Abstract
A growing interest in cerebellar function and its involvement in higher cognition have prompted much research in recent years. Cerebellar presence in a wide range of cognitive functions examined within an increasing body of neuroimaging literature has been observed. We applied a meta‐analytic approach, which employed the activation likelihood estimate method, to consolidate results of cerebellar involvement accumulated in different cognitive tasks of interest and systematically identified similarities among the studies. The current analysis included 88 neuroimaging studies demonstrating cerebellar activations in higher cognitive domains involving emotion, executive function, language, music, timing and working memory. While largely consistent with a prior meta‐analysis by Stoodley and Schmahmann (2009: Neuroimage 44:489‐501), our results extended their findings to include music and timing domains to provide further insights into cerebellar involvement and elucidate its role in higher cognition. In addition, we conducted inter‐ and intradomain comparisons for the cognitive domains of emotion, language, and working memory. We also considered task differences within the domain of verbal working memory by conducting a comparison of the Sternberg with the n‐back task, as well as an analysis of the differential components within the Sternberg task. Results showed a consistent cerebellar presence in the timing domain, providing evidence for a role in time keeping. Unique clusters identified within the domain further refine the topographic organization of the cerebellum. Hum Brain Mapp 35:593–615, 2014. © 2012 Wiley Periodicals, Inc.
Keywords: cerebellum, cognition, emotion, language, music, neuroimaging, timing, meta‐analysis
INTRODUCTION
Interest in cerebellar contributions to nonmotor functions has been building over the last decade; where past attention was solely given to its role as a motor control device, recently the focus has shifted to establishing the cerebellum's precise role in higher cognitive functions. Although cerebellar pathology with intellectual, emotional, and behavioral impairments have been reported from as early as 1831 [Combettes, as cited in Schmahmann,2004], recognition for its involvement in nonmotor‐related functions came much later. Evidence for cerebellar contributions to higher cognition has been documented in numerous clinical reports, lesion studies, and more recently, neuroimaging research. Lesion studies in nonprimates first provided evidence in determining cerebellar contributions to motor coordination. Its importance in associative motor learning was suggested in classical studies of eye‐blink conditioning in animals with cerebellar lesions [e.g., Chen et al.,1996; McCormick et al.,1982; McCormick and Thompson,1984; Knowlton et al.,1988] where the presence of cerebellar lesions disrupt the acquisition and retention of conditioned blinking. In a recent review, Timmann et al. 2010 reasoned that the cerebellum is also involved in higher order associative learning such as emotional and cognitive associative learning. For example, a classical fear conditioning study demonstrated the inability of patients with medial cerebellar lesions to associate the neutral stimulus of tone with aversive electrical shocks [Maschke et al.,2002]; unlike healthy controls, they showed no startle responses. Another study showed that patients with cerebellar degenerative disease were also unable to make cognitive associations between colors and numbers [Drepper et al.,1999].
Evidence for cerebellar role beyond motor coordination and associative learning was demonstrated by studies in visual attention. Studies by Courchesne et al. [e.g. Allen et al.,1997] using visual attention tasks provide support of the cerebellum's involvement in attention. In a recent replication of the classical blink paradigm, Schweizer et al. 2007 found rapid visual attention to be impaired in patients with focal cerebellar lesions. They found a deficit in patients' resource allocation during temporally demanding segments of the task and concluded that fast and efficient visuo‐temporal attention required an intact cerebellum. In fact, it was further suggested that the cerebellum plays a pivotal role within a neuro‐network describing visuo‐temporal attention.
More recently, studies employing various imaging methods such as PET and fMRI, provide further support for cerebellar activity in cognition. A summary of this development was presented through a meta‐analysis by Stoodley and Schmahmann 2009; their study provided systematic evidence for the cerebellum's involvement in higher cognition. This meta‐analysis consisted of 54 imaging studies that summarized a current understanding of the cerebellum's contribution in various cognitive functions via functional neuroimaging. The study also provided valuable insights to the role of cerebellum in the domains of motor, somatosensory, spatial, language, executive function, emotion, and working memory.
Cumulative evidence of the cerebellum's involvement in higher cognition has led to the development of theories which attempt to understand its precise function. One prominent theory, the cerebellar cognitive affective syndrome (CCAS), describes the cerebellum as a mediator of functions; a breakdown in the fronto‐cerebellar connection is manifested in a pattern of deficiencies in executive functioning, spatial cognition, linguistic abilities, and negative changes in personality [Schmahmann,1998].
Another well‐researched approach to understanding cerebellar role proposes an error‐driven model that describes the cerebellum as a “general‐purpose” modulator of cognitive processes that detects various patterns and its changes together with errors in cognition, updates the information and then provides adaptive feedback to the cerebral cortex [Andreasen and Pierson,2008; Bower,1997; Ito,1997; Leiner et al.,1991]. One of the theories in this approach by Ito 1997 suggests that the cerebellum is part of a network that oversees the execution of adaptive learning based on models of error‐based motor learning. Andreasen and Pierson 2008 applied this theory to their work on schizophrenia and describes a "cognitive dysmetria" where hallucinations are accounted for by cerebellar failures to detect errors in various perceptions.
Of particular interest, is an extensive model describing the role of the cerebellum as that of an internal timing device involving several processes, including the perception of speed [Ivry and Keele,1989]. Evidence which suggests the cerebellum serves as an internal timing mechanism is demonstrated in reports of patients with cerebellar damage, who have deficits in their judgments of short duration stimuli or speed of moving visual stimuli [Ivry,1997].
While perception of speed and its subsequent relation to other time‐related functions are part of time‐processing, knowledge of temporal order is another aspect of this domain. In evaluating the internal timing theory to explain cerebellum's contribution to articulatory rehearsal in verbal working memory, Ben‐Yehudah et al. 2007 highlighted evidence from an fMRI study conducted by Henson et al. 2000 that compared differing probes using a delayed match‐to‐sample paradigm. They found that when subjects were asked to assess temporal ordering information versus letter recognition, increase activations in bilateral cerebellum was elicited. Evidence from these studies suggests that the cerebellum acts as a form of internal timing device. Nonetheless, one of the challenges in examining this theory lies in distinguishing time‐related processes which are inherent in many higher cognitive functions.
Objectives of the Current Study
The overarching objective of the current study is to provide consolidated evidence in determining the role of the cerebellum in higher cognitive tasks via a meta‐analysis. A meta‐analysis provides a systematic way of identifying similarities among studies that may have inconsistent results, and specifically, regions that are consistently activated in different functions. As shown in the meta‐analysis by Stoodley and Schmahmann 2009, a functional topography of distinct cerebellar regions involved in motor, somatosensory, verbal working memory, spatial, executive function, and emotion processing tasks was identified. They found considerable overlaps in peak activations for verbal working memory and language tasks. Bilateral cerebellar activation was reported in executive functioning, suggesting cerebellar involvement across multiple domains. Their meta‐analytic study on cerebellar functions provided a platform upon which this study aimed to build upon. The present meta‐analysis extended the topography to include two other higher cognitive functions of timing and music, as well as comparisons among cognitive domains.
Music and Timing
Music is a complex function that relies on both perception of speed and knowledge of temporal order. In Peretz and Zatorre's 2005 review of brain organization in music processing, they highlighted that pitch and time relations are different dimensions, as evidenced by a body of neuropsychological tasks where patients' performance at one dimension did not affect the other. A meta‐analysis of functional neuroimaging studies on various auditory tasks by Petacchi et al. 2005 also provided support for the cerebellum to be involved in sensory auditory processing. However, the involvement of the cerebellum with pitch and time in music processing has not been systematically examined. Earlier, Sakai et al. 1999 reported metrical and non‐metrical rhythm processing as engaging specific cerebellar mechanisms. Yet, despite increasingly more studies reporting cerebellar presence in the functional imaging of music‐related tasks, none have yet to analyze this body of information. Hence, one of the main motivations for including music in the current meta‐analysis was to gain meaningful conclusions about the cerebellum's precise involvement in this complex cognitive function.
Given the inherent differences between pitch (melodic) and temporal processes, this study has chosen to focus on the rhythmic (temporal) aspects of music processing to provide a comparison with studies related to time processing. This study also evaluated the possibility of the cerebellum serving as a time‐keeping mechanism, and attempted to identify the cerebellar locations for music‐related tasks and conduct a comparison between the domains of music and timing. We expected a strong association between music processing and timing. We further addressed findings from our current study in terms of the theory of the cerebellum's role as an internal timing device. Given that time‐related processes are inherent in many higher cognitive functions, comparisons between the various domains examined here and that of timing were conducted.
Comparisons Among and Within Domains
Existing attempts at identifying both common and unique neural underpinnings of cognitive functions often involve comparisons among multiple studies. Results can get complicated due to the varied approaches adopted by each individual study. While meta‐analysis provides a systematic way of compiling information across different studies, subtle details may be lost when considering studies that employ different tasks of the same domain.
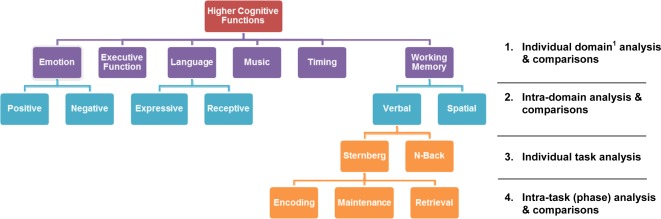
To increase our understanding of the complexities of different tasks within each domain, we performed the meta‐analysis in a “step‐down” fashion to refine each domain and further analyze tasks within individual domains. Table 1 summarizes the series of comparisons involved in the current study. We first performed meta‐analysis in examining the cerebellar involvement in six selected cognitive domains (referred to as the Individual domain analysis and comparisons in Table 1). Studies in three selected domains (emotion, language, and working memory) were further categorized into separate, specific categories (e.g., expressive and receptive language tasks, positive, and negative emotions) and were compared (referred to as the “Intra‐domain analysis and comparisons in Table 1). Within the working memory domain, individual task differences in verbal working memory were considered by a comparison of the Sternberg with the n‐back tasks (referred to as the “Individual task analysis” in Table 1). Finally, different components within the Sternberg task were also identified and analyzed separately (referred to as the intra‐task/component analysis in Table 1).
Table 1.
