Abstract
Objective
The aims was to investigate the energy-dose response effect of IES on small bowel motility, to compare the effect of forward and backward IES; to explore the possibility of using intermittent IES and mechanism of IES on intestinal motility.
Material and Methods
Five dogs implanted with a duodenal cannula and one pair of intestinal serosal electrodes were studied in 5 sessions: 1) energy-dose response study; 2) forward IES; 3) backward IES; 4) intermittent IES vs. continuous IES; 5) administration of guanethidine. The contractile activity and tonic pressure of the small intestine were recorded. The duration of sustained effect after turning off IES was manually calculated.
Results
1) IES with long pulses energy-dose dependently inhibited contractile activity and tonic pressure of the small intestine (p < 0.001). 2) The duration of sustained inhibitory effect of IES on the small intestine depended on the energy of IES delivered (p < 0.001). 3) The potency of the inhibitory effect was the same between forward and backward IES. 4) The efficacy of intermittent IES was the same as continuous IES in inhibiting motility of the small intestine. 5) Guanethidine blocked the inhibitory effect of IES on intestinal motility.
Conclusions
IES with long pulses inhibits small intestinal motility; the effect is energy-dose dependent, diffused and sustained. Intermittent IES has the same efficacy as the continuous IES in inhibiting small intestinal motility. Forward and backward IES have similar inhibitory effects on small bowel motility. This IES-induced inhibitory effect is mediated via the sympathetic pathway.
Keywords: intestinal pacing, gastrointestinal motility, sympathetic nerve, intestinal disorders, pseudo-obstruction
INTRODUCTION
Electrical stimulation as a unique modality is an attractive option for various gastrointestinal disorders refractory to conventional therapies. Gastric electrical stimulation (GES) has been used to either enhance gastric motor function in case of treating gastric motility disorders (1–4) or inhibit gastric motor function in treating obesity (5, 6) based on the pattern and energy of electrical stimulation delivered to the stomach as well as the stimulation location. It has been reported that short pulse gastric electrical stimulation improved dyspepsia symptoms in patients with gastroparesis (7), while long pulse gastric electrical stimulation affected gastric slow waves and improved gastric emptying (8, 9). Furthermore, forward GES (electrodes located at the corpus along the great curvature) or sequential GES (annular electrodes encircled the distal two thirds of the stomach) increased gastric emptying, while backward GES (electrodes located at the antrum) delayed gastric emptying.
Compared to GES, the effects of intestinal electrical stimulation (IES) on intestinal contractile activity are less understood. IES has been used to treat patients with short bowel syndrome (10–12), patients with dumping syndrome post-gastrectomy (13, 14), and patients with Roux stasis syndrome (15). In animal experiments, the results of IES on gastrointestinal motility were conflicting. Cranley showed IES on Roux limb delayed gastric emptying (15) while Sawchuk showed IES on Roux limb enhanced gastric emptying (16). Chen et al showed IES accelerated small intestinal transit slowed by fat in the ileum (17) whereas Gladen et al showed IES decreased small intestinal output and consequently enhancing water and glucose absorption (11). However, little is known on the effects and mechanisms of IES on intestinal contractile activity and tonic pressure (18, 19).
Forward and backward GES showed opposite effects on gastric motility. It is unclear whether it is also true for IES. It has been postulated that the inconsistency in the effect of IES on small bowel motility might be attributed to different energies of IES delivered and/or forward or backward IES applied (17, 20). However, there has been no data showing the energy dose response of contractile activity of the small intestine to IES and there have been no published studies comparing the effects on the small intestinal contractile activity and tone between forward IES and backward IES. In order to better understand the effect of IES on contractile activity of the small intestine, more information is needed to fulfill the gaps of our knowledge regarding IES. It was also of great interest and clinical significance to know whether IES would have an excitatory or inhibitory effect on contractile activity of the small intestine; whether this effect would be localized or diffused; and whether the effect of IES would be sustained
The aims of this study were, therefore, (1) to investigate the energy dose dependent effect of IES on contractile activity of the small intestine; (2) to compare the effects of backward IES and forward IES on small intestinal motility and (3) to study the sustained effect of IES on small intestinal motility, (4) to investigate the possible mechanism by which the effect of IES on intestinal motility is mediated.
METHODS
Animal Preparation
The procedures used in this study were approved by institutional animal care and use committee at the University of Texas Medical Branch at Galveston, Texas, USA. Five healthy female hound dogs (17.4~25.2 Kg) were included in the study. After an overnight fast, the dog was operated under general anesthesia. The anesthesia was induced with Pentothal (sodium thiopental 5 mg/kg, intravenous, Abbott Laboratories, North Chicago, Illinois) and maintained on IsoFlo (isoflurane 1.5%, inhalation anesthesia, Abbott) in oxygen-nitrous oxide (1:1) carrier gases delivered from a ventilator following endotracheal intubation. The dog was monitored with the assessment of tongue color, pulse rate, and breath rate. All dogs were surgically prepared with a chronic duodenal fistula located 20 cm beyond the pylorus. The fistula was fitted with a modified Thomas cannula. Just proximal and distal to the fistula, a Tygon tubing with a diameter of 2 mm was looped around the intestine to create a tent, and fixed by sutures through the visceral peritoneal to the intestinal wall. The length of tubing was individualized to be as short as possible without producing a tightening effect on the lumen. The cannula was brought out through the abdominal wall and fixed to prevent rotation. Two 28-guage cardiac pacing electrodes (A & E Medical, Farmingdale, NJ) were implanted 1 cm apart on the serosal surface of small intestine 30 cm distal to the duodenal cannula. The electrodes penetrated the subserosa and were affixed to the intestinal serosa by non-absorbable sutures. The electrode wires were subcutaneously tunneled through the anterior abdominal wall along the right side of the trunk and were placed outside the skin around the right hypochondrium for attachment to the electrical stimulator. Following completion of the operation, the anesthetic gases were discontinued. Extubation was performed after the airway reflexes were retained. The dog received medication for postoperative pain control and was transferred to a recovery cage. The study was initialized after the dogs were completely recovered from the surgery, usually after 2 weeks.
Experimental Protocols
All dogs were fast overnight before the experiments. Each dog was studied in 5 randomized sessions on 5 separate days (a minimum of three days apart). All experiments were performed in the fed state after the ingestion of 8 oz of standard dog can food. In session 1, the contractile activity and tone of the small intestinal segment distal to IES and the duration of sustained inhibitory effect of IES were recorded during IES with different stimulation parameters as the following combinations of pulse width (ms) with pulse amplitude (mA) and orders: 50 ms with 2.5 mA; 50 ms with 5 mA; 50 ms with 7.5 mA; 50 ms with 10 mA; 100 ms with 2.5 mA; 100 ms with 5 mA; 100 ms with 7.5 mA; 100 ms with 10 mA; 200 ms with 2.5 mA; 200 ms with 5 mA; 200 ms with 7.5 mA; 200 ms with 10 mA; 300 ms with 2.5 mA; 300 ms with 5 mA; 300 ms with 7.5 mA; 300 ms with 10 mA. In session 2, the contractile activity and tone of small intestinal segment proximal to IES was recorded for 40 minutes, 20-min at baseline and 20-min during IES. In session 3, the contractile activity and tone of small intestinal segment distal to IES was recorded for 40 minutes, 20-min for baseline and 20-min after IES. In session 4, the contractile activity and tone of small intestinal segment distal to IES was recorded for 20-min as baseline and 20-min during intermittent IES (see intestinal electrical stimulation in experimental session 4). In session 5, the contractile activity of small intestinal segment distal to IES was sequentially recorded for 20-min as baseline, 20-min after the administration of adrenergic blocking agent guanethidine (3 mg/kg for 5-min), 20-min during IES and 20-min for recovery.
