Abstract
Purpose
To determine the maximum tolerated dose per day of silybin phosphatidylcholine (Siliphos) in patients with advanced hepatocellular carcinoma (HCC) and hepatic dysfunction.
Experimental Design
Patients with advanced HCC not eligible for other therapies based on poor hepatic function were enrolled in a phase I study of silybin phosphatidylcholine. A standard phase I design was used with 4 planned cohorts, dose escalating from 2, 4, 8, to 12 g per day in divided doses for 12 weeks.
Results
Three participants enrolled in this single institution trial. All enrolled subjects consumed 2 g per day of study agent in divided doses. Serum concentrations of silibinin and silibinin glucuronide increased within 1 to 3 weeks. In all 3 patients, liver function abnormalities and tumor marker α-fetoprotein progressed, but after day 56 the third patient showed some improvement in liver function abnormalities and inflammatory biomarkers. All 3 participants died within 23 to 69 days of enrolling into the trial, likely from hepatic failure, but it could not be ruled out that deaths were possibly due to the study drug.
Conclusion
Short-term administration of silybin phosphatidylcholine in patients with advanced HCC resulted in detectable increases in silibinin and its metabolite, silibinin glucuronide. The maximum tolerated dose could not be established. Since patients died soon after enrollment, this patient population may have been too ill to benefit from an intervention designed to improve liver function tests.
Keywords: phase I clinical trial, milk thistle, hepatocellular carcinoma, herbal supplement, dietary supplement
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide. In 2012, it was predicted that more than 22 000 new HCC cases will be diagnosed in the United States.1 Approximately 80% of HCC patients in the United States have underlying cirrhosis, which often complicates treatment. Some patients with localized disease benefit from resection or liver transplantation. Patients with more advanced HCC often receive local and systemic treatments, but these usually are not curative.2
Current treatment options for metastatic HCC include sorafenib, a targeted oral multikinase inhibitor for patients with unresectable HCC, or participation in clinical trials.3,4 However, patients with elevated liver function enzymes (ie, total bilirubin, aspartate transaminase [AST], alanine transaminase [ALT]) secondary to underlying cirrhosis, tumor involvement, and/or adverse effects of prior therapy often are ineligible to receive chemotherapy and/or to participate in clinical trials. For patients with advanced HCC and elevated liver enzymes, there is no standard treatment, and therapies are usually limited to supportive care (ie, pain control and relief of ascites).
Milk thistle (Silybum marianum) is an herb that has been used for more than 2000 years to treat or prevent liver and biliary diseases. It has been studied in patients with chronic liver diseases and toxin-mediated acute liver disease, and its effects on elevated liver function enzymes have been evaluated.5,6 Silymarin, the main active constituent of milk thistle, is a mixture of polyphenols, including flavonolignans and flavonoids. Silybin (also known as silybinin) is the main flavonolignan of silymarin, and in traditional extracts comprises 60% to 70% of active silymarin.7,8 Silymarin has also been shown to have anti-inflammatory and antiviral effects. However, a recent study found that among patients with chronic hepatitis C who were refractory to interferon, ALT levels did not decline more in those who took milk thistle than those who took placebo.9
Doses of up to 2.1 g per day of silymarin extracts have been found to be safe in patients with chronic hepatitis C without cirrhosis.10 Siliphos is a commercially available formulation (Indena S.p.A, Milan, Italy) of silybin extract combined with phosphatidylcholine to increase absorption (1:2 ratio of silybin:phosphatidylcholine). Ladas et al5 at our institution conducted a randomized, controlled trial of 50 children with acute lymphoblastic leukemia and hepatic toxicity using up to 320 mg per day (based on weight) of silybin phosphatidlycholine. Hepatic toxicity was defined as grade 2 or greater of ALT, AST, or total bilirubin as assessed by the National Cancer Institute Common Toxicity Criteria, version 2.0.11 The authors reported that patients who received Siliphos had marginally significant reductions in liver toxicity compared with those who received placebo. Flaig et al12 conducted a phase I study of silybin phosphatidlycholine in patients with prostate cancer (and otherwise normal liver function) and determined the maximum tolerated dose (MTD) to be 13 g daily.12
We hypothesized that in patients with advanced HCC and significant liver disease, treatment with silybin would lower liver function tests (including total bilirubin, AST, and ALT levels) enough to allow them to receive and/or resume chemotherapy. To our knowledge, no published clinical trials have evaluated the clinical efficacy of silybin in patients with advanced HCC, and only a few trials of silybin have been conducted in cancer patients in general.13,14 As preliminary work toward a trial that would test our hypothesis, we conducted a phase I trial to identify the MTD of silybin phosphatidlycholine (Siliphos) in patients with advanced HCC. We planned to evaluate 4 dose levels of Siliphos: 2, 4, 8, and 12 g daily in 3 divided doses over 12 weeks.