Summary of the cognitive functions and the series of comparisons involved in current meta‐analysis study
|
METHODS
Literature Review and Inclusion Criteria
Imaging studies published between 1993 and 2011 were identified using the following databases: EBSCOHost, JSTOR, MEDLINE, Pubmed, PsycInfo, ScienceDirect Online, Scopus, and SpringerLink, with keywords “cerebellum,” “cerebellar,” “cognition,” “imaging,” as well as keywords that describe each of the domains: “emotion,” “executive function,” “language,” “music, rhythm,” “timing,” and “working memory”. Out of ∼430 articles identified, 88 studies fulfilling the following criteria were included in the meta‐analysis: (1) specific nonmotor‐related cognitive functions were examined; (2) coordinates of activation maxima in standardized stereotaxic space were provided; and (3) imaging method used was either PET or fMRI. In addition to all the studies examined in Stoodley and Schmahmann's meta‐analysis, 53 studies were uniquely included in this analysis. Only coordinates of interest from normal healthy subjects were included in this meta‐analysis; patient group information from studies which recruited both patient and normal healthy subjects were excluded. In addition, only coordinates of interest from tasks which controlled for motor responses were selected. Appendix A provides the full list of studies in this article. While most studies had whole brain coverage, several studies either did not specify or were unable to achieve whole brain coverage due to the study's focus on frontal regions.
Procedure
A Java‐based software, GingerALE 1.2 [Laird et al.,2005], was used to perform the activation likelihood estimation (ALE) meta‐analyses reported in this study. The ALE meta‐analytic procedure was originally developed by Turkeltaub et al. 2002 and further refined by Laird et al. 2005. The ALE method attempts to reveal interstudy consistencies. Based on the collection of activation foci (observed maxima) reported in studies identified in a meta‐analysis, the probability that at least one of the activation foci lies within a voxel (the ALE statistic) is estimated. Such computation is repeated at each voxel in the brain and results in an ALE map.
To test the significance of the results, GingerALE implements a nonparametric permutation test. It tests the null hypothesis that the activation foci are spread uniformly throughout the brain (i.e., random clustering). Rejection of the null suggests the presence of real signal (i.e., nonrandom clustering). Laird et al. 2005 further proposed a technique for making statistical comparisons between two ALE maps using a permutation method. The difference in ALE value between two ALE images, which measures the difference in convergence between two maps, is first computed at each voxel. Statistical significance is then tested by the permutation method. More details on these permutation tests can be found in Turkeltaub et al. [2002, p 769–772] and Laird et al. 2005, p 156–159). To address the multiple comparisons issue, GingerALE implements two methods: (1) a single threshold test based on the null distribution of the maximal statistic for controlling family‐wise error rate, and (2) a false discovery rate procedure.
Coordinates from studies that used the Talaraich template were all transformed to MNI space using one of three options [(1)Talairach to MNI (SPM)for conversions using the Lancaster transform; (2) Brett: Talairach to MNI; (3) Talairach to MNI (Other) for programs other than SPM or FSL] available within GingerALE. For example, coordinates in Talairach space from studies using SPM for normalization which specified a conversion from MNI space using the Brett transform were converted back to MNI space using the "Brett: Talairach to MNI" option. Foci were then sorted according to one of six selected domains: emotion, executive function, language, music, timing, and working memory (see Table 2 for summary of tasks included in each domain). Eighteen studies were included in the emotion domain, with a total of 62 foci. Thirteen studies were included in the executive function domain with a total of 58 foci. Fifteen studies were included in the language domain with a total of 68 foci. Seven studies were included in the music domain with 57 foci. Nine studies were included in the timing domain with 36 foci and 26 studies were included in the working memory domain with 116 foci. Appendix A provides a complete list of studies and details on foci from each study included in our analysis.
Table 2.
Description of tasks in each higher cognitive domain
| Higher cognitive domain | Tasks included |
|---|---|
| Emotion | IAPS picture viewing; Facial emotions identification; Emotional intonation identification |
| Executive function | Decision making; Random number generation; Task switching; Arithmetic and Memory task; Go‐No‐Go task; Tower of London |
| Language | Verb generation; Verb reading; Covert word repetition; Semantic discrimination task |
| Music (Rhythm) | Rhythmic synchronization task; Temporal sequence performance (paced and trained); Audio‐paced rhythmic performance; Rhythm perception and production, Rhythmic learning |
| Timing | Irregular timing; Timing vs. Order task; Temporal‐spatial perceptual prediction; Temporal reproduction timing task; Time Interval Production task |
| Working memory | Sternberg working memory; PASAT; n‐back/2‐back task; linear increases in activation with increasing memory load; oculomotor delayed response task |
Analysis of each domain was conducted following the procedures used by Stoodley and Schmahmann 2009. Text files of coordinates for each of the six domains were created and activation likelihood estimates [ALE; Turkeltaub et al.,2002] for each voxel were computed at a FWHM of 12 mm, with permutation tests of 5,000 and a false discovery rate of P = 0.001. Cluster analysis with a minimum cluster of 150 mm3 was performed on the final thresholded map. GingerALE was employed to conduct statistical comparisons between two sets of foci by testing for significant differences in the convergence of activated regions. Foci for each domain were compared to determine unique activation areas by loading GingerALE with two sets of foci corresponding to the domains at the same time and were analyzed using the same parameters, output and visualization procedures as described previously.
Studies in three selected domains (emotion, language, and working memory) were further categorized into the following for an intradomain analysis: positive and negative emotions; expressive and receptive language tasks; verbal and spatial working memory. Comparisons of these categories within the same domain were performed, following the same parameters, output and visualization procedures described previously. In addition, individual task differences within the domain of verbal working memory were considered by a comparison of the Sternberg with the n‐back tasks. Components within the Sternberg task were analyzed separately (an intratask analysis), with foci sorted under encoding, maintenance and retrieval components based on information provided in the original studies. This systematic breakdown of the various domains and tasks examined is summarized in Table 1.
RESULTS
Individual Domain Peak Coordinates
As Figure 1 illustrates, emotional processing tasks activated right Lobules IV/V, VI, IX, and bilateral Lobules VIII and Crus 1. Executive function tasks were found to activate Crus 1 bilaterally, left Crus 2 right Lobule VI and midline Lobule VII. Peak activation coordinates were found bilaterally in Lobules VI, midline Lobule VIII, left Crus 1 and right Crus 2 in language tasks. Activation was found in bilateral Lobule VI for music and timing tasks, but music tasks also activated bilateral Lobule VIII, and right Lobule IV/V. Activations for working memory tasks were found primarily in bilateral Crus 1, as well as left Lobule IV/V and right Lobule VIII. The cluster sizes, peak coordinates, significant ALE values, and cerebellar locations of each domain are presented in Appendix B.
Figure 1.
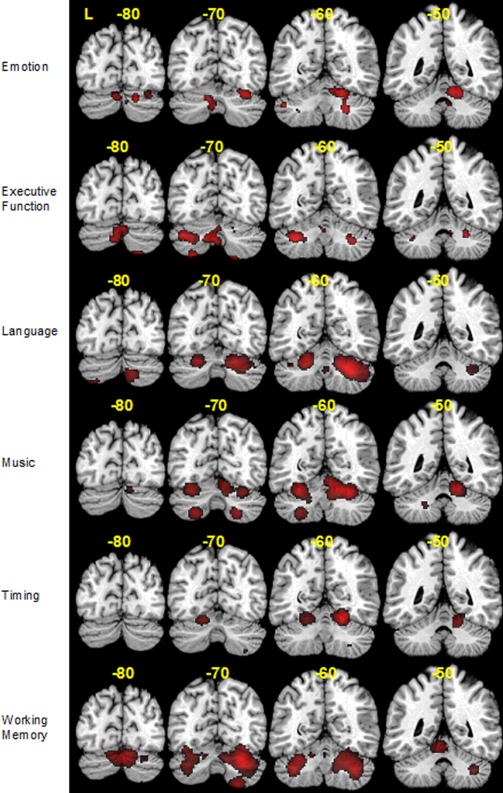
ALE activation maps for emotion, executive function, language, music, timing and working memory at four different slices (y = −80, −70, −60, −50) mapped onto coronal sections of the Colin27 brain.
Activation Clusters in Domain Comparisons
Overlapping regions across several domains provided the impetus for comparisons between domains. Unique clusters of activations emerged for each domain and results are summarized in Table 3. Results have been presented such that regions unique to a particular domain are reported within the column; each row reflects the domain with which a comparison was made. A detailed listing of these results are included in Appendix C. Clusters of activation in bilateral Lobule IV/V were found to be significantly different in domain of emotion (A) when compared with executive function (B), language (C), and working memory (F), and in bilateral Crus 1 when compared with music (D) and timing (E). Consistent clusters of activation were found to be uniquely different for executive function (B) in left Crus 1 when compared with three other domains (C, D, and E) and left lobule VIIB when compared with domains C, E, and F. Clusters of activation for language (C) were consistently found in right Crus 1 and 2 when compared with domains A, B, and D, and in bilateral Lobule VI when compared with domains A, B, C, and E. Activation clusters for music tasks (D) were consistently found in a wider number of areas, including right Lobule IV/V, bilateral VI and left Lobule VIII when compared with all four other domains. Timing tasks (E) were found to uniquely activate either bilateral Lobule VI (when compared with domains A and B) or right lobule VI (when compared with domains C, D, and E). Activation clusters for working memory tasks (F) were also found in a wide number of areas, including left Lobule IV/V and VI, right Lobule VIII, right Crus 1 and left Crus 2.
Table 3.