Intestinal Electrical Stimulation
Various IES stimulation parameters have been tested in different experimental sessions as indicated below. Preliminary testing was performed before the following systematic study to make sure that none of the parameter sets to be tested would induce animal behaviors suggestive pain or discomfort or retching or vomiting.
Experimental session 1. IES was delivered using following different stimulation parameters. The stimulation frequency was fixed at 20 cpm, which was slightly higher than the frequency of the intestinal slow wave in dogs (about 18 cpm). Four different pulse widths (50 ms, 100ms, 200ms, and 300ms) and four different pulse amplitudes (2.5 mA, 5 mA, 7.5 mA, and 10 mA) were tested. The stimulation energy was calculated as a×b2, where a was the value of pulse width in ms and b was the value of pulse amplitude in mA. IES with each set of parameters was applied for 90 seconds and a period without IES was provided between two consecutive IES for the intestinal motility to recover to the baseline postprandial level. An adjustitable electrical stimulator (Model A 301, World Precision Instruments, Sarasota, FL, USA) was used for stimulation.
Experimental session 2, 3 and 5. IES was delivered with a frequency of 20 cpm, pulse widths of 200ms and amplitude of 10 mA. These parameters of IES were selected based on preliminary experiments that showed the most reliable and reproducible effect on intestinal motility during IES.
Experimental session .4 IES was performed intermittently using the same parameters as in experimental sessions 2 and 3. However, the stimulator was turned ‘on’ for a period of 90 seconds and ‘off’ for a period of 30 seconds during the 20-min IES period.
Measurement and analysis of small intestinal contractile activity and tone
Small intestinal contractile activity and tone were recorded using a manometric method via a catheter with an 8 ml balloon at the end, and 4 side holes at an interval of 5 cm (Medtronic, Synectic Medical AB, Stockholm, Sweden). The catheter was inserted through the duodenal cannula to the small intestinal segment with the balloon positioned either 5 cm distal to the IES site in experimental sessions 1, 2 and 4 or 5 cm proximal to the IES site in experimental session 3. The manometric assembly was continuously perfused by a pneumohydraulic capillary infusion system.
The manometric recording was made in the following sequence: 1) the animal was fed with one can of dog food; 2) 10 min after eating, a continuous recording of intestinal contractile activity was made for 20 min and this recording was considered as baseline); 3) various segmental recordings with or without IES as indicated in the experimental protocols for various sessions.
The contractile activity of small intestine was assessed by using the mean area under the contractile wave curve (mean AUC) per second. The mean AUC was computed by using the Polygram function testing software (software Medtronic, Synectic Medical AB, Stockholm, Sweden). A higher value in the mean AUC was indicative of increased contractile activities of the small intestine.
The tone of the small intestine was assessed from the basal pressure measured from the balloon channel. The mean basal pressure of the balloon channel was visually determined by drawing the best-fit line on the tracing every one-minute and the information regarding whether IES was performed was made unavailable during the assessment.
Sustained duration of IES