Methods
Subjects
Subjects were identified by members of the liver transplant team and medical oncology at Columbia University Medical Center (CUMC). Inclusion criteria included the following: age 18 years or older; diagnosis of advanced HCC based either on American Association for the Study of Liver Diseases guidelines15 or on biopsy; diagnosis of HCC not amenable to other therapies after multidisciplinary review; Child–Pugh class B or C,16 anticipated survival time of 3 months or greater based on the investigator’s impression. Other criteria included, total bilirubin >1.5 times the upper limit of normal, or serum AST >2.5 times the upper limit of normal; measurable disease by RECIST (response evaluation criteria in solid tumors) criteria; failed, refused, or not a candidate for sorafenib or local therapy, and ECOG performance status of 0–3. The protocol was approved by the CUMC institutional review board, and written informed consent was obtained from all subjects.
Silybin Phosphatidylcholine and Dosing
Siliphos powder is a 1:2 ratio of silybin to phosphatidylcholine, which increases drug absorption. An IND (investigational new drug) was obtained from the Food and Drug Administration (#107662). Powdered Siliphos was obtained from the manufacturer (Indena S.p.A, Milan, Italy) by the US-based distributor (Thorne Research, Sandpoint, ID), weighed by the CUMC Research Pharmacy, and distributed in glassine envelope packets. The raw material was analyzed by an independent contractor to screen for heavy metals (arsenic, cadmium, mercury, lead) and microbes (total viable count, yeast and mold, coliform, Escherichia coli, Salmonella, Staphylococcus aureus). The raw material was stored at the CUMC Research Pharmacy in a controlled environment. A single Siliphos batch was used for the entire study. Patients received premeasured powder containing Siliphos to take 3 times a day mixed with 4 ounces of applesauce with each doze.
Statistical Design
We used a standard phase I open label dose escalation design to define the MTD of silybin phosphatidylcholine in subjects with advanced HCC and elevated liver function tests over a 12-week intervention period, followed by an additional 12 months of observational data collection. The study had a planned maximum enrollment of 30 patients. The MTD was defined as the highest dose at which 0 of 3 patients, or fewer than 2 of 6 patients, experienced a dose-limiting toxicity (DLT).
The primary endpoint of this study was to identify the MTD of Siliphos in patients with advanced HCC and hepatic dysfunction. Secondary aims were to assess changes in the following measures from baseline to 3 months at each dose level: (a) mean intrapatient percentage change in AST, ALT, and total serum bilirubin levels; (b) quality of life as measured by the FACT (Functional Assessment of Cancer Therapy)–Hepatobiliary questionnaire; (c) plasma concentrations of silibinin and silibinin glucuronide; (d) mean intrapatient percentage change in serum inflammatory biomarkers; and (e) tumor response as measured by RECIST criteria and α-fetoprotein (AFP) concentrations. Exploratory aims were to assess (a) tumor response as measured by RECIST criteria and AFP concentrations and (b) survival at 12 months.