Table listing results of pair‐wise task activation comparisons, where locations for presence of unique activations are provided
| In comparison with | Regions unique to: | |||||
|---|---|---|---|---|---|---|
| A (Emotion) | B (EXE Function) | C (language) | D (Music) | E (Timing) | F (Working MEM) | |
| A | X | Left Crus 1 and 2 | Right Crus 1 and 2; Bilateral Lobule VI | Midline and Right Lobule IV/V; Bilateral Lobule VI; Left Lobule VIII | Bilateral Lobule VI | Midline Lobule IV/V; Right Lobule VIII; Right Crus 1 and Left Crus 2 |
| B | Right Lobule IV/V; Right Lobule VI | X | Bilateral Lobule VI; Right Crus 1 and 2 | Right Lobule IV/V; Bilateral Lobule VI and VIII | Bilateral Lobule VI | Left Lobule IV/V; Bilateral Lobule VI; Bilateral Crus 2, Right Crus 1, Lobule VIII and VIIB |
| C | Right Lobule IV/V; Lobule X | Left Crus 1; Midline and Left Lobule VIIB | X | Right Lobule IV/V; Bilateral Lobule VI; Left Lobule VIII | Right Lobule VI | Bilateral Crus 1; Left Crus 2, Lobule IV/V and VI; Right Lobule VIII and VIIB |
| D | Bilateral Crus 1 | Left Crus 1 and 2 | Right Crus 1 and 2; Bilateral Lobule VI | X | Right Lobule VI | Bilateral Crus 1 and Left Crus 2 and Lobule IV/V |
| E | Right Lobule VI; Bilateral Crus 1; Left Crus 2 | Bilateral Crus 1; Midline Lobule VII; Left Lobule VIIB | Right Crus 2; Bilateral Lobule VI | Right Lobule IV/V; Bilateral Lobule VI and VIII | X | Bilateral Crus 1; Left Lobule IV/V and VI |
| F | Bilateral lobule IV/V | Left Lobule VIIB | Left Lobule VI | Right Lobule IV/V; Bilateral Lobule VI and VIII | Right Lobule VI | X |
All locations are determined using the MRI atlas of the Human Cerebellum [Schmahmann et al.,2000].
Peak Coordinates and Activation Clusters in Intradomain Category Comparisons
Studies in three selected domains (emotion, language, and working memory) were further categorized into the following categories: positive and negative emotions; expressive and receptive language tasks; verbal and spatial working memory. Peak activation for tasks examining positive emotions was found in right Lobule VI while a wider range of activated areas were found for tasks examining negative emotions, including right Lobule IV/V, left Lobule VI and bilateral Crus 1. Tasks examining expressive language activated clusters in bilateral Lobule VI, Crus 1 and Crus 2, as well as midline Lobule VIII, while receptive language tasks activated left Lobule VI and right Crus 1. Spatial working memory primarily activated left Lobule VI, while activations for verbal working memory was primarily found in right lobule VI, VIIB, and left Crus 1. A summary of these results is presented in Table 4.
Table 4.
Summary of intradomain analyses for emotion, language and working memory
| Task type/cluster # | Cluster size | Local extrema | Location | ALE value (x10−3) | ||
|---|---|---|---|---|---|---|
| Emotions (negative) | ||||||
| 1 | 1976 | 14 | −50 | −18 | Right Lobule IV/V | 7.61 |
| 2 | 1368 | −6 | −74 | −28 | Left Crus 1 | 7.21 |
| −10 | −68 | −26 | Left Lobule VI | 6.41 | ||
| 3 | 456 | −50 | −66 | −28 | Left Crus 1 | 7.44 |
| 4 | 368 | 26 | −66 | −36 | Right Crus 1 | 6.64 |
| Emotions (positive) | ||||||
| 1 | 600 | 32 | −72 | −20 | Right Lobule VI | 4.65 |
| Language (expressive) | ||||||
| 1 | 10368 | 24 | −66 | −24 | Right Lobule VI | 19.41 |
| 2 | −64 | −32 | Mid Lobule VIII | 7.85 | ||
| 52 | −68 | −36 | Right Crus 1 | 7.78 | ||
| 2 | 3152 | −22 | −68 | −20 | Left Lobule VI | 16.92 |
| 3 | 496 | 14 | −86 | −32 | Right Crus 2 | 6.93 |
| 4 | 248 | −42 | −60 | −24 | Left Crus 1 | 6.20 |
| 5 | 208 | −34 | −86 | −40 | Left Crus 2 | 7.13 |
| 6 | 152 | 54 | −60 | −26 | Right Crus 1 | 5.09 |
| Language (receptive) | ||||||
| 1 | 488 | 32 | −66 | −32 | Right Crus 1 | 4.24 |
| 44 | −68 | −32 | Right Crus 1 | 4.11 | ||
| 2 | 424 | −20 | −60 | −18 | Left Lobule VI | 4.41 |
| −24 | −64 | −20 | Left Lobule VI | 4.38 | ||
| 3 | 176 | 40 | −56 | −36 | Right Crus 1 | 4.09 |
| Working Memory (spatial) | ||||||
| 1 | 800 | −34 | −72 | −22 | Left Lobule VI | 6.07 |
| Working Memory (verbal) | ||||||
| 1 | 20352 | 28 | −70 | −22 | Right Lobule VI | 22.10 |
| 10 | −78 | −22 | Right Lobule VI | 15.15 | ||
| −2 | −84 | −20 | Left Crus 1 | 11.98 | ||
| 26 | −78 | −48 | Right Lobule VIIb | 11.94 | ||
| 2 | 5368 | −28 | −64 | −28 | Left Lobule VI | 12.47 |
| −38 | −62 | −38 | Left Crus 1 | 11.92 | ||
| 3 | 1496 | −4 | −48 | −16 | Left Lobule IV/V | 12.41 |
| 4 | 320 | 12 | −56 | −38 | Right Lobule IX | 7.57 |
Peak ALE coordinates are given in MNI space and locations were determined using the MRI atlas of the Human Cerebellum [Schmahmann et al.,2000].
A comparison of the intradomain categories for emotion (positive vs. negative) found unique clusters of activation in bilateral Crus 1 and right lobule IV/V for negative emotions only. The comparison of intradomain categories for language (expressive vs. receptive) revealed clusters of activation in bilateral Lobule VI and Crus 2, as well as midline Lobule VIII and right Crus 1, for expressive language only. The comparison of verbal versus spatial working memory found clusters of activation in bilateral Lobule VI and Crus 1, as well as right Lobule VIIB, left Crus 2, and Lobule IV/V, to be unique for verbal working memory only. A summary of these results is presented in Table 5.
Table 5.
Summary of intradomain comparisons for positive (EmoP) vs. negative emotions (EmoN); expressive (LangE) vs. receptive language (LAngR); and verbal (WMVerb) vs. spatial working memory (WMSpat)
| Task Type/Cluster # | Cluster Size | Local Extrema | Location | ALE value (x10−3) | ||
|---|---|---|---|---|---|---|
| EmoN in comparison with EmoP | ||||||
| 1 | 1896 | −6 | −74 | −28 | Left Crus 1 | 7.21 |
| 2 | 1352 | 14 | −50 | −18 | Right Lobule IV/V | 7.48 |
| 3 | 576 | 26 | −66 | −36 | Right Crus 1 | 6.46 |
| 4 | 488 | −50 | −66 | −28 | Left Crus 1 | 7.40 |
| 5 | 336 | 24 | −86 | −20 | Right Crus 1 | 5.43 |
| EmoP in comparison with EmoN | ||||||
| N/A | ||||||
| LangE in comparison with LangR | ||||||
| 1 | 6944 | 22 | −66 | −24 | Right Lobule VI | 18.02 |
| 2 | −64 | −32 | Lobule VIII | 7.84 | ||
| 2 | 1832 | −20 | −68 | −20 | Left Lobule VI | 13.70 |
| 3 | 888 | 52 | −68 | −36 | Right Crus 1 | 6.95 |
| 4 | 512 | −34 | −86 | −40 | Left Crus 2 | 7.13 |
| 5 | 456 | 14 | −86 | −32 | Right Crus 2 | 6.93 |
| 6 | −90 | −26 | Right Crus 2 | 4.82 | ||
| LangR in comparison with LangE | ||||||
| N/A | ||||||
| WMSpat in comparison with WMVerb | ||||||
| N/A | ||||||
| WMVerb in comparison with WMSpat | ||||||
| 1 | 16816 | 28 | −70 | −22 | Right Lobule VI | 21.14 |
| 10 | −78 | −22 | Right Lobule VI | 15.12 | ||
| 26 | −78 | −50 | Right Lobule VIIb | 11.94 | ||
| 2 | 3080 | −38 | −62 | −40 | Left Crus 2 | 10.84 |
| −28 | −64 | −28 | Left Lobule VI | 10.78 | ||
| 3 | 1312 | −4 | −48 | −16 | Left Lobule IV/V | 11.71 |
| 4 | 816 | −2 | −84 | −20 | Left Crus 1 | 10.41 |
| 5 | 152 | 12 | −56 | −38 | Right Lobule IX | 7.57 |
Peak ALE coordinates are given in MNI space and locations were determined using the MRI atlas of the Human Cerebellum [Schmahmann et al.,2000].
Activation Clusters in Sternberg Task Phase Analysis and Phase Comparison
So far, a significant number of studies on cerebellar damage has examined the contributions of the cerebellum to verbal working memory. For example, studies by Chiricozzi et al. 2008, Justus et al. 2005 collectively demonstrate that verbal working memory and related functions are impeded in the presence of cerebellar damage. Of particular interest were the cerebro‐cerebellar networks for verbal working memory that Chen and Desmond 2005b proposed: an articulatory control system involving frontal and superior cerebellar activations, and a phonological storage system involving parietal and inferior cerebellar activations. By employing the error‐driven theory embedded within Baddeley's 1992 working memory framework, they demonstrated involvement of the superior cerebellum in the initial encoding of a verbal task while the involvement of the inferior cerebellum was shown in the maintenance phase of the task.
The above line of investigation prompted a further analysis of task categorization for working memory in this study. To understand the role of the cerebellum in this domain, we chose to focus on two tasks that were included within the same domain—the Sternberg and n‐back tasks. Coordinates from the n‐back and Sternberg tasks were analyzed separately and peak coordinates from both tasks were then compared. Figure 2 illustrates clusters of peak activated coordinates for the n‐back task in both left and right Crus 1, while clusters for the Sternberg task were found bilaterally in Crus 2 and right Lobules VI, VIII, and IX. A direct comparison of the coordinates from these two tasks revealed greater activated clusters (in left Crus 2, right Lobule VI, VIII, and IX) only in the Sternberg versus n‐back contrast. That is, no activated clusters in the n‐back were found to have greater ALEs than those in the Sternberg task.
Figure 2.
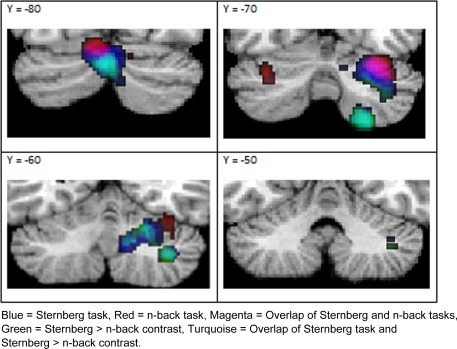
Overlay map of ALE peak activation results from individual task analyses of Sternberg and n‐back only; and direct comparison of Sternberg versus n‐back tasks. The contrast of n‐back>Sternberg did not yield any significant positive clusters.