Sustained duration of IES was defined as the duration in seconds between the cessation of IES and the time when the contractive activity or the basal pressure returned to the baseline level before IES.
Statistics
In experimental session 1, the contractile activity, basal tone of the small intestine, and duration of sustained inhibitory effect during IES with 14 different sets of stimulation parameters were analyzed by One-way ANOVA. Bonferroni (All-Pairwise) Multiple Comparison Test was used to test significant difference between each two different conditions. Linear regression analysis was used to assess the correlation of two parameters. Paired t-test was used to compare two test conditions. All data are expressed as mean±SD. P value less than 0.05 was considered a statistically significant difference.
RESULTS
The study was well-tolerated by the animals. No animal behaviors suggestive pain or discomfort was noted. No adverse events were reported during and after the study.
Energy-dose-dependent inhibitory effect of IES on small intestinal motility
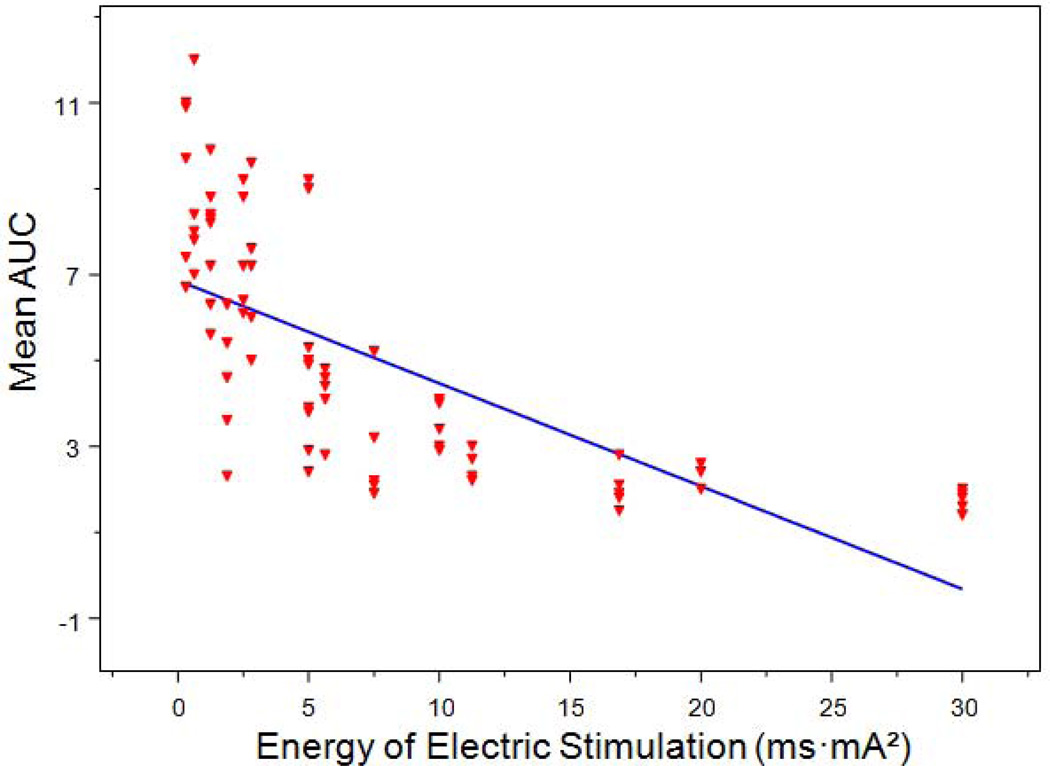
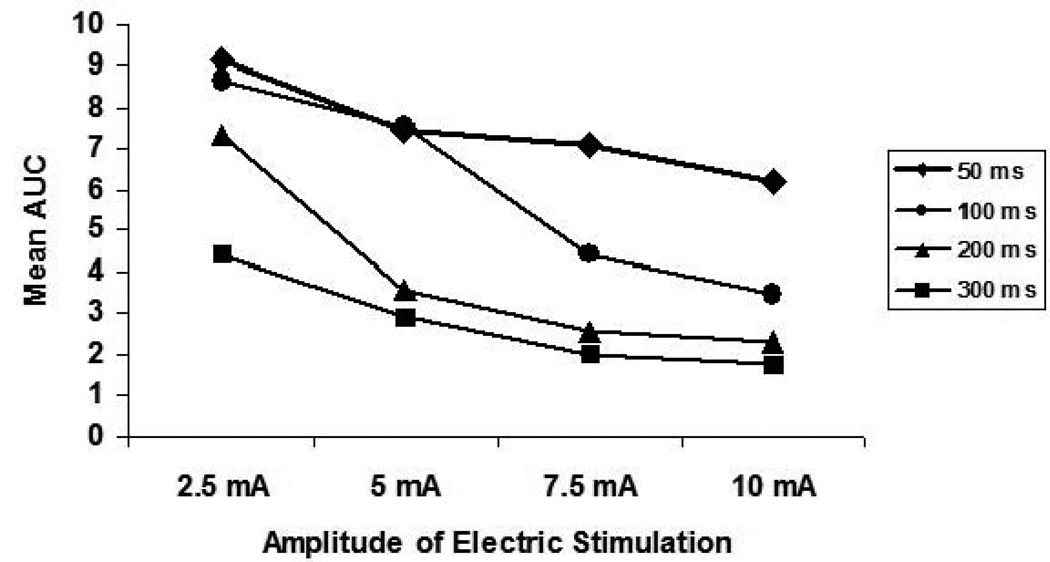
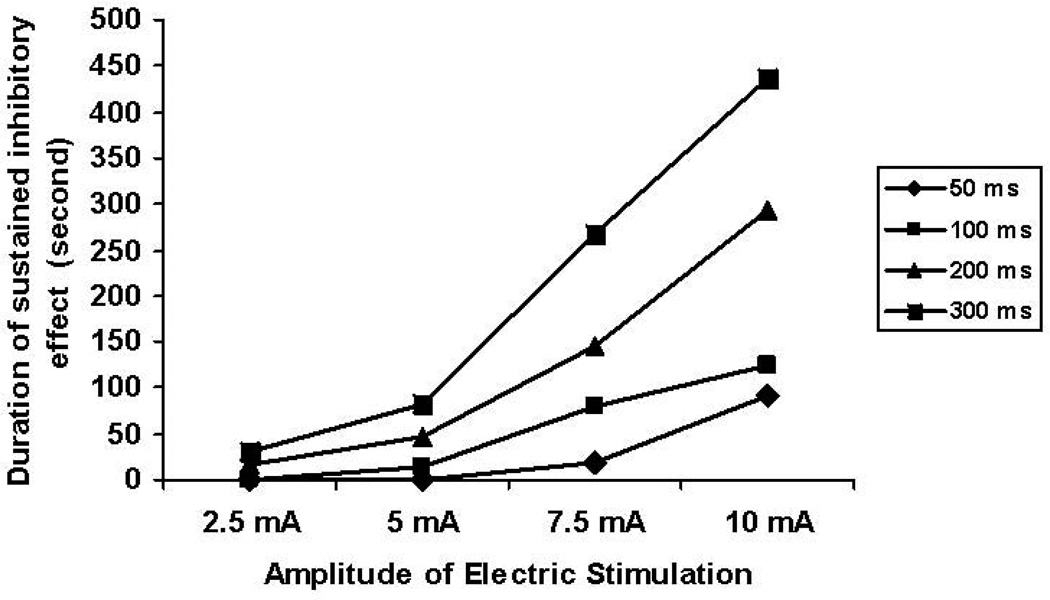
IES inhibited small intestinal contractile activity in an energy-dose–dependent manner. As shown in Table 1, the contractile activity of the small intestine presented as the mean AUC was 9.1 ± 2.0 during IES with the lowest energy to 1.7 ± 0.2 during IES with the highest energy (p < 0.001, one-way ANOVA). Linear regression analysis of 80 individual test periods from 5 dogs showed that IES inhibited small intestinal contractile activity in a manner that was dependent on energy-dose (r = −0.698, p < 0.001) (Figure 1). IES with energy ranged from 2.5 to 17 ms·mA2 showed reliable and reproducible inhibitory effects on small intestinal contractile activity. The inhibitory effect of IES on small intestinal contractile activity was less predictable if the stimulation energy was less than 5 ms·mA2 as shown in Figure 1. On the other hand, the maximum inhibition was reached when the stimulation energy was higher 17 ms·mA2. Figure 2 showed the effects of IES on the intestinal contractions with pulse width ranging from 50 ms to 300ms and pulse amplitude ranging from 2.5 mA to 10 mA.
Table.
IES energy-dose-dependently inhibited small intestinal motility
| Energy delivered to small intestine by IES (ms·mA2) |
Contractile activity during IES (mean AUC) |
Tonic Pressure Decreased during IES (mmHg) |
Duration of Sustained Inhibitory effect (Second) |
|---|---|---|---|
| 0.312 | 9.1 ± 2.0 | 0 ± 0 | 0 ± 0 |
| 0.625 | 8.6 ± 1.9 | 1.4 ± 3.1 | 0 ± 0 |
| 1.250 | 7.4 ± 1.6 | 1.5 ± 2.8 | 9.8 ± 17.3 |
| 1.880 | 4.4 ± 1.6 | 6.8 ± 2.0 | 92.6 ± 34.4 |
| 2.500 | 7.5 ± 1.4 | 3.4 ± 3.2 | 13.4 ± 18.6 |
| 2.810 | 7.1 ± 1.7 | 3.2 ± 2.9 | 16.3 ± 16.6 |
| 5.000 | 4.8 ± 2.5 | 5.9 ± 4.7 | 54.7 ± 37.9 |
| 5.630 | 4.1 ± 0.8 | 8.6 ± 3.2 | 46.0 ± 13.4 |
| 7.500 | 2.9 ± 1.4 | 11.2 ± 4.4 | 124.6 ± 38.6 |
| 10.00 | 3.5 ± 0.6 | 10.2 ± 4.0 | 81.2 ± 17.9 |
| 11.25 | 2.6 ± 0.3 | 7.8 ± 3.7 | 144.8 ± 81.3 |
| 16.88 | 2.0 ± 0.5 | 13.4 ± 5.1 | 294.4 ± 213.8 |
| 20.00 | 2.3 ± 0.3 | 12.0 ± 5.5 | 267.2 ± 156.9 |
| 30.00 | 1.7 ± 0.2 | 19.2 ± 11.4 | 436.8 ± 304 6 |
Figure 1.
Correlation of small intestinal contractile activity (mean AUC) with IES energy (ms·mA2). R2 = 0.487, p < 0.001.
Figure 2.
Dose-dependent curves of IES on small intestinal contractile activity (mean AUC) with four different long pulse IES (50 ms, 100 ms, 200 ms and 300 ms) combined with four different amplitudes of IES (2.5 mA, 5 mA, 7.5 mA, and 10 mA).