Dose-Limiting Toxicity
A DLT was defined as any non-hepatic grade 3 or grade 4 (severe) toxicity, or grade 4 hepatic toxicity (moderate-severe encephalopathy, potentially life-threatening, total bilirubin >10 times the upper limit of normal, and alkaline phosphatase (ALP) >20 times the upper limit of normal). A grade 3 AST or ALT elevation also qualified as a DLT since the purpose of the trial was to lower transaminases (AST/ALT >5 to 20 times the upper limit of normal, or >5 times the upper limit of normal for over 2 weeks). We used the CTCAE (Common Toxicity Criteria for Adverse Events) version 4.0 for toxicity assessments.7
Data Collection
Fasting morning blood samples were scheduled to be collected at baseline, week 1, week 3, week 6, week 9, and week 12. On the day of the blood draw, participants were asked to take their first silybin phosphatidylchole dose of the day supervised in the research clinic and blood samples were be collected 1 hour after dosing. Patients were scheduled for clinic visits at weeks 1, 3, 6, 9, and 12 and were evaluated using CTCAE criteria.7 Study subjects were scheduled to complete the FACT-Hepatobiliary questionnaire at weeks 1, 6, and 12.17 Study subjects were scheduled to be followed via standard clinical care until one year after last dose of study medication or until mortality, whichever came first.
Laboratory Methods
Silibinin and Silibinin Glucuronide Analysis in Plasma
Silibinin, naringenin, saccharic acid, β-glucuronidase, and silibinin were obtained from Sigma (St. Louis, MO). All other chemicals and solvents used in the analysis were of reagent or higher quality and obtained from Fisher Scientific (Pittsburgh, PA). An initial silibinin stock (1 mg/mL) was prepared in 1:1 acetonitrile (ACN)/methanol with 0.1% formic acid followed by subsequent dilutions in ACN. Naringenin stock solution was prepared in methanol at 1 mg/mL and then diluted in ACN to a final concentration of 10 µg/mL. Samples were prepared using 100 µL of plasma. For the analysis of silibinin in plasma, each sample was spiked with 10 µL ACN or 10 µL of the appropriate silibinin standard (resulting in a standard curve from 50 to 7500 ng/mL), and 10 µL of 10 µg/mL naringenin as an internal standard followed by vortexing briefly. Then, 200 µL of ACN with 2% formic acid was added to precipitate proteins. Samples were then vortex mixed continuously for 10 minutes followed by centrifugation for 10 minutes at 20 000 × g. The supernatant was collected and analyzed via liquid chromatography–tandem mass spectrometry. The metabolite, silibinin glucuronide, was determined indirectly by determining the increase in concentration of silibinin following incubation of samples with 500 units of β-glucuronidase.18
Negative ion electrospray ionization mass spectra were obtained with MDS Sciex 3200 Q-TRAP triple quadrupole mass spectrometer (Applied Biosystems, Inc, Foster City, CA) with a turbo ionspray source interfaced to a Shimadzu LC-20AD High Performance Liquid Chromatograph system (Shimadzu Corporation, Kyoto, Japan). Samples were chromatographed with a Waters XBridge phenyl, 2.5 µm, 4.6 × 50 mm column (Waters Corporation, Milford, MA) protected by a C18 guard cartridge, 4.0 × 2.0 mm (Phenomenex, Torrance, CA). An LC gradient was employed with mobile phase A consisting of 10 mM ammonium acetate and mobile phase B consisting of acetonitrile. Chromatographic resolution was achieved by linearly holding the B solvent at 20% for 1 minute. The solvent mixture was then altered by increasing mobile phase B linearly from 20% to 95% between 1 and 2 minutes, maintaining at 95% between 2 and 3.25 minutes, and then decreasing linearly from 95% to 20% between 3.25 and 4 minutes, followed by reequilibration of the column at 20% mobile phase B from 4 to 5 minutes. The LC flow rate was 650 µL/min, the sample injection volume was 20 µL, and the analysis run time was 5 minutes.