The Sternberg task has three distinct phases (encoding, maintenance, retrieval) which is thought to illustrate the regions activated at each stage of the task. Of the three studies which used this task, two reported coordinates corresponding to each phase. Coordinates from these two studies were used for further analysis of clusters found within each task phase. Results from three separate analyses demonstrate that different clusters of activation can be found at each phase. During encoding, peak activation clusters can be found bilaterally in Crus 1, left Crus 2, and right Lobule VI. During maintenance, activation clusters are found bilaterally in Crus 1, left Crus 2, and right Lobule VIII. During the retrieval phase, right Crus 1, Lobules VI and VIII are activated.
DISCUSSION
This study provides further consolidation of results from a growing body of literature on cerebellar involvement in higher cognitive functions. This study builds upon a prior meta‐analysis [Stoodley and Schmahmann,2009] and provides further insights to the role of cerebellum in the following domains: emotion, executive function, language, music, timing, and working memory. Both meta‐analyses demonstrate that topographical organization of function takes place in the cerebellum. A summary of the results and comparisons from both meta‐analyses is provided in Appendix D; locations associated with each task are listed and common regions found in both studies are listed. A pictorial summary of results from both meta‐analyses is presented in Figure 3.
Figure 3.
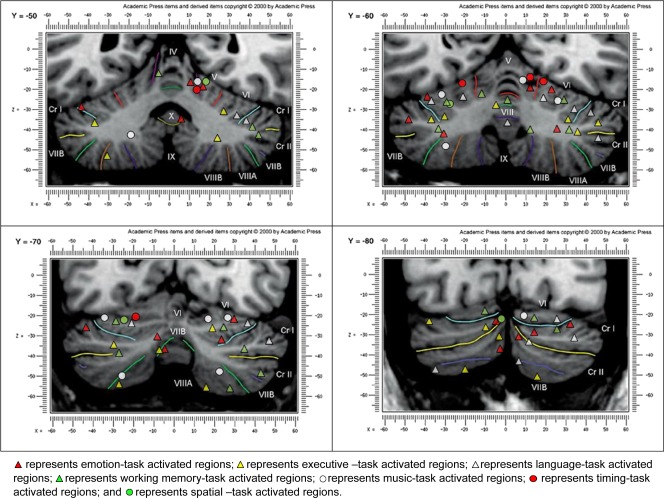
A pictorial summary of the combined results from both Stoodley and Schmahmann's (2009) meta‐analysis and current study; presented on Schmahmann et al.'s (2000) MRI atlas at coronal slices Y = −50, −60, −70, and −80.
Emotion
Cerebellar involvement in emotional processes has been described by an early hypothesis, cerebellar cognitive affective syndrome [CCAS; Schmahmann,1998] and identified in recent studies [e.g., Tavano, et al.,2007]. This involvement was described in the CCAS as encompassing an affective regulatory loop including the frontal lobes and limbic system. The current meta‐analysis and that of Stoodley and Schmahmann's 2009 meta‐analysis demonstrate not only the cerebellum's involvement in emotional processing but also show that consistent activations unique to emotional processing can be found in right Lobules VI, IV/V, and bilateral Crus 1. In a review by Schutter and Honk 2005, it was suggested that particular forms of emotional processes may be reflected differentially within the cerebellum. By categorizing within the domain of emotion, this study found unique clusters of activation in bilateral Crus 1 and right Lobule IV/V for processing of negative emotions when compared with positive emotions. While it may suggest that differential regions within the cerebellum could be related to the processing of multifarious emotions, the result of this comparison was limited by the unequal number of existing studies which examined positive and negative emotions; a total of 28 foci from 11 studies examining negative emotions was compared with a total of 9 foci from 5 studies examining positive emotions.
Executive Function
Comparisons of activation between executive function and domains involving emotion, music, and timing showed consistently greater associations in left Crus 1 and Crus 2 for executive function, while greater activation for executive function was consistently found in left Lobule VIIB when compared with domains of language, timing, and working memory. This is fairly consistent with findings from Stoodley and Schmahmann's 2009 meta‐analysis where the difficulty in identifying purely executive regions was addressed. Executive function comprises multiple processes, some of which overlap with that of other domains, such as working memory, emotion and language processing. Previous studies [e.g., Kelly and Strick, 2003; O'Reilly, et al., 2010; Strick et al., 2009] demonstrate the involvement of Crus 2 in executive function; both Kelly and Strick's [2003] transneuronal tracing study and O'Reilly et al. [2010] resting state connectivity study found projections from Crus 2 to regions involved in executive function. Results from the current study also suggest that although Crus 1 and 2 consistently appear to have greater activation in executive function when compared to other domains, these regions are also strongly activated for domains such as emotion, language, and working memory.
Language
This study found a significant cluster of coordinates in bilateral lobule VI and right Crus 1 for tasks in the language domain, while Stoodley and Schmahmann 2009 found significant clusters of coordinates in right Crus 1, 2, and lobule VIIAt. We have attributed this to the inclusion of additional articles and the resultant differences in coordinates entered into the final analysis. These results are, nonetheless, largely consistent with existing studies which described lateralized activation in the posterior regions of the cerebellum for language tasks. The current study also identified two categories within the language domain and performed a comparison of expressive versus receptive language. We found clusters of activation in bilateral Lobule VI and Crus 2, as well as midline Lobule VIII and right Crus 1, unique to expressive language only. However, these results are limited by the uneven number of foci included in each category; 48 foci from seven studies examining expressive language were compared with nine foci from four studies examining receptive language.
Music and Timing
Music processing is highly complex and involves a number of processes, including temporal and pitch processing, which belong to different dimensions [Peretz and Zatorre,2005]. This has made it difficult to try to categorize music related tasks into a single domain of music processing. Likewise, time‐related processes appear to share overlaps with many other cognitive domains and is hence, difficult to isolate time‐related tasks into a clear domain. In light of this complexity, this study chose to focus on a specific aspect of each domain—rhythmic aspects of music processing and interval estimation in time processing. Tasks that required rhythmic paced performance, rhythmic learning via perception and reproduction of rhythms were included in the music domain, while tasks that examined reproduction of temporal intervals and temporal order were included in the timing domain.
This first attempt to examine tasks related to music and timing revealed that while both aspects of the domain are intrinsically similar in that knowledge of temporal order is involved, tasks in these two domains recruit unique activations. On the basis of the theory where the cerebellum is seen as an internal timing device and evidence suggesting timing to involve knowledge of temporal order, we expected to find that activations for timing would represent a subset of activations for the rhythmic aspects of music processing. However, results in this meta‐analysis revealed recruitment of distinct regions, with music related tasks consistently demonstrating significant activation in right Lobule IV/V, bilateral Lobules VI and VIII, and timing uniquely activating the right Lobule VI.
Working Memory
In Stoodley and Schmahmann's 2009 meta‐analysis, it was suggested that overlaps with regions associated with language during verbal working memory support existing models of verbal rehearsal in Baddeley's 1992 model. Their study also found that differential components within tasks explain the distributed areas and pattern of activation. This study found activations in bilateral Lobule VI and left Crus 2 to be consistently associated with working memory; this corresponds partly with results presented in the previous meta‐analysis. The inclusion of 18 additional studies in the current analysis provided positive verification of the involvement of bilateral Lobule VI in working memory.
Although most of the studies in this domain had whole brain coverage, five studies in this domain either did not specify or were unable to achieve whole brain coverage due to the study's focus on frontal regions. This has likely contributed to the absence of inferior cerebellar activations in our results. Further analysis of working memory categories (spatial and verbal), as well as the identification and comparison of specific tasks and components within verbal working memory, using the n‐back and Sternberg tasks, were found to shed more light on the involvement of the cerebellum in this domain.
Verbal Versus Spatial Working Memory
A comparison of spatial and verbal working memory found clusters of activation in bilateral Lobule VI and Crus 1, as well as right lobule VIIB, left Crus 2 and Lobule IV/V, to be unique for verbal working memory. While it suggests that categories within a domain can indeed be topographically represented within the cerebellum, the results are restricted by the disparate number of foci included for comparison; 91 foci from 16 verbal working memory studies were compared with just nine foci from four studies which examined spatial working memory.
Differentiating Between Two Verbal Working Memory Tasks
Watter et al. 2001 explored the n‐back task as a dual task and highlighted the differences between the n‐back task and the classical Sternberg task. While there are many variants of the n‐back task, it typically requires participants to view a series of stimuli and recall a stimulus presented n trials prior to the current one. In theorizing that the n‐back task might be a dual task, they suggested that there are two distinct subcomponents, one of which involves a complex updating mechanism in working memory and the other concerns the process of searching for a match of the stimuli. The Sternberg, on the other hand, involves a simple maintenance of information and subsequent retrieval; participants are presented with sets of items (e.g., digits) to remember and are subsequently required to recall whether the test item had been previously presented. Results from our study revealed a wider range of activated clusters in the Sternberg compared to the n‐back task. Although the n‐back task showed clusters of activation in the left crus I and superior portions of lobule VI (see figure 2), these clusters did not survive the n‐back versus Sternberg contrast analysis. These variations could reflect the different processes involved in the two working memory tasks. However, it should be noted that the disparity in coordinates available for comparison (9 for n‐back and 32 for Sternberg) may have also contributed to this finding.
Examining the Phases Within the Sternberg Task
A significant number of studies on cerebellar damage has examined the contributions of the cerebellum to verbal working memory [e.g., Chiricozzi et al.,2008; Justus et al.,2005; Kirschen et al.,2008; Peterburs et al., 2010; Ravizza et al., 2006; Silveri et al., 1998]. For example, Justus et al. 2005 observed diminished phonological similarity effects in patients with cerebellar damage while Chiricozzi et al. 2008 found phonological short‐term store deficits in an individual with bilateral cerebellar lesion. These studies collectively demonstrate that verbal working memory and related functions are impeded in the presence of cerebellar damage, although the exact nature of the cerebellum's involvement had yet to be identified.
Chen et al. [2005a, 2005b] suggest that two separate but related loops are activated during verbal working memory; one, an articulatory control system involving frontal and superior cerebellar activations and the other, a phonological storage system involving the parietal and inferior cerebellum. Activation of these loops is dependent upon the phases of the task. Consistent with this proposal, this study found activation clusters in Crus 1 and Lobule VI during encoding and activations in Lobule VIII during maintenance.