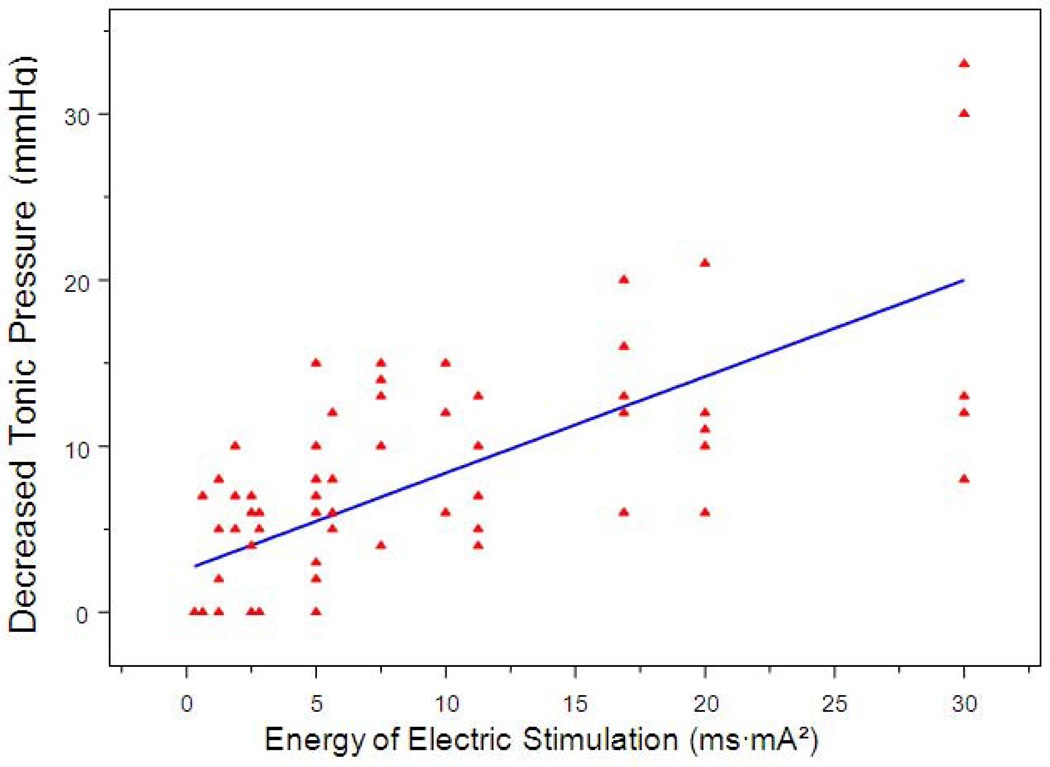
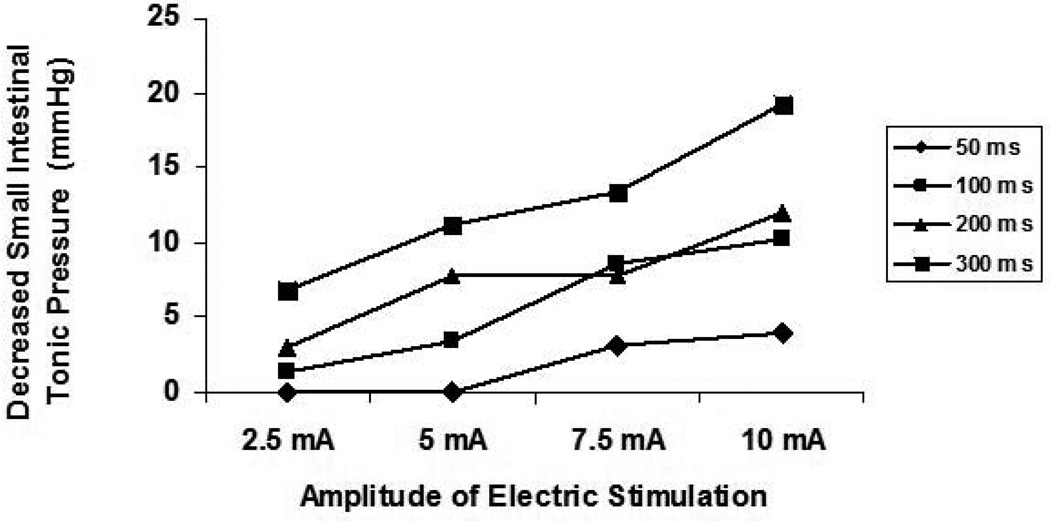
Similarly, IES reduced the tonic pressure of the small intestine in an energy-dose-dependent manner. As shown in table 1, the basal tonic pressure of the small intestine decreased from 0±0 mmHg during IES with the lowest energy to 19.2±11.4 mmHg during IES with the highest energy (p < 0.001, one-way ANOVA). Linear regression analysis of 80 individual test periods from the 5 dogs showed that IES decreased the tonic pressure of the small intestine in a manner that was dependent on energy dose (r= 0.710, p < 0.001, Figures 3). Figure 4 showed the effects of IES on tonic pressure changes with pulse width ranging from 50 ms to 300 ms and the pulse amplitude ranging from 2.5 mA to 10 mA.
Figure 3.
Correlation of decreased tonic pressure of the small intestine with IES energy (ms·mA2). R2 = 0.504, p < 0.001.
Figure 4.
Dose-dependent curves of IES on decreased tonic pressure of the small intestine with four different long pulse IES (50 ms, 100 ms, 200 ms and 300 ms) combined with four different amplitudes of IES (2.5 mA, 5 mA, 7.5 mA, and 10 mA).
Diffused inhibitory effects of IES on small intestinal motility
IES with long pulses (pulse width of 200 ms, amplitude of 10 mA and frequency of 20 cpm) significantly and substantially inhibited the contractile activity and tonic pressure of the small intestine. The mean AUC measured from the location either proximal or distal to the stimulation site during IES was 1.9±0.7 mmHg and 2.4±0.3 mmHg, which were substantially lower than that during baseline of 10.2±3.5 mmHg and 15.4±2.6 mmHg, respectively. The mean tonic pressure measured from the location either proximal or distal to the stimulation site during IES was 2.0±0.4 mmHg and 2.0±0.5 mmHg, which were substantially lower than that during baseline of 13.4±1.7 mmHg and 12.9±2.3 mmHg, respectively.
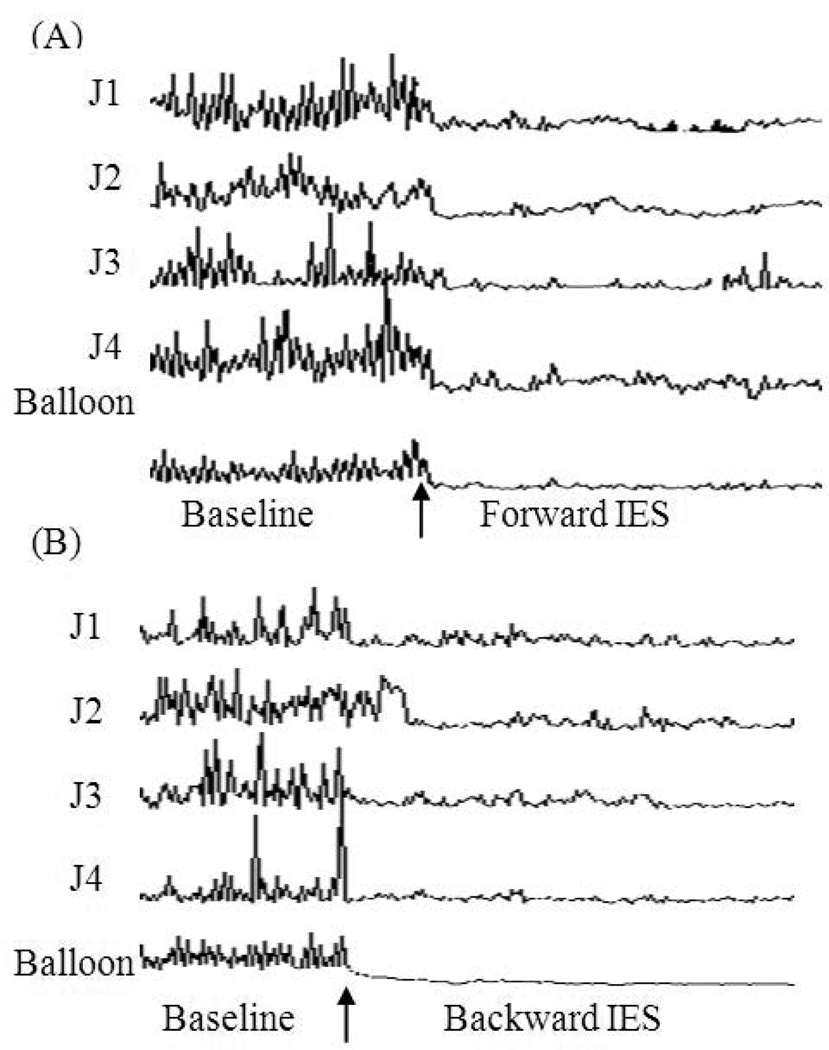
No difference was noted in the effect of IES on the contractile activity or tonic pressure of the small intestine between the forward stimulation and the backward stimulation. The mean AUC of the contractions measured from the small intestinal segment proximal to the IES site was inhibited by 81% compared to the mean AUC at baseline, whereas the mean AUC of the contraction of small intestinal segment distal to the IES was decreased by 85% compared to the mean AUC at baseline. These two values were not statistically different (p > 0.05). Similarly, the IES-induced inhibitions in the tonic pressure measured from the small intestine proximal to the IES site and distal to the IES site showed no difference (p > 0.05).