The mass spectrometer settings were optimized as follows: turbo ionspray temperature 500°C; ionspray needle voltage, −4500 V; curtain gas, N2, (CUR), 10 units; collision gas, N2 , (CAD), medium; nebulizer gas, N2 , 60 units; and auxiliary gas, N2 , 60 units. Samples were quantified by the internal standard reference method in the MRM mode by monitoring and summing the transitions of m/z 481.1 to m/z 125.2 and m/z 481.1 to m/z 300.96 for silibinin and m/z 271 to m/z 119 for naringenin (internal standard). The compound-specific parameters for the two transitions used to quantify silibinin (m/z 481.1 to m/z 125.2 and m/z 481.1 to m/z 300.96) were as follows, respectively: declustering potential (DP) −51 and −48 V; entrance potential (EP) −6.9 and −3.8 V; collision cell entrance potential (CEP) −16 and −14; collision energy (CE) −40 and −32; collision cell exit potential (CXP) −0.5 and −1.2. The compound-specific parameters for naringenin were as follows: DP −55 V; EP −6.0 V; CEP −21; CE −37; CXP −0.5. Quantitation of silibinin was based on standard curves in prepared matrix using the ratio of silibinin peak area sum to naringenin peak area with 1/χ2 weighting of linear regression.
Inflammatory Cytokines in Serum
Serum was assayed for 6 cytokines (IL-1b, IL-6, IL-10, IL-12p70, IFN-γ, TNF-α) simultaneously in a 96-well format using a human high sensitivity cytokine/chemokine kit (Milliplex Map Kit, EMD Millipore Corporation). The kit contains 6 sets of micro-spheres, each containing a single capture antibody and a unique mixture of fluorescent dyes. The plate was read in a Luminex 100 Analyzer (Luminex, Austin, TX) controlled by StarStation 2.0 software (Applied Cytometry, Sheffield, UK). Two fluorescent signals were measured from individual beads; one identified the bead type and the other measured the median fluorescent intensity of the bound phycoerythrin.
Results
Patient Recruitment and Characteristics
From August 2010 to February 2012, three patients were enrolled in this trial. Accrual to the trial was much lower than anticipated. We did not record the number of patients who were initially approached to participate in the trial, but many preferred to use an off-label antineoplastic agent (ie, bevacizumab or FOLFOX), rather than take sorafenib. Once patients progressed on these other therapies, they were usually too sick to be enrolled on this current trial. Four patients were screen failures because of the following reasons: grade 4 bilirubin (n = 1), preference for trying other chemotherapy agent (n = 1), and death prior to scheduled screening day (n = 2). Characteristics of enrolled patients are shown in Table 1. All 3 patients reported low functional well-being per the FACT-Hepatobiliary questionnaire.
Table 1.
Patient Baseline Characteristics.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age | 54 | 47 | 60 |
| Gender | Male | Male | Male |
| ECOG performance status | 2 | 3 | 3 |
| Underlying liver disease | Hepatitis B | Hepatitis B | NASH/alcohol |
| Child–Pugh score | 12 | 9 | 10 |
| Tumor grade | Moderate 2–3 | Moderate/severe 3–4 | |
| Total bilirubin (mg/dL) | 4.2 | 5.4 | 9.2 |
| AST (IU/L) | 519 | 154 | 63 |
| ALT (IU/L) | 285 | 54 | 23 |
| AFP (ng/mL) | 89 | 50 | 66 282 |
| Prior treatment | None | TACE | Resection (November 2009, April 2011), TACE
× 10, sorafenib + IGFRab, gemcitabine + oxaliplatin |
| Survival postenrollment (days) | 39 | 23 | 69 |
| FACT-Hepatobiliary questionnaire |
|||
| Physical well-being | 3.0 | 2.7 | 2.0 |
| Social well-being | 3.4 | 2.7 | 3.3 |
| Emotional well-being | 4.0 | 2.8 | 2.5 |
| Functional well-being | 0.0 | 0.1 | 0.6 |
| Additional concerns | 2.4 | 1.8 | 1.4 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NASH, nonalcoholic steatohepatitis; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, α-fetoprotein; TACE, transarterial chemoembolization; IGFR, insulin-like growth factor receptor; FACT, Functional Assessment of Cancer Therapy.