The distinct clusters of activations identified in this study for each task and for the different phases of the Sternberg task suggest that individual phases within a domain can be functionally organized within the cerebellum. This suggestion is consistent with the conclusions made by the previous meta‐analysis [Stoodley and Schmahmann,2009] and also provides the impetus for further investigation into the roles of the cerebellum during verbal working memory. Results also suggest that cerebellar role in verbal working memory may be closely related to its role in language and speech. A recent review by Marvel and Desmond 2010a discusses how cerebellar support of speech in turn plays a part in enhancing working memory processes.
The Cerebellum and Its Role as a Timing Device
Although it is clear that topographical organization of function takes place within the cerebellum, its precise role remains to be determined. Different theories have suggested different roles; one postulates that the cerebellum serves as an internal timing mechanism, another suggests that it serves an error‐correction or adjustment role for specific projections to the cerebrum as an universal transform, while yet another considers it a general modulator.
In this study, the consistent involvement of the bilateral Lobule VI across all tasks in timing provides a strong evidence to support time‐keeping as an essential function of the cerebellum. It has been hypothesized that the cerebellum may operate as a form of internal timing system by providing some form of temporal knowledge in various cognitive tasks. Supportive evidence from a recent study by Koch et al. 2007, using repetitive transcranial magnetic stimulation, a modality not included in the current meta‐analysis, found areas in the cerebellum to be essential to the explicit timing of millisecond intervals.
In a review of studies examining the cerebellar cellular organization, Ben‐Yehudah et al. 2007 suggested that its role in timing of millisecond intervals is due to the creation of a functional “tapped delay line”; a wave that is propagated along a single parallel fiber and reaches consecutive Purkinje‐cells at incremental delays from the onset of the wave. They provided neuropsychological evidence consistent with their theory, including the breakdown of motor action timing in patients with cerebellar damage [e.g., Timmann et al., 1999]. Predictions based on the timing theory found that cerebellar lesions result in a variety of speech impairments such as prolonged speech utterances and distorted categorical perceptions of temporally cued speech sounds.
The cerebellum is also thought to be part of a network which codes ordinal position in memory. Ben‐Yehudah et al. have suggested that further investigations on tasks requiring serial order maintenance as well as examinations of the role prearticulatory speech codes during different stages of a working memory task would shed further light upon the precise role of the cerebellum as an internal timing device. In another review of cerebellar function, Hogan 2004 suggested that if the cerebellum is a time‐keeping mechanism and is necessary for the coordination of inputs and outputs from varied sources during processing, then a disruption of this system should result in deficits present across all functions that demand such coordination.
Ivry et al. [1996, 1997, 2002] theory of the cerebellum's important role in event timing has since been examined in various studies [e.g., Dreher and Grafman,2002; Harrington, et al.,2010]. Dreher and Grafman's 2002 study demonstrated the activation in the cerebellum to be associated with timing irregularity and that right Lobules VI, VII, and Crus 1 seem to be critical for timing processes. They concur that the nature of this role is related more to error adjustment and that the cerebellum adjusts to timing information. A recent study by Harrington et al. 2010 examined timing in various phases of a cognitive task. They were unable to provide a definitive conclusion about the cerebellum's precise temporal role, but suggest that it is likely an adaptive one. Nonetheless, it is clear that the cerebellum's relation to temporal processing can be mapped topographically; as suggested by Dreher and Grafman 2002 and consistent with our results, the right Lobule VI seems to play a critical role. The difficulty in identifying processes specific to the temporal experience from other related processes poses a challenge in determining the temporal role of the cerebellum. By comparing the role of cerebellum in timing and music, this study has provided a basis for further investigations into the subtle differences between these two domains. This is pertinent to our understanding of the precise role of the cerebellum in timing.
Issues and Future Directions
This study was limited by the lack of published papers available for comparison across differential domains. While some domains included foci from more than 15 studies, others only included fewer than 10 (music and timing). In particular, distinguishing between timing and music domains proved to be a challenge; studies which could be considered within either domain were included within the analysis due to the general lack of studies within the field. The unique activations associated with each domain needs to be verified with the inclusion of studies that can be better distinguished into either the timing or music domain.
Our study was also limited by the unequal representation of differential task categories within each domain (4 spatial working memory studies vs. 18 verbal working memory studies; 4 receptive language studies vs. 7 expressive language studies; and 5 positive emotion studies vs. 11 negative emotion studies). Despite recent interest in deepening current understanding of a nonmotor cerebellum, it is apparent that further research needs to be conducted. A particular challenge this study faced was the meager number of studies and subsequently, coordinates, available for an adequate analysis of manifold tasks and their components. However, this preliminary foray into an examination of task categorization demonstrates the possibility of identifying specific cerebellar involvement in different categories within a domain and at discrete stages of a complex cognitive function. This study provides a platform upon which further investigations can be made.
This analysis included more studies than the meta‐analysis conducted by Stoodley and Schmahmann 2009. Although the results from both meta‐analyses were similar, they were not identical. The inclusion of more studies could have generated different peak activation clusters, where peak activation clusters reflect regions that were most consistently activated across different studies. This suggests that the replication of peak activated regions in this study would reflect the consistency of the region's role in the associated cognitive domain. Our results have demonstrated a topographical representation of higher cognitive functions in the cerebellum. However, the role of the cerebellum within these related cognitive networks is still inconclusive. Recent resting state connectivity studies demonstrate the existence of distinct functional regions within the cerebellum. O'Reilly et al. [2010] in their resting state functional connectivity study found two functional zones, the primary sensorimotor and supramodal, which are distinct but share overlapping functional maps. Another study on intrinsic connectivity networks (ICN) conducted by Habas et al. 2009 found cerebellar contributions in all four of their ICNs: the sensorimotor network, default mode network, executive network and salience network. Krienen and Buckner 2009 also identified four topographically distinct networks involving the cerebellum and the motor cortex, the dorsolateral prefrontal cortex, medial prefrontal cortex and the anterior prefrontal cortex in their study.
More recently, using intrinsic functional connectivity, Buckner et al. [2011] were able to conclude that the cerebellum is functionally linked to a number of cerebral networks and these functions can be topographically organized. Work by these researchers suggests that investigations into the nature of cerebellar role can take diverse forms. However, the nature of interactions between the cerebellum and the cortex may be better described using effective connectivity methods. Further research mapping functional topography of the cerebellum using effective connectivity in higher cognitive functions such as language and working memory will likely serve to increase understanding of the cerebellum's precise role in cognition.
CONCLUSIONS
The main aims of this study were to help determine the cerebellum's contribution in different cognitive tasks and to expand on existing functional topography of the cerebellum by considering two other higher cognitive domains of music and timing. We also systematically examined intradomain tasks differences and performed individual task and intratask analysis. The consistent presence of cerebellar activations in the timing domain indicates a time‐keeping role. However, its role as a general modulator or error‐adjustment mechanism cannot be effectively ruled out due to the presence of cerebellar activations across all higher cognitive functions and the specific topography seen for various task domains. Further investigations where the role of the cerebellum can be considered within theoretical models of cognitive functions may provide better understanding of its involvement in higher cognition.
Acknowledgments
This study was supported by the Tier2 Academic Research Fund (MOE2011‐T2‐1‐031), Ministry of Education, Singapore and the National Institutes of Health (R01 AA018694), USA.
Table A1.