There was a diffused and consistent inhibitory effect as shown in the sample tracing (Figure 5): the phasic contractions were significantly suppressed in all four channels, spreading both forward (distal) and backward (proximal) from the IES site along the small intestine. Similar inhibitory effect of IES on tonic pressure of the small intestine was seen in balloon channel of the sample tracing (Figure 5).
Figure 5.
The sample manometric recordings of the small intestinal segment distal to IES site (A) and proximal to IES site (B) at base line and with long pulse IES of 200 ms, 10 mA of amplitude, and 20 cpm of frequency. Balloon 25 cm distal to IES site. J1: small intestine 5 cm from balloon. J2: small intestine 10 cm from balloon. J3: small intestine 15 cm from balloon. J4: small intestine 20 cm from balloon.
Sustained inhibitory effect of IES on small intestinal motility
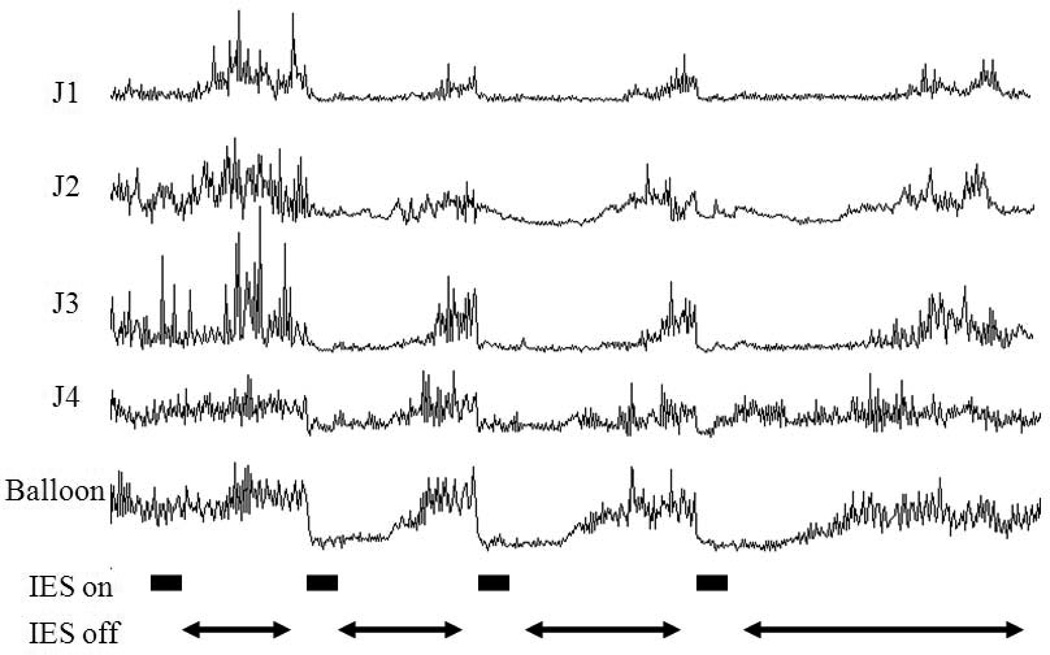
A sustained inhibitory effect of IES on small intestinal motility was observed (Figure 6). The duration of the sustained inhibitory effect of IES on the small intestinal contractile activity was increased with the increased stimulation energy and these two variables were significantly correlated with each other (r = 0.769, p < 0.001, table 1). The duration of the sustained effect ranged from 0 second with IES of the lowest energy to about 436 seconds with IES of the highest energy. (p < 0.001, one-way ANOVA). Figure 7 shows the different dose response curves of IES with pulse width ranging from 50 ms to 300ms with pulse amplitude ranging from 2.5 mA to 10 mA on the duration of the sustained inhibitory effect.
Figure 6.
Manometric recording of the small intestine with IES of different energy ranged from 1.25 to 20.0 (ms·mA2). IES was on for 2 min shown in block lines and the off for a period times shown in arrows until the contraction and basal tonic pressure of the small intestine were restored back to baseline level before next IES delieved. J1: small intestine 20 cm from balloon. J2: small intestine 15 cm from balloon. J3: small intestine 10 cm from balloon. J4: small intestine 5 cm from balloon.
Figure 7.
Dose-dependent curves of duration of sustained inhibitory effect (seconds) with four different long pulse IES (50 ms, 100 ms, 200 ms and 300 ms) combined with four different amplitudes of IES (2.5 mA, 5 mA, 7.5 mA, and 10 mA).
Efficacy of intermittent IES
Based on the sustained effect of IES, we proposed a method of intermittent IES, that is, IES was programmed to be alternatively and repetitively “on” for a certain period and “off” for another period. The duration of the “off” time was determined according to the duration of the sustained effect of IES with a particular set of parameters. It was found that such a designed intermittent IES was as effective as the continuous IES but apparently consumed less energy. Intermittent IES for 20 min (repetitively 90s-on and 30s-off) inhibited contractile activity of the small intestine by 65.0±14.3% compared to the baseline (p < 0.05, paired t-test) and significantly decreased the tone of the small intestine by 8.8 ± 5.8 mmHg compared to relative baseline (p<0.01). The mean AUC was 4.2±3.1 during continuous IES compared to the mean AUC of 12.7±4.0 in the baseline and the mean AUC was 4.5±1.4 during the intermittent IES compared to the mean AUC of 13.6±2.2 during the baseline. The mean decreased tonic pressure of the small intestine was 10.1±5.8 during continuous IES and the mean decreased tonic pressure of the small intestine was 8.7±5.8 during the intermittent IES. The potency of the inhibitory effects of IES on the contract tile activity and the tone of the small intestine was not different between the intermittent IES and the continuous IES (p > 0.05, paired t-test).