Maximum Tolerated Dose
All 3 patients received the initial total daily dose of 2 g silybin phosphatidylcholine in 3 divided doses per day. All 3 patients died before the full length of time for administration (12 weeks) and the study was stopped because of reaching the stopping rule for adverse events that were potentially related to the study drug. Therefore, an MTD could not be established for this patient population.
Toxicity and Adverse Event Profile
Adverse events are summarized in Table 2, and included typical signs of late-stage liver disease, including ascites, increased bilirubin, transaminases, and hyponatremia. Patient 1 died at home, most likely of cardiopulmonary arrest (day 39), patient 2 died of a lower gastrointestinal bleed (day 23), and patient 3 died of hemoptysis (day 69).
Table 2.
Adverse Events.
| Adverse Event | All Grades; n (%) | Grades 3–4; n (%) |
|---|---|---|
| Ascites | 3 (100) | 1 (33) |
| Asthenia | 1 (33) | 0 (0) |
| Constipation | 1 (33) | 0 (0) |
| Depression | 1 (33) | 0 (0) |
| Lower back pain | 1 (33) | 0 (0) |
| Lower extremity edema | 1 (33) | 0 (0) |
| Pruritis | 1 (33) | 0 (0) |
| Rash | 1 (33) | 0 (0) |
| Dyspnea | 1 (33) | 0 (0) |
| Nausea | 2 (66) | 0 (0) |
| Vomit | 1 (33) | 0 (0) |
| Rectal bleeding | 1 (33) | 1 (33) |
| Urinary tract infection | 1 (33) | 1 (33) |
| Increased creatinine | 1 (33) | 0 (0) |
| Increased INR | 1 (33) | 0 (0) |
| Hyperkalemia | 2 (66) | 0 (0) |
| Increased AST | 3 (100) | 2 (66) |
| Increased ALT | 0 (0) | 0 (0) |
| Increased lipase | 1 (33) | 1 (33) |
| Increased glucose | 1 (33) | 1 (33) |
| Hyponatremia | 1 (33) | 1 (33) |
| Anemia | 1 (33) | 1 (33) |
| Thrombocytopenia | 2 (66) | 0 (0) |
Abbreviations: INR, International Normalized Ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Change in Plasma Concentrations of Silybinin and Silibinin Glucuronide
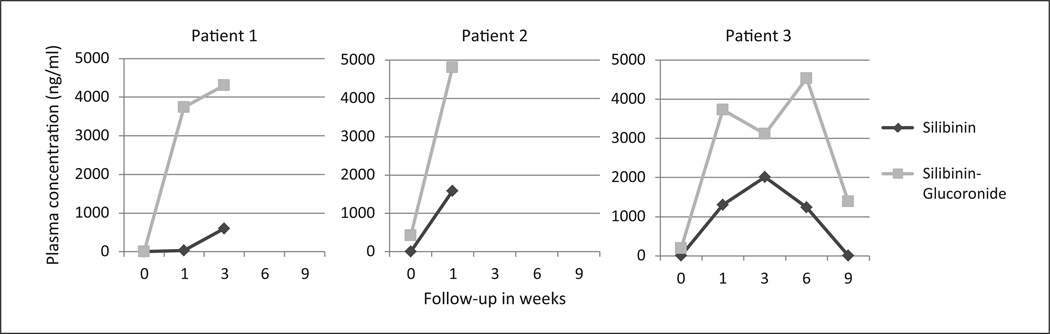
Increases in plasma silybinin were detectable after 1 week of administration and plasma silibinin glucuronide was detectable after 1 to 3 weeks of administration (Figure 1).
Figure 1. Change in plasma concentrations of silibinin and silibinin glucuronide.
Patient 1 died on day 39, patient 2 on day 23, and patient 3 on day 69.