Full list of studies and number of foci included in this article
| Task domain (X/Y) | Imaging modality | N | Task description | No. of foci |
|---|---|---|---|---|
| Emotion (18/9): | 62 | |||
| Imaizumi et al. 1997 | PET | Emotion in speaker voice vs. Speaker identification | 5 | |
| Lane et al. 1997 | PET | IAPS pictures | 2 | |
| Paradiso et al. 1999 | PET/1.5T fMRI | IAPS pictures | 10 | |
| Simpson et al. 2000* | 1.5T fMRI | IAPS pictures | 2 | |
| Abel et al. 2003* | 1.5T fMRI | Ketamin and facial emotion recognition | 2 | |
| Gündel et al. 2003 | 1.5T fMRI | Grief | 5 | |
| Markowitsch et al. 2003* | 1.5T fMRI | Happy vs. sad autobiographical memory and fMRI | 2 | |
| Ueda et al. 2003* | 1.5T fMRI | Expectancy of emotional stimuli (warned reaction task) | 1 | |
| Canli et al. 2004* | 3T fMRI | Lexical decision task for neutral, happy, sad, and threat‐related words | 2 | |
| Lee et al. 2004 | 1.5T fMRI | Unpleasant vs. neutral IAPS pictures | 3 | |
| Najib et al. 2004* | 1.5T fMRI | Women who had romantic relationship breakup | 8 | |
| Takahashi et al. 2004 | 1.5T fMRI | IAPS pictures | 1 | |
| Habel et al. 2005 | 1.5T fMRI | Happy vs. sad faces | 2 | |
| Bermpohl et al. 2006 | 3T fMRI | IAPS pictures | 2 | |
| Hofer et al. [2006]* | 1.5T fMRI | Gender difference in fMRI images during the perception of emotion | 6 | |
| Hofer et al. 2007 | 1.5T fMRI | IAPS pictures | 6 | |
| Jollant et al. 2008* | 1.5T fMRI | IAPS pictures (angry, happy vs. neutral), suicidal behavior | 2 | |
| Park et al. 2010* | 1.5T fMRI | Integration of emotional information from different sources | 1 | |
| Executive function (13/6): | 58 | |||
| Rao et al. 1997 | 1.5T fMRI | Conceptual reasoning vs. sensorimotor control | 3 | |
| Dagher et al. 1999)* | PET | Tower of London task | 2 | |
| Jahanshahi et al. 2000 | PET | Random number generation vs. Counting | 2 | |
| Liddle et al. 2001 | 1.5T fMRI | Go‐No‐Go task | 15 | |
| Dreher et al. 2002* | 1.5T fMRI | Letter discrimination with task‐switching conditions | 4 | |
| Ernst et al. 2002 | PET | Risk taking task | 10 | |
| Schall et al. 2003* | PET/1.5T fMRI | Tower of London task | 2 | |
| Daniels et al. 2003 | 1.5T fMRI | Random number generation | 2 | |
| Beauchamp et al. 2003* | PET | Tower of London | 3 | |
| Blackwood et al. 2004 | 1.5T fMRI | Decision making (uncertain vs. certain conditions) | 2 | |
| Kondo et al. 2004* | 1.5T fMRI | Arithmetic and memory tasks | 6 | |
| Harrington et al. 2004 | 1.5T fMRI | Decision making (interval timing) | 4 | |
| Gilbert et al. 2008* | 3T fMRI | Random generation task | 3 | |
| Language (15/4): | 68 | |||
| Ojemann et al. 1998 | PET and 1.5T fMRI | Word stem completion (covert) vs. fixation | 7 | |
| Schlosser et al. 1998 | 1.5T fMRI | Verbal fluency | 6 | |
| Lurito et al. 2000 | fMRI | Word generation vs. viewing nonletter symbols | 3 | |
| Seger et al. 2000 | 1.5T fMRI | Verb generation | 13 | |
| Gurd et al. (2002) | 1.5T fMRI | Semantic fluency (categories) vs. overlearned sequence fluency | 1 | |
| Noppeney et al. 2002 | PET | Semantic decision | 2 | |
| Xiang et al. 2003 | 1.5T fMRI | Semantic discrimination | 1 | |
| McDermott et al. 2003 | 1.5T fMRI | Semantic vs. Phonological word lists | 3 | |
| Seki et al. 2004 | 3T fMRI | Vowel exchange vs. Reading words and nonwords | 2 | |
| Tieleman et al. 2005 | 1.5T fMRI | Semantic vs. perceptual categorization | 3 | |
| Callan et al. 2006* | 3T fMRI | Listening and covert production of singing relative to speech | 2 | |
| Frings et al. 2006 | 1.5T fMRI | Verb generation | 17 | |
| Rauschecker et al. 2008* | 3T fMRI | Test of nonword repetition | 4 | |
| Sweet et al. 2008* | 1.5T fMRI | Effects of phonological similarity on verbal working memory (2‐back and 0‐back) | 2 | |
| Durisko et al. 2010* | 3T fMRI | Contribution of cerebellum to verbal working memory | 2 | |
| Music* (7): | 57 | |||
| Zatorre et al. 1994 | PET | Auditory processing of melody | 3 | |
| Ramnani et al. [2001] | PET | Rhythmic learning | 3 | |
| Pope et al. 2005 | 3T fMRI | Rhythmic sequence production | 10 | |
| Grahn et al. 2007 | 3T fMRI | Rhythm and beat perception | 2 | |
| Chen et al. 2008 | 1.5T fMRI | Rhythm perception and production | 26 | |
| Thaut et al. 2008 | 1.5T fMRI | Rhythmic production/formation | 7 | |
| Karabanov et al. 2009 | 1.5T fMRI | Rhythmic production | 6 | |
| Timing* (9): | 36 | |||
| Tracy et al. 2000 | 1.5T fMRI | Time interval production task | 5 | |
| Jäncke et al. 2000 | 1.5T fMRI | Paced finger‐tapping task | 4 | |
| Dreher et al. 2002 | 1.5T fMRI | Timing prediction task | 2 | |
| Smith et al. 2003 | 1.5T fMRI | Temporal discrimination task | 1 | |
| Jantzen et al. 2005 | 1.5T fMRI | Self‐paced rhythmic timing task | 12 | |
| Tregellas et al. 2006 | 3T fMRI | Auditory temporal discrimination task | 2 | |
| Stevens et al. 2007 | 3T fMRI | Temporal reproduction | 3 | |
| Jantzen et al. 2007 | 1.5T fMRI | Continuation paradigm | 2 | |
| O'Reilly et al. 2008 | 3T fMRI | Temporal perception task | 5 | |
| Working memory(26/18): | 116 | |||
| Fiez et al. 1996* | PET | Working memory and PET | 5 | |
| Schumacher et al. 1996* | PET | Verbal working memory and its modality‐specific representations | 1 | |
| Jonides et al. 1998* | PET | Role of parietal cortex in verbal working memory | 3 | |
| LaBar et al. 1999 | 1.5T fMRI | 2‐back task; spatial WM | 1 | |
| Thomas et al. 1999* | 1.5T fMRI | Spatial working memory – children and adults | 2 | |
| Honey et al. 2000 | 1.5T fMRI | 2‐back vs. Control | 1 | |
| Gruber et al. 2001 | 3T fMRI | Letter memory vs. Uppercase/lowercase judgment | 1 | |
| Cairo et al. 2004 | fMRI | Sternberg working memory | 8 | |
| Kirschen et al. 2005 | 3T fMRI | Regions of linear and quadratic increases in activation with increasing memory load | 6 | |
| Chen et al. 2005b | 3T fMRI | Sternberg working memory | 13 | |
| Chen et al. 2005a* | 3T fMRI | Verbal working memory | 9 | |
| Tomasi et al. 2005 | 4T fMRI | n‐back | 3 | |
| Valera et al. 2005 | 1.5T fMRI | n‐back | 1 | |
| Woodward et al. 2006* | 1.5T fMRI | Verbal working memory | 5 | |
| Geier et al. 2007* | 3T fMRI | Spatial working memory (oculomotor delayed response task) | 3 | |
| Hayter et al. 2007* | 3T fMRI | Verbal working memory (Paced Auditory Serial Addition Test, PASAT) | 2 | |
| Tomasi et al. 2007* | 4T fMRI | n‐back and visual attention tasks | 3 | |
| Yeh et al. 2007* | 1.5T fMRI | Visuospatial working memory for change detection | 1 | |
| O'Hare et al. 2008* | 3T fMRI | Parametric verbal Sternberg working memory task | 4 | |
| Scheuerecker et al. 2008* | 1.5T fMRI | 2‐back vs. 0‐back | 5 | |
| Hautzel et al. 2009* | 1.5T fMRI | 2‐back paradigm and extracting %change in BOLD signal | 19 | |
| Koelsch et al. 2009* | 3T fMRI | Functional architecture of verbal and tonal working memory | 1 | |
| Durisko et al. 2010* | 3T fMRI | Delayed serial recall (DSR) task | 7 | |
| Schulze et al. 2011* | 3T fMRI | Verbal and tonal working memory (musicians and nonmusicians) | 5 | |
| Marvel et al. 2010b* | 3T fMRI | Sternberg working memory | 2 | |
| Kirschen et al. 2010* | 3T fMRI | Load‐dependent verbal working memory | 5 |
X, total number of studies included in each domain; Y, total number of articles not included in previous meta‐analysis; N, total number of foci included in each domain, and task type and articles marked with an asterisk (*) are ones not included in the previous meta‐analysis conducted by Stoodley and Schmahmann 2009.
Table B1.
Peak ALE coordinates for individual task category
| Task type/cluster # | Cluster size (mm3) | Local extrema (x, y, z) | Location | ALE value (x10−3) | ||
|---|---|---|---|---|---|---|
| EMOTION | ||||||
| 1 | 6,656 | 16 | −54 | −20 | Right Lobule IV/V | 11.02 |
| 34 | −72 | −24 | Right Lobule VI | 8.16 | ||
| 24 | −60 | −42 | Right Lobule VIIIA | 8.07 | ||
| 6 | −52 | −34 | Right Lobule IX | 7.31 | ||
| 2 | 1,664 | −12 | −66 | −28 | Left Lobule VI | 6.77 |
| −6 | −82 | −24 | Left Crus 2 | 6.53 | ||
| −6 | −68 | −38 | Left Lobule VIIIB | 6.15 | ||
| 3 | 368 | 18 | −78 | −28 | Right Crus 1 | 7.89 |
| 4 | 176 | −48 | −60 | −36 | Left Crus 1 | 7.66 |
| Executive function | ||||||
| 1 | 3,992 | −8 | −74 | −38 | Left Crus 2 | 9.14 |
| 4 | −76 | −26 | Lobule VII | 7.33 | ||
| 0 | −62 | −26 | Lobule VII | 7.07 | ||
| 2 | 3,752 | −32 | −62 | −36 | Left Crus 1 | 10.77 |
| 3 | 1,104 | 26 | −50 | −32 | Right Lobule VI | 7.30 |
| 30 | −62 | −40 | Right Crus 1 | 7.24 | ||
| Language | ||||||
| 1 | 13,664 | 30 | −58 | −36 | Right Lobule VI | 20.35 |
| 22 | −62 | −30 | Right Lobule VI | 20.10 | ||
| 32 | −76 | −46 | Right Crus 2 | 6.932 | ||
| 2 | −62 | −36 | Lobule VIII | 6.75 | ||
| 32 | −74 | −40 | Right Crus 2 | 6.33 | ||
| 2 | 3,920 | −22 | −64 | −28 | Left Lobule VI | 18.36 |
| 3 | 2,496 | 12 | −80 | −42 | Right Crus 2 | 10.25 |
| 4 | 152 | −40 | −56 | −30 | Left Crus 1 | 6.27 |
| Music | ||||||
| 1 | 13,664 | 16 | −54 | −22 | Right Lobule IV/V | 22.05 |
| 28 | −62 | −26 | Right Lobule VI | 19.61 | ||
| 10 | −72 | −22 | Right Lobule VI | 10.89 | ||
| 2 | 5,128 | −30 | −64 | −24 | Left Lobule VI | 21.15 |
| 3 | 2,584 | −26 | −66 | −50 | Left Lobule VIII | 16.28 |
| 4 | 1,728 | 22 | −70 | −50 | Right Lobule VIII | 12.67 |
| Timing | ||||||
| 1 | 4,536 | 20 | −58 | −18 | Right Lobule VI | 22.53 |
| 2 | 2,896 | −16 | −66 | −20 | Left Lobule VI | 9.86 |
| Working memory | ||||||
| 1 | 32,536 | 28 | −66 | −32 | Right Crus 1 | 28.92 |
| −36 | −62 | −38 | Left Crus 1 | 20.01 | ||
| 8 | −76 | −26 | Right Crus 1 | 19.20 | ||
| 24 | −70 | −58 | Right Lobule VIII | 15.95 | ||
| 2 | 2,280 | −4 | −48 | −14 | Left Lobule IV/V | 13.51 |
Coordinates are given in MNI space and locations were determined using the MRI atlas of the Human Cerebellum [Schmahmann et al.,2000].
Table C1.
