SH, Cheng G, Green CB, Nuessen JA, Yeater K, et al. ALS3 and ALS8 represent a single locus that encodes a Candida albicans adhesin; functional comparisons between Als3p and Als1p. Microbiology. 2004 Jul;150(Pt 7):2415–28. doi: 10.1099/mic.0.26943-0. [DOI] [PubMed] [Google Scholar]
- 12.Nobile CJ, Mitchell AP. Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol. 2006 Sep;8(9):1382–91. doi: 10.1111/j.1462-5822.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 13.Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, et al. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007 Mar;5(3):e64. doi: 10.1371/journal.pbio.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almeida RS, Brunke S, Albrecht A, Thewes S, Laue M, Edwards JE, et al. The hyphal-associated adhesin and invasin Als3 of Candida albicans mediates iron acquisition from host ferritin. PLoS Pathog. 2008 Nov;4(11):e1000217. doi: 10.1371/journal.ppat.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spellberg BJ, Ibrahim AS, Avanesian V, Fu Y, Myers C, Phan QT, et al. Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J Infect Dis. 2006 Jul 15;194(2):256–60. doi: 10.1086/504691. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim AS, Spellberg BJ, Avenissian V, Fu Y, Filler SG, Edwards JE., Jr Vaccination with recombinant N-terminal domain of Als1p improves survival during murine disseminated candidiasis by enhancing cell-mediated, not humoral, immunity. Infect Immun. 2005 Feb;73(2):999–1005. doi: 10.1128/IAI.73.2.999-1005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spellberg B, Ibrahim AS, Lin L, Avanesian V, Fu Y, Lipke P, et al. Antibody titer threshold predicts anti-candidal vaccine efficacy even though the mechanism of protection is induction of cell-mediated immunity. J Infect Dis. 2008 Apr 1;197(7):967–71. doi: 10.1086/529204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009 Dec;5(12):e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin L, Ibrahim AS, Baquir B, Avanesian V, Fu Y, Spellberg B. Immunological surrogate marker of rAls3p-N vaccine-induced protection against Staphylococcus aureus. FEMS Immunol Med Microbiol. 2009 Apr;55(3):293–5. doi: 10.1111/j.1574-695X.2008.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conti HR, Gaffen SL. Host responses to Candida albicans: Th17 cells and mucosal candidiasis. Microbes Infect. Jul;12(7):518–27. doi: 10.1016/j.micinf.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Veerdonk FL, Kullberg BJ, Netea MG. Pathogenesis of invasive candidiasis. Curr Opin Crit Care. Oct;16(5):453–9. doi: 10.1097/MCC.0b013e32833e046e. [DOI] [PubMed] [Google Scholar]
- 22.van de Veerdonk FL, Netea MG. T-cell Subsets and Antifungal Host Defenses. Curr Fungal Infect Rep. Dec;4(4):238–43. doi: 10.1007/s12281-010-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yano J, Noverr MC, Fidel PL., Jr Cytokines in the host response to Candida vaginitis: Identifying a role for non-classical immune mediators, S100 alarmins. Cytokine. 2012 Apr;58(1):118–28. doi: 10.1016/j.cyto.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chow YH, Chiang BL, Lee YL, Chi WK, Lin WC, Chen YT, et al. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J Immunol. 1998 Feb 1;160(3):1320–9. [PubMed] [Google Scholar]
- 25.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008 Jan;83(1):64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 26.Luo G, Gebremariam T, Lee H, French SW, Wiederhold NP, Patterson TF, et al. Efficacy of liposomal amphotericin B and posaconazole in intratracheal models of murine mucormycosis. Antimicrob Agents Chemother. 2013;57(7):3340–7. doi: 10.1128/AAC.00313-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gukovsky I, Lugea A, Shahsahebi M, Cheng JH, Hong PP, Jung YJ, et al. A rat model reproducing key pathological responses of alcoholic chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2008 Jan;294(1):G68–79. doi: 10.1152/ajpgi.00006.2007. [DOI] [PubMed] [Google Scholar]