Change in Liver Function Tests and α-Fetoprotein
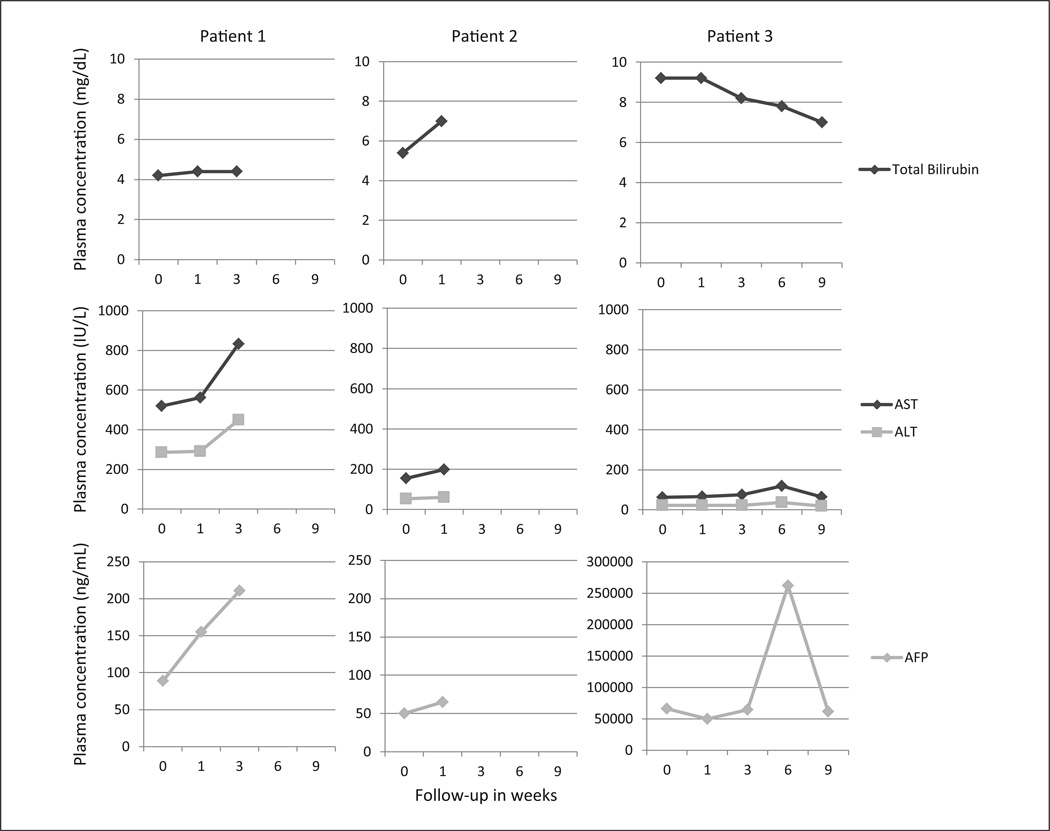
For the first 2 patients, their liver function tests and AFP progressed while on milk thistle (Figure 2). Interestingly, the third patient’s liver function tests and AFP slowly progressed until day 56; then values improved.
Figure 2. Change in liver function tests and AFP levels.
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, α-fetoprotein.
Y-axis scale for AFP levels for patient 3 is different from the scales for patients 1 and 2. Patient 1 died on day 39, patient 2 on day 23, and patient 3 on day 69.
Change in Inflammatory Biomarkers
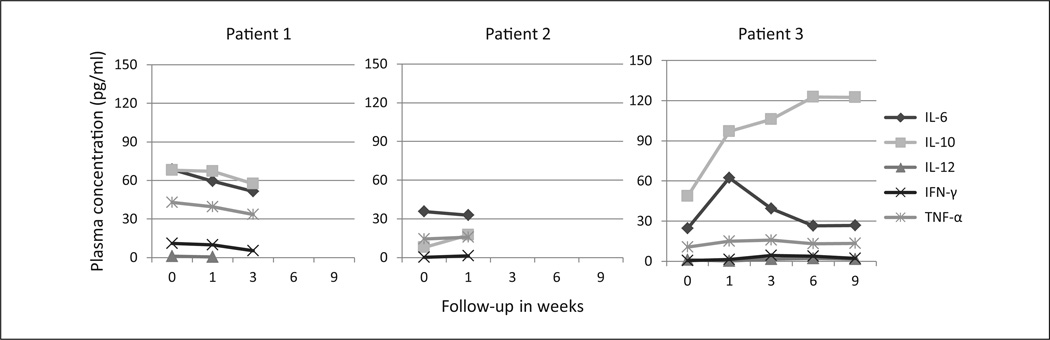
No clear patterns were observed in changes in inflammatory biomarkers although IL-6 trended during all points (Figure 3).