Peak ALE coordinates for interdomain comparisons
| Task type/cluster # | Cluster size | Local extrema | Location | ALE value | ||
|---|---|---|---|---|---|---|
| Emotion in comparison with Executive Function | ||||||
| 1 | 2,048 | 16 | −54 | −20 | Right Lobule IV/V | 10.50 |
| 2 | 1,032 | 34 | −72 | −24 | Right Lobule VI | 7.92 |
| Emotion in comparison with Language | ||||||
| 1 | 2,200 | 14 | −52 | −20 | Right Lobule IV/V | 10.28 |
| 20 | −44 | −24 | Right Lobule IV/V | 7.66 | ||
| 6 | −52 | −32 | Lobule X | 6.19 | ||
| Emotion in comparison with Music | ||||||
| 1 | 344 | −48 | −60 | −36 | Left Crus 1 | 7.49 |
| 2 | 256 | 34 | −76 | −24 | Right Crus 1 | 6.64 |
| 3 | 216 | −6 | −80 | −24 | Left Crus 1 | 6.32 |
| −10 | −74 | −28 | Left Crus 1 | 6.15 | ||
| Emotion in comparison with Timing | ||||||
| 1 | 680 | 32 | −72 | −22 | Right Lobule VI | 7.15 |
| 2 | 280 | 18 | −78 | −28 | Right Crus 1 | 7.64 |
| 3 | 200 | −4 | −82 | −24 | Left Crus 2 | 6.17 |
| 4 | 168 | −48 | −60 | −36 | Left Crus 1 | 7.66 |
| Emotion in comparison with working memory | ||||||
| 1 | 848 | 14 | −52 | −20 | Left Lobule IV/V | 9.37 |
| 20 | −42 | −26 | Right Lobule IV/V | 7.42 | ||
| Executive Function in comparison with Emotion | ||||||
| 1 | 992 | −36 | −66 | −36 | Left Crus 1 | 7.92 |
| −30 | −66 | −38 | Left Crus 2 | 7.77 | ||
| Executive Function in comparison with Language | ||||||
| 1 | 1,312 | −32 | −64 | −38 | Left Crus 1 | 8.96 |
| 2 | 496 | −10 | −72 | −38 | Left Lobule VIIB | 7.95 |
| 0 | −70 | −42 | Midline Lobule VIII | 5.63 | ||
| Executive function in comparison with music | ||||||
| 1 | 1,816 | −8 | −74 | −38 | Left Crus 2 | 9.12 |
| 2 | 1,704 | −34 | −62 | −36 | Left Crus 1 | 9.50 |
| Executive Function in comparison with Timing | ||||||
| 1 | 5,600 | −34 | −62 | −36 | Left Crus 1 | 10.55 |
| −10 | −72 | −38 | Left Lobule VIIB | 8.77 | ||
| 4 | −76 | −26 | Midline Lobule VII | 7.05 | ||
| 0 | −62 | −26 | Midline Lobule VII | 6.05 | ||
| 2 | 616 | 30 | −62 | −40 | Right Crus 1 | 6.90 |
| 3 | 192 | 48 | −58 | −36 | Right Crus 1 | 5.83 |
| Executive function in comparison with working memory | ||||||
| 1 | 352 | −10 | −72 | −40 | Left Lobule VIIB | 7.88 |
| Language in comparison with Emotion | ||||||
| 1 | 7,664 | 32 | −58 | −36 | Right Crus 1 | 18.32 |
| 20 | −64 | −30 | Right Lobule VI | 16.71 | ||
| 46 | −62 | −42 | Right Crus 2 | 8.14 | ||
| 2 | 2,608 | −22 | −64 | −26 | Left Lobule VI | 15.61 |
| 3 | 1,120 | 14 | −82 | −44 | Right Crus 2 | 9.74 |
| Language in comparison with executive function | ||||||
| 1 | 5,984 | 22 | −62 | −30 | Right Lobule VI | 17.24 |
| 32 | −58 | −36 | Right Crus 1 | 15.34 | ||
| 2 | 2,472 | −22 | −64 | −26 | Left Lobule VI | 16.31 |
| 3 | 1,312 | 14 | −82 | −44 | Right Crus 2 | 9.98 |
| Language in comparison with Music | ||||||
| 1 | 6,288 | 32 | −58 | −38 | Right Crus 1 | 18.67 |
| 20 | −64 | −32 | Right Lobule VI | 14.76 | ||
| 46 | −62 | −44 | Right Crus 2 | 8.21 | ||
| 2 | 1,968 | 14 | −82 | −42 | Right Crus 2 | 10.12 |
| 3 | 896 | −20 | −64 | −28 | Left Lobule VI | 10.69 |
| Language in comparison with Timing | ||||||
| 1 | 10,920 | 30 | −58 | −36 | Right Lobule VI | 20.27 |
| 22 | −62 | −32 | Right Lobule VI | 19.38 | ||
| 30 | −76 | −42 | Right Crus 2 | 6.30 | ||
| 2 | 1,840 | −22 | −64 | −28 | Left Lobule VI | 14.52 |
| 3 | 1,296 | 14 | −82 | −44 | Right Crus 2 | 10.01 |
| Language in comparison with Working Memory | ||||||
| 1 | 312 | −20 | −62 | −26 | Left Lobule VI | 9.51 |
| Music in comparison with Emotion | ||||||
| 1 | 6,040 | 28 | −60 | −26 | Right Lobule VI | 14.57 |
| 16 | −54 | −24 | Right Lobule IV/V | 11.52 | ||
| 6 | −62 | −14 | Midline Lobule IV/V | 10.96 | ||
| 10 | −72 | −22 | Midline Lobule VI | 10.10 | ||
| 2 | 2,720 | −30 | −64 | −22 | Left Lobule VI | 20.04 |
| 3 | 1,448 | −26 | −66 | −50 | Left Lobule VIII | 15.33 |
| Music in comparison with Executive Function | ||||||
| 1 | 8,096 | 16 | −54 | −22 | Right Lobule IV/V | 21.15 |
| 30 | −62 | −24 | Right Lobule VI | 18.44 | ||
| 10 | −72 | −20 | Right Lobule VI | 7.24 | ||
| 2 | 3,144 | −30 | −64 | −22 | Left Lobule VI | 20.59 |
| 3 | 1,072 | 22 | −70 | −50 | Right Lobule VIII | 11.84 |
| 4 | 1,064 | −26 | −64 | −50 | Left Lobule VIII | 12.30 |
| Music in comparison with Language | ||||||
| 1 | 6,056 | 16 | −54 | −22 | Right Lobule IV/V | 20.36 |
| 28 | −58 | −24 | Right Lobule VI | 10.87 | ||
| 10 | −74 | −22 | Right Lobule VI | 8.86 | ||
| 2 | 2,264 | −26 | −64 | −50 | Left Lobule VIII | 16.13 |
| 3 | 1,344 | −32 | −64 | −22 | Left Lobule VI | 16.34 |
| Music in comparison with Timing | ||||||
| 1 | 7,896 | 30 | −62 | −26 | Right Lobule VI | 18.35 |
| 14 | −54 | −24 | Right Lobule IV/V | 11.88 | ||
| 10 | −72 | −22 | Right Lobule VI | 10.67 | ||
| 6 | −64 | −16 | Midline Lobule VI | 10.53 | ||
| 2 | 2,680 | −26 | −64 | −50 | Left Lobule VIII | 16.14 |
| −20 | −54 | −42 | Left Lobule VIII | 6.91 | ||
| 3 | 2,664 | −30 | −64 | −24 | Left Lobule VI | 17.61 |
| 4 | 1,312 | 22 | −70 | −50 | Right Lobule VIII | 12.03 |
| Music in comparison with working memory | ||||||
| 1 | 3,792 | 16 | −54 | −22 | Right Lobule IV/V | 18.29 |
| 30 | −58 | −24 | Right Lobule VI | 8.66 | ||
| 2 | 1,312 | −26 | −66 | −50 | Left Lobule VIII | 14.70 |
| 3 | 696 | −30 | −62 | −22 | Left Lobule VI | 13.39 |
| 4 | 160 | 22 | −70 | −48 | Right Lobule VIII | 8.40 |
| Timing in comparison with Emotion | ||||||
| 1 | 1,536 | 20 | −58 | −16 | Right Lobule VI | 16.72 |
| 2 | 736 | −18 | −64 | −20 | Left Lobule VI | 8.30 |
| Timing in comparison with Executive Function | ||||||
| 1 | 3,312 | 20 | −58 | −18 | Right Lobule VI | 22.31 |
| 2 | 1,248 | −18 | −64 | −20 | Left Lobule VI | 8.18 |
| Timing in comparison with Language | ||||||
| 1 | 2,920 | 20 | −58 | −18 | Right Lobule VI | 19.88 |
| Timing in comparison with Music | ||||||
| 1 | 960 | 22 | −58 | −16 | Right Lobule VI | 14.95 |
| Timing in comparison with Working Memory | ||||||
| 1 | 1,808 | 20 | −58 | −16 | Right Lobule VI | 18.77 |
| Working Memory in comparison with Emotion | ||||||
| 1 | 12,936 | 28 | −66 | −32 | Right Crus 1 | 22.15 |
| 8 | −76 | −26 | Right Crus 1 | 17.26 | ||
| 24 | −70 | −58 | Right Lobule VIII | 12.15 | ||
| 2 | 5,000 | −36 | −62 | −40 | Left Crus 2 | 17.55 |
| 3 | 1,216 | −2 | −48 | −14 | Midline Lobule IV/V | 12.00 |
| Working Memory in comparison with Executive Function | ||||||
| 1 | 14,344 | 28 | −66 | −30 | Right Lobule VI | 23.62 |
| 10 | −76 | −26 | Right Crus 1 | 13.09 | ||
| 38 | −62 | −46 | Right Lobule VIIB | 10.96 | ||
| 26 | −70 | −58 | Right Lobule VIII | 8.69 | ||
| 2 | 3,960 | −36 | −62 | −42 | Left Crus 2 | 12.69 |
| −28 | −62 | −28 | Left Lobule VI | 12.21 | ||
| −34 | −70 | −22 | Left Lobule VI | 9.86 | ||
| 3 | 2,224 | −4 | −48 | −14 | Left Lobule IV/V | 13.45 |
| Working memory in comparison with language | ||||||
| 1 | 7,520 | 8 | −78 | −26 | Right Crus 1 | 17.28 |
| 30 | −68 | −32 | Right Crus 1 | 13.84 | ||
| −12 | −80 | −24 | Left Crus 1 | 9.57 | ||
| 36 | −64 | −46 | Right Lobule VIIB | 9.24 | ||
| 2 | 4,512 | −36 | −62 | −40 | Left Crus 2 | 19.00 |
| −36 | −70 | −20 | Left Lobule VI | 9.44 | ||
| 3 | 2,176 | −4 | −48 | −14 | Left Lobule IV/V | 13.50 |
| 856 | 24 | −70 | −58 | Right VIII | 11.23 | |
| Working memory in comparison with music | ||||||
| 1 | 14,632 | 28 | −68 | −34 | Right Crus 1 | 19.50 |
| 2 | −80 | −28 | Left Crus 2 | 15.68 | ||
| 2 | 3,088 | −36 | −62 | −38 | Left Crus 1 | 19.50 |
| 3 | 1,920 | −4 | −48 | −14 | Left Lobule IV/V | 12.82 |
| Working memory in comparison with timing | ||||||
| 1 | 18,008 | 28 | −66 | −32 | Right Crus 1 | 25.41 |
| 8 | −76 | −26 | Right Crus 1 | 18.77 | ||
| 2 | 6,304 | −36 | −62 | −38 | Left Crus 1 | 19.98 |
| −36 | −70 | −20 | Left Lobule VI | 9.60 | ||
| 3 | 2,104 | −4 | −48 | −14 | Left Lobule IV/V | 13.20 |
Coordinates are given in MNI space and locations were determined using the MRI atlas of the Human Cerebellum [Schmahmann et al.,2000].