- 28.Tsuchimori N, Sharkey LL, Fonzi WA, French SW, Edwards JE, Jr, Filler SG. Reduced virulence of HWP1-deficient mutants of Candida albicans and their interactions with host cells. Infect Immun. 2000 Apr;68(4):1997–2002. doi: 10.1128/iai.68.4.1997-2002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman D, Mistry M, Thavaraj S, Challacombe SJ, Naglik JR. Murine model of concurrent oral and vaginal Candida albicans colonization to study epithelial host-pathogen interactions. Microbes Infect. 2007 Apr;9(5):615–22. doi: 10.1016/j.micinf.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClellan SA, Huang X, Barrett RP, van Rooijen N, Hazlett LD. Macrophages restrict Pseudomonas aeruginosa growth, regulate polymorphonuclear neutrophil influx, and balance pro- and anti-inflammatory cytokines in BALB/c mice. J Immunol. 2003 May 15;170(10):5219–27. doi: 10.4049/jimmunol.170.10.5219. [DOI] [PubMed] [Google Scholar]
- 31.Ismail HF, Fick P, Zhang J, Lynch RG, Berg DJ. Depletion of neutrophils in IL-10(−/−) mice delays clearance of gastric Helicobacter infection and decreases the Th1 immune response to Helicobacter. J Immunol. 2003 Apr 1;170(7):3782–9. doi: 10.4049/jimmunol.170.7.3782. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt CS, White CJ, Ibrahim AS, Filler SG, Fu Y, Yeaman MR, et al. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine. 2012;30(52):7594–600. doi: 10.1016/j.vaccine.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sobel JD, Faro S, Force RW, Foxman B, Ledger WJ, Nyirjesy PR, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998 Feb;178(2):203–11. doi: 10.1016/s0002-9378(98)80001-x. [DOI] [PubMed] [Google Scholar]
- 34.Parish CR. Immune response to chemically modified flagellin. II. Evidence for a fundamental relationship between humoral and cell-mediated immunity. J Exp Med. 1971 Jul 1;134(1):21–47. doi: 10.1084/jem.134.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parish CR. Immune response to chemically modified flagellin. I. Induction of antibody tolerance to flagellin by acetoacetylated derivatives of the protein. J Exp Med. 1971 Jul 1;134(1):1–20. doi: 10.1084/jem.134.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parish CR, Liew FY. Immune response to chemically modified flagellin. 3. Enhanced cell-mediated immunity during high and low zone antibody tolerance to flagellin. J Exp Med. 1972 Feb 1;135(2):298–311. doi: 10.1084/jem.135.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Bernardis F, Boccanera M, Adriani D, Spreghini E, Santoni G, Cassone A. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect Immun. 1997 Aug;65(8):3399–405. doi: 10.1128/iai.65.8.3399-3405.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han Y, Morrison RP, Cutler JE. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect Immun. 1998 Dec;66(12):5771–6. doi: 10.1128/iai.66.12.5771-5776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacquot S, Macon-Lemaitre L, Paris E, Kobata T, Tanaka Y, Morimoto C, et al. B cell co-receptors regulating T cell-dependent antibody production in common variable immunodeficiency: CD27 pathway defects identify subsets of severely immuno-compromised patients. Int Immunol. 2001 Jul;13(7):871–6. doi: 10.1093/intimm/13.7.871. [DOI] [PubMed] [Google Scholar]
- 40.Spellberg B, Edwards JE., Jr Type 1/Type 2 immunity in infectious diseases. Clin Infect Dis. 2001 Jan;32(1):76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 41.Fidel PL, Jr, Luo W, Steele C, Chabain J, Baker M, Wormley F., Jr Analysis of vaginal cell populations during experimental vaginal candidiasis. Infect Immun. 1999 Jun;67(6):3135–40. doi: 10.1128/iai.67.6.3135-3140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fidel PL, Jr, Barousse M, Espinosa T, Ficarra M, Sturtevant J, Martin DH, et al. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun. 2004 May;72(5):2939–46. doi: 10.1128/IAI.72.5.2939-2946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009 Feb 16;206(2):299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernandez-Santos N, Huppler AR, Peterson AC, Khader SA, McKenna KC, Gaffen SL. Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol. Dec 19; doi: 10.1038/mi.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yano J, Kolls JK, Happel KI, Wormley F, Wozniak KL, Fidel PL., Jr The acute neutrophil response mediated by S100 alarmins during vaginal Candida infections is independent of the Th17-pathway. PLoS One. 7(9):e46311. doi: 10.1371/journal.pone.0046311. [DOI] [PMC free article] [PubMed] [Google Scholar]