Figure 3. Change in plasma concentrations of inflammatory biomarkers.
Abbreviations: IL, interleukin; IFN-γ, interferon-gamma; TNF-α, tumor necrosis factor-α.
IL-12 levels for patient 2 are missing. Patient 1 died on day 39, patient 2 on day 23, and patient 3 on day 69.
Evaluation of Disease Progression and Response
The study protocol stated that imaging would be performed at baseline and 3 months to evaluate changes in RECIST criteria. Unfortunately, none of the three patients lived to the 3-month follow-up.
Conclusions
Our phase I trial did not enable us to determine an MTD for silybin phosphatidylcholine in this population of HCC patients with advanced liver disease. We selected subjects with expected survival time of 3 months, but none of our patients lived long enough to assess our primary endpoint.
Because the third patient showed improvement in laboratory values after being on the trial for some time, he may have derived some benefit from milk thistle. However, he ultimately had a lower gastrointestinal bleed, which precipitated his death. The second patient died from hemoptysis. A review of platelets and coagulation factors did not show significant changes in either of these patients while on study (data not shown). It is unclear if milk thistle increases the risk of bleeding in patients with end-stage liver disease, or if the bleeding was a complication from their underlying liver disease. To our knowledge, there are no published reports of milk thistle agents being associated with increased bleeding. However, there have been reports of milk thistle being associated with elevated liver enzymes, which could exacerbate the risk of bleeding.13 Since only one patient appeared to have improved liver function tests and AFP, the number is too few to comment on the significance. To our knowledge, this is the first dose escalation trial of a milk thistle compound that was halted because of potential adverse events. It is unclear whether this is reflective of the patient population or the investigational agent.
As shown in Table 1, one patient was Child–Pugh B and one was Child–Pugh C. The third patient (who was Child Pugh C) was on the trial for nearly 3 months. His liver function tests and AFP trended up initially but started to fall after week 6, as did some inflammatory biomarkers (IL-6 and TNF-α). The cause of these changes is unclear. However, our observation suggests that if we were able to select patients who lived slightly longer, it is possible that they may benefit from milk thistle. In the previous study in children by Ladas et al,5 there also was not a significant change in liver function tests at day 28, but improvements were seen at day 56. Milk thistle may have a role for helping to limit further hepatic injury, but may be less beneficial for individuals with severe/established damage. The average survival of Child–Pugh C patients is approximately 3 months, whereas that of Child–Pugh B patients is approximately 6 months.19
Future studies could assess subjects who were Child– Pugh B 8 or 9, rather than C. This group also has no standard treatment options and might be more likely to show benefit from milk thistle. There are now observational data suggesting that sorafenib may be administered safely to Child–Pugh B patients, although it is not clear whether it prolongs survival in this population.20 Alternatively, a phase I trial with sorafenib in Child–Pugh B patients may also be an interesting next step.
Acknowledgment
We thank the study participants and their families.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Lotte and John Hecht Memorial Foundation, Herbert Irving Comprehensive Cancer Center, NIH National Center for Advancing Translational Sciences (UL1 TR000040), NIH/NCI K23CA141052 (to HG) and NIH/NCI K23CA149084-01A1 (to ABS), and Steven J. Levinson Medical Research Foundation (to ABS).