Aadrenergic mechanisms of IES
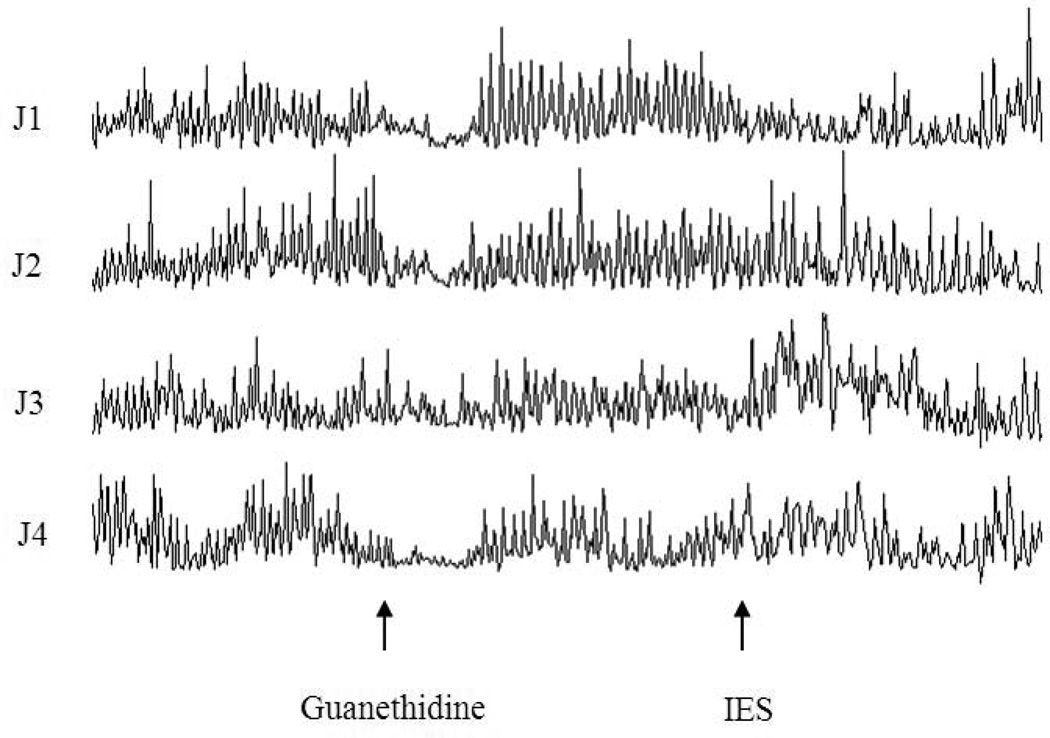
The inhibitory effect of IES on intestinal motility was blocked by guanethidine. As it can be seen from Figure 8 that in the session 5, regular intestinal contractions were recorded at baseline, not affected by guanethidine (except an initial brief inhibition) and not altered even with IES. The mean AUC was 14.5±1.0 after guanethidine infusion without IES and maintained unchanged during IES (14.3±1.9, p > 0.05, paired t-test).
Figure 8.
Manometric tracings showing the effects of IES with long pulses on small intestinal motility in session 5. Guanethidine prevented the inhibitory effect of IES on small intestinal motility.
DISCUSSION
While the effect of IES on intestinal transit and absorption has been previous studied, little is known on the effect of IES on intestinal contractions and tone. In this study, we found that 1) IES significantly inhibited the contractile activity and decreased the tonic pressure of the small intestine; 2) these inhibitory effects were diffused as they were noted from both segments proximal and distal to the IES site; 3) the potency and duration of the sustained inhibitory effects of IES on the small intestine were energy dose-dependent; 4) the effects of appropriately designed intermittent IES on the contractile activity and tonic pressure of the small intestine were the same as the continuous IES; 5) the inhibitory effect of IES on intestinal contractions was blocked by guanethidine, suggesting the involvement of the sympathetic pathway.
In this study, we used an established chronic canine model with a duodenal cannula and the clinically established manometric method to comprehensively and systematically determine the effect of IES on intestinal contractile and tonic activity. To the best of our knowledge, this was the first systematic study investigating the effects of intermittent IES on intestinal contractions. Previous studies have investigated the effects of IES on intestinal transit (17) and absorption (10, 21) and gastric emptying (16) without actual assessment of intestinal phasic and tonic activities. Moreover, none of previous studies has systematically investigated the dose/energy response. Contrast to the data presented in this study, it had previously been assumed that backward IES and forward IES had opposite effects on intestinal motility (14, 16, 22, 23).
A number of significant findings were reported in this study regarding the effects of IES on intestinal motility, which have not been reported elsewhere. Firstly, the effect of IES on intestinal motility was found inhibitory and energy-dose dependent. Secondly, the inhibitory effect of IES on intestinal motility was sustained after the cessation of IES and the sustained duration was also proportional to the stimulation energy. Thirdly, based on this sustained effect, an intermittent IES was proposed and the efficacy of the proposed intermittent IES on intestinal motility was the same as the continuous IES. If a battery is used as the energy source for IES, one-third energy will be preserved by using the proposed intermittent IES, which will be translated into prolongation of one-third of battery life. In addition, the proposed intermittent IES is superior to the continuous IES with regarding to possible muscle fatigue or tissue adaptation to IES. Finally, IES showed the same inhibitory effect on the contractile activity and tonic pressure of the small intestinal segments both proximal and distal to the IES site. This suggests that the effect of IES on small intestinal motility may not be a local myogenic effect but a diffused, and possibly a neurogenic reflexive phenomenon. This finding is also critical to understand why IES is able to extend its inhibitory effect to the stomach (24).
Previous studies have suggested different effects of IES on gastrointestinal transit described as “forward pacing” or “backward pacing”. Forward pacing was used to enhance the transit of the small intestine segment that located caudally to the IES site, such as to treat Roux stasis syndrome (25, 26). In contrast, backward pacing was used to inhibit transit of the small intestinal segment that located cranially to the IES site (13, 22, 27–31). However, a few studies showed that both forward and backward IES enhanced glucose and water absorption in both the intact and the transected small intestinal loop (22, 27, 30–32). A few recent studies showed that IES accelerated intestinal transit in a canine model of ileal brake (17) and decreased fat absorption in a rat model (33). In this study, the inhibitory effects of IES was found to be unrelated to the stimulation direction, whether forward or backward. It should be mentioned that none of previous studies systematically compared the performance difference between forward and backward stimulation. Most often, the stimulation was performed using electrodes either proximal to or distal to the testing segment but not both (22, 30, 34).
The mechanisms of diffused, sustained inhibitory effects of IES on motility of the small intestine are not fully understood. Nitric oxide (35–37), neuropeptide YY (38–40), VIP (41–44), and sympathetic pathway (45, 46) are potential mediators of the IES-induced inhibitory effect on small bowel motility. The diffused inhibitory effect of IES suggests that the IES-induced inhibitory effect on small bowel motility might involve certain neural reflex (46, 47). Guanethidine is an adrenergic blocker for preventing release of norepinephrine and the present study showed that the administration of guanethidine prevented the inhibitory effect of IES on intestinal motility, indicating that the inhibitory effect of IES on intestinal motility was mediated via the sympathetic nerve activation. This was in agreement with a previous study in which the inhibitory effect of IES on intestinal contractions was found to be mediated via sympathetic but not nitrergic, serotoninergic 5-HT3 or opiate pathway (48). The diffused and inhibitory effect of IES reported in this study suggests that IES may have a therapeutic potential for severe motility disorders featured with hypertensive and/or uncoordinated contractions, such as neuropathic intestinal pseudo-obstruction and spastic colon.
Based on its inhibitory effects on intestinal motility observed in this study, IES is anticipated to have a number of clinical applications in the future. Firstly, inhibited intestinal motility is expected to delay intestinal transit and therefore, the IES method presented in this study may be effectively applied to treat dumping or short bowel syndromes. Although this application was previously explored by Kelly and his colleagues (15, 16), more effective methodologies may be derived based on the findings of the present study. Secondly, in a subgroup of patients with intestinal pseudo-obstruction, intestinal motility is featured with uncoordinated hypertensive intestinal contractions (49). The inhibitory IES may be applied to treat this group of patients with intestinal pseudo-obstruction. Thirdly, it is known that, neurotransmitters, peptide YY and Glucagon-like peptide-1 (GLP-1), secreted in the distal intestine, play an important role in the control of satiety and food intake (50, 51). IES may be performed at the distal intestine to delay intestinal transit (52) and enhance the release of these satiety-related peptides, leading to an increased satiety. In addition, a separate study performed in our lab has shown that in addition to its inhibitory effects on intestinal motility, IES with appropriate parameters also inhibits gastric contractions and delays gastric empting in normal dogs (24). Taken together, IES is expected to have a therapeutic potential for obesity as shown in a recent study in dogs and humans (53, 54).