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Olsen SK, Brown RS, Siegel AB. Hepatocellular carcinoma: review of current treatment with a focus on targeted molecular therapies. Therap Adv Gastroenterol. 2010;3:55–66. doi: 10.1177/1756283X09346669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 4.Siegel AB, Olsen SK, Magun A, Brown RS., Jr. Sorafenib: where do we go from here? Hepatology. 2010;52:360–369. doi: 10.1002/hep.23633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ladas EJ, Kroll DJ, Oberlies NH, et al. A randomized, controlled, double-blind, pilot study of milk thistle for the treatment of hepatotoxicity in childhood acute lymphoblastic leukemia (ALL) Cancer. 2010;116:506–513. doi: 10.1002/cncr.24723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagoner J, Morishima C, Graf TN, et al. Differential in vitro effects of intravenous versus oral formulations of silibinin on the HCV life cycle and inflammation. PloS One. 2011;6(1):e16464. doi: 10.1371/journal.pone.0016464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroll DJ, Shaw HS, Oberlies NH. Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther. 2007;6:110–119. doi: 10.1177/1534735407301825. [DOI] [PubMed] [Google Scholar]
- 8.Hogan FS, Krishnegowda NK, Mikhailova M, Kahlenberg MS. Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer. J Surg Res. 2007;143:58–65. doi: 10.1016/j.jss.2007.03.080. [DOI] [PubMed] [Google Scholar]
- 9.Fried MW, Navarro VJ, Afdhal N, et al. Silymarin in NASH and C Hepatitis (SyNCH) Study Group. Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis C unsuccessfully treated with interferon therapy: a randomized controlled trial. JAMA. 2012;308:274–282. doi: 10.1001/jama.2012.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawke RL, Schrieber SJ, Soule TA, et al. SyNCH Trial Group. Silymarin ascending multiple oral dosing phase I study in noncirrhotic patients with chronic hepatitis C. J Clin Pharmacol. 2010;50:434–449. doi: 10.1177/0091270009347475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cancer Institute Common Toxicity Criteria. Bethesda, MD: National Cancer Institute; [Google Scholar]
- 12.Flaig TW, Gustafson DL, Su L-J, et al. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs. 2007;25:139–146. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- 13.Flaig TW, Glode M, Gustafson D, et al. A study of high-dose oral silybin-phytosome followed by prostatectomy in patients with localized prostate cancer. Prostate. 2010;70:848–855. doi: 10.1002/pros.21118. [DOI] [PubMed] [Google Scholar]
- 14.Vidlar A, Vostalova J, Ulrichova J, et al. The safety and efficacy of a silymarin and selenium combination in men after radical prostatectomy—a six month placebo-controlled double-blind clinical trial. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010;154:239–244. doi: 10.5507/bp.2010.036. [DOI] [PubMed] [Google Scholar]
- 15.Sherman M, Bruix J, Porayko M, Tran T. AASLD Practice Guidelines Committee. Screening for hepatocellular carcinoma: the rationale for the American Association for the Study of Liver Diseases recommendations. Hepatology. 2012;56:793–796. doi: 10.1002/hep.25869. [DOI] [PubMed] [Google Scholar]
- 16.Hollebecque A, Cattan S, Romano O, et al. Safety and efficacy of sorafenib in hepatocellular carcinoma: the impact of the Child-Pugh score. Aliment Pharmacol Ther. 2011;34:1193–1201. doi: 10.1111/j.1365-2036.2011.04860.x. [DOI] [PubMed] [Google Scholar]
- 17.Heffernan N, Cella D, Webster K, et al. Measuring health-related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer therapy-hepatobiliary questionnaire. J Clin Oncol. 2002;20:2229–2239. doi: 10.1200/JCO.2002.07.093. [DOI] [PubMed] [Google Scholar]
- 18.Guo Y, Lu P, Farrell E, et al. In silico and in vitro pharmacogenetic analysis in mice. Proc Natl Acad Sci U S A. 2007;104:17735–17740. doi: 10.1073/pnas.0700724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llovet JM, Di Bisceglie AM, Bruix J, et al. Panel of Experts in HCC-Design Clinical Trials. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 20.Lencioni R, Kudo M, Ye SL, et al. First interim analysis of the GIDEON (Global Investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafeNib) non-interventional study. Int J Clin Pract. 2012;66:675–683. doi: 10.1111/j.1742-1241.2012.02940.x. [DOI] [PubMed] [Google Scholar]