In conclusions, IES with long pulses inhibits motility of the small intestine mediated by the sympathetic pathway. This inhibitory effect is energy-dose dependent, diffused and sustained. IES performed intermittently based on the sustained duration is as effective as continuous IES. More studies are needed to explore clinical potentials of IES in treating various disorders related to intestinal motility or obesity.
Acknowledgement
This study was support in part by a grant from National Institute of Digestive Diseases and Kidney (DK063733).
Abbreviations
- IES
intestinal electrical stimulation
- GES
gastric electrical stimulation
- AUC
area under the curve
Footnotes
Conflict of Interest: none
Authorship Statement:
Dr. Zhao designed and conducted the study, including performing experiments, collecting and analyzing data, Dr. Zhao prepared the manuscript. Dr. Yin conducted the study, collected and analyzed the data, revised the manuscripts. Ms. Wang was in charge of collecting data. Dr. Chen designed and conducted study, interpreted the data and finalized the manuscript. All authors approved the final manuscript. National Institute of Digestive Diseases and Kidney provided funding for the study.
REFERENCES
- 1.McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998;114:456–461. doi: 10.1016/s0016-5085(98)70528-1. [DOI] [PubMed] [Google Scholar]
- 2.Hocking MP. Postoperative gastroparesis and tachygastria--response to electric stimulation and erythromycin. Surgery. 1993;114:538–542. [PubMed] [Google Scholar]
- 3.Song GQ, Chen JD. Gastric electrical stimulation on gastric motility in dogs. Neuromodulation. 2011;14:271–277. doi: 10.1111/j.1525-1403.2011.00338.x. [DOI] [PubMed] [Google Scholar]
- 4.Song GQ, Lei Y, Xu X, Chen JD. Gastric electrical stimulation with long pulses in humans and animals: can data obtained in animals be replicated in humans? Neuromodulation. 2010;13:87–92. doi: 10.1111/j.1525-1403.2009.00241.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen J. Mechanisms of Action of the Implantable Gastric Stimulator for Obesity. Obes Surg. 2004;14:S28–S32. doi: 10.1007/BF03342135. [DOI] [PubMed] [Google Scholar]
- 6.Cigaina V. Gastric pacing as therapy for morbid obesity: preliminary results. Obes Surg. 2002;12:S12–S16. doi: 10.1007/BF03342141. [DOI] [PubMed] [Google Scholar]
- 7.Yin J, Abell TD, McCallum RW, Chen JD. Gastric neuromodulation with Enterra system for nausea and vomiting in patients with gastroparesis. Neuromodulation. 2012;15:224–231. doi: 10.1111/j.1525-1403.2012.00429.x. [DOI] [PubMed] [Google Scholar]
- 8.Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 9.Chen JD, Qian L, Ouyang H, Yin J. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology. 2003;124:401–409. doi: 10.1053/gast.2003.50048. [DOI] [PubMed] [Google Scholar]
- 10.Gladen HE, Kelly KA. Electrical pacing for short bowel syndrome. Surg Gynecol Obstet. 1981;153:697–700. [PubMed] [Google Scholar]
- 11.Gladen HE, Kelly KA. Enhancing absorption in the canine short bowel syndrome by intesitnal pacing. Surgery. 1980;88:281–286. [PubMed] [Google Scholar]
- 12.Devine RM, Kelly KA. Surgical therapy of the short bowel syndrome. Gastroenterol Clin North Am. 1989;18:603–618. [PubMed] [Google Scholar]
- 13.Morrison PD, Kelly KA. Increasing antidumping effect of intestinal pacing with motor-active agents. Dig Dis Sci. 1986;31:422–427. doi: 10.1007/BF01311680. [DOI] [PubMed] [Google Scholar]
- 14.Becker JM, Sava P, Kelly KA, Shturman L. Intestinal pacing for canine postgastrectomy dumping. Gastroenterology. 1983;84:383–387. [PubMed] [Google Scholar]
- 15.Cranley B, Kelly KA, Go VL, McNichols LA. Enhancing the anti-dumping effect of Roux gastrojejunostomy with intestinal pacing. Ann Surg. 1983;198:516–524. doi: 10.1097/00000658-198310000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawchuk A, Canal D, Grosfeld JL, et al. Electrical pacing of the Roux limb resolves delayed gastric emptying. J Surg Res. 1987;42:635–641. doi: 10.1016/0022-4804(87)90007-2. [DOI] [PubMed] [Google Scholar]
- 17.Chen JD, Lin HC. Electrical pacing accelerates intestinal transit slowed by fat-induced ileal brake. Dig Dis Sci. 2003;48:251–256. doi: 10.1023/a:1021911023155. [DOI] [PubMed] [Google Scholar]
- 18.Yin J, Chen JD. Mechanisms and potential applications of intestinal electrical stimulation. Dig Dis Sci. 2009;55:1208–1220. doi: 10.1007/s10620-009-0884-3. [DOI] [PubMed] [Google Scholar]
- 19.Sevcencu C. Gastrointestinal mechanisms activated by electrical stimulation to treat motility dysfunctions in the digestive tract: a review. Neuromodulation. 2007;10:100–112. doi: 10.1111/j.1525-1403.2007.00098.x. [DOI] [PubMed] [Google Scholar]
- 20.Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of gastroparesis with electrical stimulation. Dig Dis Sci. 2003;48:837–848. doi: 10.1023/a:1023099206939. [DOI] [PubMed] [Google Scholar]
- 21.Sawchuk A, Nogami W, Goto S, et al. Reverse electrical pacing improves intestinal absorption and transit time. Surgery. 1986;100:454–460. [PubMed] [Google Scholar]
- 22.Layzell T, Collin J. Retrograde electrical pacing of the small intestine--a new treatment for the short bowel syndrome? Br J Surg. 1981;68:711–713. doi: 10.1002/bjs.1800681012. [DOI] [PubMed] [Google Scholar]
- 23.Reiser SB, Weiser HF, Schusdziarra V, Siewert JR. Effect of pacing on small intestinal motor activity and hormonal response in dogs. Dig Dis Sci. 1989;34:579–584. doi: 10.1007/BF01536336. [DOI] [PubMed] [Google Scholar]
- 24.Zhao X, Yin J, Chen J, et al. Inhibitory effects and mechanisms of intestinal electrical stimulation on gastric tone, antral contractions, pyloric tone, and gastric emptying in dogs. Am J Physiol Regul Integr Comp Physiol. 2009;296:R36–R42. doi: 10.1152/ajpregu.90627.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eagon JC, Kelly KA. Effects of gastric pacing on canine gastric motility and emptying. Am J Physiol. 1993;265:G767–G774. doi: 10.1152/ajpgi.1993.265.4.G767. [DOI] [PubMed] [Google Scholar]
- 26.Miedema BW, Kelly KA. The Roux stasis syndrome. Treatment by pacing and prevention by use of an 'uncut' Roux limb. Arch Surg. 1992;127:295–300. doi: 10.1001/archsurg.1992.01420030057011. [DOI] [PubMed] [Google Scholar]
- 27.Layzell T, Collin J, Johnston ID. Effect of retrograde electrical pacing on jejunal absorption of xylose and vitamin C. JPEN J Parenter Enteral Nutr. 1981;5:103–105. doi: 10.1177/0148607181005002103. [DOI] [PubMed] [Google Scholar]
- 28.Monson JR, Keane FB, Byrne PJ, Fry G, Hennessy TP. Retrograde electrical pacing and its influence on the migrating motor complex of the canine jejunum. Ir J Med Sci. 1984;153:161–165. doi: 10.1007/BF02939818. [DOI] [PubMed] [Google Scholar]
- 29.Morrison P, Miedema BW, Kohler L, Kelly KA. Electrical dysrhythmias in the Roux jejunal limb: cause and treatment. Am J Surg. 1990;160:252–256. doi: 10.1016/s0002-9610(06)80017-6. [DOI] [PubMed] [Google Scholar]
- 30.Collin J, Kelly KA, Phillips SF. Enhancement of absorption from the intact and transected canine small intestine by electrical pacing. Gastroenterology. 1979;76:1422–1428. [PubMed] [Google Scholar]
- 31.Collin J, Kelly KA, Phillips SF. Absorption from the jejunum is increased by forward and backward pacing. Br J Surg. 1979;66:489–492. doi: 10.1002/bjs.1800660712. [DOI] [PubMed] [Google Scholar]
- 32.Collin J, Kelly KA, Phillips SF. Increased canine jejunal absorption of water, glucose, and sodium with intestinal pacing. Am J Dig Dis. 1978;23:1121–1124. doi: 10.1007/BF01072888. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Chen J. Intestinal electric stimulation decreases fat absorption in rats: therapeutic potential for obesity. Obes Res. 2004;12:1235–1242. doi: 10.1038/oby.2004.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiser SB, Schusdziarra V, Bollschweiler E, Holscher AH, Siewert JR. Effect of enteric pacing on intestinal motility and hormone secretion in dogs with short bowel. Gastroenterology. 1991;101:100–106. doi: 10.1016/0016-5085(91)90465-w. [DOI] [PubMed] [Google Scholar]
- 35.llescher HD, Tougas G, Vergara P, Lu S, Daniel EE. Nitric oxide as a putative nonadrenergic noncholinergic inhibitory transmitter in the canine pylorus in vivo. Am J Physiol. 1992;262:G695–G702. doi: 10.1152/ajpgi.1992.262.4.G695. [DOI] [PubMed] [Google Scholar]
- 36.Boeckxstaens GE, Pelckmans PA, Ruytjens IF, et al. Bioassay of nitric oxide released upon stimulation of non-adrenergic non-cholinergic nerves in the canine ileocolonic junction. Br J Pharmacol. 1991;103:1085–1091. doi: 10.1111/j.1476-5381.1991.tb12304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990;345:346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi T, Yamamura T, Utsunomiya J. Human pancreatic polypeptide, neuropeptide Y and peptide YY reduce the contractile motility by depressing the release of acetylcholine from the myenteric plexus of the guinea pig ileum. Gastroenterol Jpn. 1992;27:327–333. doi: 10.1007/BF02777750. [DOI] [PubMed] [Google Scholar]
- 39.Al-Saffar A, Hellstrom PM, Nylander G. Correlation between peptide YY-induced myoelectric activity and transit of small-intestinal contents in rats. Scand J Gastroenterol. 1985;20:577–582. doi: 10.3109/00365528509089699. [DOI] [PubMed] [Google Scholar]
- 40.Browning KN, Travagli RA. Neuropeptide Y and peptide YY inhibit excitatory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Physiol. 2003;549:775–785. doi: 10.1113/jphysiol.2003.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goyal RK, Rattan S, Said SI. VIP as a possible neurotransmitter of non-cholinergic non-adrenergic inhibitory neurones. Nature. 1980;288:378–380. doi: 10.1038/288378a0. [DOI] [PubMed] [Google Scholar]
- 42.Kishi M, Takeuchi T, Suthamnatpong N, et al. VIP- and PACAP-mediated nonadrenergic, noncholinergic inhibition in longitudinal muscle of rat distal colon: involvement of activation of charybdotoxin- and apamin-sensitive K+ channels. Br J Pharmacol. 1996;119:623–630. doi: 10.1111/j.1476-5381.1996.tb15719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamura T, Tanobe Y, Fujioka H, Ayajiki K, Toda N. Mechanism of neurogenic relaxation and modification of the response by enteric substances in isolated dog colon. Eur J Pharmacol. 1998;358:245–252. doi: 10.1016/s0014-2999(98)00624-4. [DOI] [PubMed] [Google Scholar]
- 44.Maggi CA, Giuliana S, Santicioli P, et al. Vasoactive intestinal polypeptide (VIP) and the specific motor response to capsaicin of the human isolated ileum. Adv Exp Med Biol. 1991;298:213–217. doi: 10.1007/978-1-4899-0744-8_19. [DOI] [PubMed] [Google Scholar]
- 45.Bjorck S, Kelly KA, Phillips SF. Mechanisms of enhanced canine enteric absorption with intestinal pacing. Am J Physiol. 1987;252:G548–G553. doi: 10.1152/ajpgi.1987.252.4.G548. [DOI] [PubMed] [Google Scholar]
- 46.Glise H, Lindahl BO, Abrahamsson H. Reflex adrenergic inhibition of gastric motility by nociceptive intestinal stimulation and peritoneal irritation in the cat. Scand J Gastroenterol. 1980;15:673–681. doi: 10.3109/00365528009181514. [DOI] [PubMed] [Google Scholar]
- 47.Glise H, Abrahamsson H. Reflex vagal inhibition of gastric motility by intestinal nociceptive stimulation in the cat. Scand J Gastroenterol. 1980;15:769–774. doi: 10.3109/00365528009181528. [DOI] [PubMed] [Google Scholar]
- 48.Liu S, Liu JS, Chen JDZ. Intestinal electrical stimulation inhibits intestinal motility via adrenergic not nitrergic pathway in dogs. Neurogastroenterology and Motility. 2006;18:62–68. doi: 10.1111/j.1365-2982.2005.00739.x. [DOI] [PubMed] [Google Scholar]
- 49.Jones MP, Wessinger S. Small intestinal motility. Curr Opin Gastroenterol. 2006;22:111–116. doi: 10.1097/01.mog.0000203867.33008.52. [DOI] [PubMed] [Google Scholar]
- 50.Beglinger C, Degen L. Gastrointestinal satiety signals in humans--physiologic roles for GLP-1 and PYY? Physiol Behav. 2006;89:460–464. doi: 10.1016/j.physbeh.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 51.Huda MS, Wilding JP, Pinkney JH. Gut peptides and the regulation of appetite. Obes Rev. 2006;7:163–182. doi: 10.1111/j.1467-789X.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- 52.Hoepfner MT, Kelly KA, Sarr MG. Pacing the canine ileostomy. Surgery. 1988;104:476–481. [PubMed] [Google Scholar]
- 53.Yin J, Ouyang H, Chen JD. Potential of intestinal electrical stimulation for obesity: a preliminary canine study. Obesity (Silver Spring) 2007;15:1133–1138. doi: 10.1038/oby.2007.615. [DOI] [PubMed] [Google Scholar]
- 54.Liu J, Qiao X, Hou X, Chen JD. Effect of intestinal pacing on small bowel transit and nutrient absorption in healthy volunteers. Obes Surg. 2009;19:196–201. doi: 10.1007/s11695-008-9533-8. [DOI] [PubMed] [Google Scholar]