Table D1.
Summary of results from the current and Stoodley and Schmahmann's 2009 meta‐analysis
| Task type | Location |
|---|---|
| Emotion | Left Crus I; Right lobule VI; Left VIIAt; Right lobules VIIIA; IV/V; IX; Left lobules VI; VIIIB; Right Crus I; Left Crus II |
| Executive function | Bilateral Crus I; Left lobule VI; Left VIIB; Left Crus II; Midline lobule VIIAt; Right lobule VI |
| Language | Bilateral lobule VI; Right Crus II; Right Crus I; Right lobule VIIAt; Midline lobule VIII; Left Crus I |
| Music | Right lobule V; Bilateral lobule VI; Bilateral lobule VIIIA |
| Timing | Right lobule VIIIA; Right lobule VIIIB; Right Crus I; Midline lobule IX |
| Working memory | Bilateral Crus I; Right lobule VIIIA; Bilateral lobule VI; Left lobule VI/Crus I; Left lobule IV/V |
Locations identified in both meta‐analysis are highlighted in bold; locations found only in Stoodley and Schmahmann's 2009 study are italicized; locations found only in this study have been placed last in regular style.
REFERENCES
- Abel KM, Allin MP, Kucharska‐Pietura K, David E, Andrew C, Williams S, Brammer MJ, Philips ML (2003): Ketamine alters neural processing of facial emotion recognition in healthy men: An fMRI study. Neuroreport 14:387–391. [DOI] [PubMed] [Google Scholar]
- Allen G, Buxton RB, Wong EC, Courchesne E (1997): Attentional activation of the cerebellum independent of motor involvement. Science 275:1940–1943. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R (2008): The role of the cerebellum in schizophrenia. Biol Psychiatry 64:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell PJ, Ivarsson M, Millar L, Yeo CH (2002): Cerebellar mechanisms in eyeblink conditioning. Ann NY Acad Sci 978:79–92. [DOI] [PubMed] [Google Scholar]
- Baddeley A (1992).Working memory. Science 255:556–559. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Dagher A, Aston JAD, Doyon J (2003): Dynamic functional changes associated with cognitive skill learning of an adapted version of the Tower of London task. Neuroimage 20:1649–1660. [DOI] [PubMed] [Google Scholar]
- Ben‐Yehudah G, Guediche S, Fiez JA (2007).Cerebellar contributions to verbal working memory: Beyond cognitive theory. The Cerebellum 6:193–201. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Pascual‐Leone A, Amedi A, Merabet LB, Fregni F, Gaab N, Alsop D, Schlaug G, Northoff G (2006): Dissociable networks for the expectancy and perception of emotional stimuli in the human brain. Neuroimage 30:588–600. [DOI] [PubMed] [Google Scholar]
- Blackwood N, Ffytche D, Simmons A, Bentall R, Murray R, Howard R (2004): The cerebellum and decision making under uncertainty. Cognitive Brain Res 20:46–53. [DOI] [PubMed] [Google Scholar]
- Bower JM (1997): Control of sensory data acquisition In: Schmahmann JD, editor.The Cerebellum and Cognition.San Diego:Academic Press; pp489–513. [DOI] [PubMed] [Google Scholar]
- Cairo TA, Liddle PF, Woodward TS, Ngan ET (2004): The influence of working memory load on phase specific patterns of cortical activity. Brain Res Cogn Brain Res 21:377–387. [DOI] [PubMed] [Google Scholar]
- Callan DE, Tsytasrev V, Hanakawa T, Callan AM, Katsuhara M, Fukuyama H, Turner R (2006).Song and speech: Brain regions involved with perception and covert production. NeuroImage 31:1327–1342. [DOI] [PubMed] [Google Scholar]
- Canli T, Sivers H, Thomason ME, Whitfield‐Gabrieli S, Gabrieli JD, Gotlib IH (2004).Brain activation to emotional words in depressed vs. healthy subjects. Neuroreport 15:2585–2588. [DOI] [PubMed] [Google Scholar]
- Chen JL, Penhune VB, Zatorre RJ (2008): Listening to musical rhythms recruits motor regions of the brain. Cerebral Cortex 18:2844–2854. [DOI] [PubMed] [Google Scholar]
- Chen SHA, Desmond JE (2005a): Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage 24:332–338. [DOI] [PubMed] [Google Scholar]
- Chen SHA, Desmond JE (2005b): Temporal dynamics of cerebro‐cerebellar network recruitment during a cognitive task. Neuropsychologia 43:1227–1237. [DOI] [PubMed] [Google Scholar]
- Chen L, Bao S, Lockard JM, Kim JJ, Thompson RF (1996): Imparid classical eyeblink conditioning in cerebellar‐lesions and Purkinje cell degeneration (pcd) mutant mice. J Neurosci 16:2829–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiricozzi FR, Clausi S, Molinari M, Leggio MG (2008).Phonological short‐term store impairment after cerebellar lesion: A single case study. Neuropsychologia 46:1940–1953. [DOI] [PubMed] [Google Scholar]
- Dagher A, Owen AM, Boecker H, Brooks DJ (1999): Mapping the network for planning: A correlational PET activation study with the Tower of London task. Brain 122:1973–1987. [DOI] [PubMed] [Google Scholar]
- Daniels C, Witt K, Wolff S, Jansen O, Deuschl G (2003): Rate dependency of the human cortical network subserving executive functions during generation of random number series—A functional magnetic resonance imaging study. Neuroscience Letters 345:25–28. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Grafman J (2002): The roles of the cerebellum and basal ganglia in timing and error prediction. Eur J Neurosci 16:1609–1619. [DOI] [PubMed] [Google Scholar]
- Drepper J, Timmann D, Kolb FP, Diener HC (1999): Non‐motor associative learning in patients with isolated degenerative cerebellar disease. Brain 122:897. [DOI] [PubMed] [Google Scholar]
- Durisko C, Fiez JA (2010).Functional activation in the cerebellum during working memory and simple speech tasks. Cortex 46:896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, London ED (2002): Decision‐making in a risk‐taking task: A PET study. Neuropsychopharmacology 26:682–691. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Petersen SE (1996).A positron emission tomography study of the short‐term maintenance of verbal information. J Neurosci 16:808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings M, Dimitrova A, Schorn CF, Elles HG, Hein‐Kropp C, Gizewski ER, Diener HC, Timmann D (2006): Cerebellar involvement in verb generation: An fMRI study. Neurosci Lett 409:19–23. [DOI] [PubMed] [Google Scholar]
- Geier CF, Garver KE, Luna B (2007).Circuitry underlying temporally extended spatial working memory. NeuroImage 35:904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Bird G, Brindley R, Frith CD, Burgess PW (2008): Atypical recruitment of medial prefrontal cortex in autism spectrum disorders: An fMRI study of two executive function tasks. Neuropsychologia 46:2281–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn JA, Brett M (2007): Rhythm and beat perception in motor areas of the brain. J Cogn Neurosci 19:893–906. [DOI] [PubMed] [Google Scholar]
- Gruber O (2001): Effects of domain‐specific interference on brain activation associated with verbal working memory task performance. Cerebral Cortex 11:1047–1055. [DOI] [PubMed] [Google Scholar]
- Gündel H, O'Connor MF, Littrell L, Fort C, Lane RD (2003): Functional neuroanatomy of grief: An fMRI study. Am J Psychiatry 160:1946–1953. [DOI] [PubMed] [Google Scholar]
- Gurd JM, Amunts K, Weiss PH, Zafiris O, Zilles K, Marshall JC, Fink GR (2002): Posterior parietal cortex is implicated in continuous switching between verbal fluency tasks: An fMRI study with clinical implications. Brain 125:1024–1038. [DOI] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, et al (2009).Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29:8586–8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habel U, Klein M, Kellermann T, Shah NJ, Schneider F (2005): Same or different? Neural correlates of happy and sad mood in healthy males. Neuroimage 26:206–214. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Boyd LA, Mayer AR, Sheltraw DM, Lee RR, Huang M, Rao SM (2004): Neural representation of interval encoding and decision making. Cogn Brain Res 21:193–205. [DOI] [PubMed] [Google Scholar]
- Harrington Dl, Zimbelmann JL, HInton SC, Rao SM (2010): Neural modulation of temporal encoding, maintenance, and decision processes. Cerebral Cortex 20:1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautzel H, Mottaghy FM, Specht K, Muller HW, Krause BJ (2009): Evidence of a modality‐dependent role of the cerebellum in working memory? An fMRI study comparing verbal and abstract n‐back tasks. NeuroImage 47:2073–2082. [DOI] [PubMed] [Google Scholar]
- Hayter AL, Langdon DW, Ramnani N (2007): Cerebellar contributions to working memory. NeuroImage 36:943–954. [DOI] [PubMed] [Google Scholar]
- Henson R, Bugess N, Frith C (2000): Recoding, storage, rehearsal and grouping in verbal short‐term memory: An fMRI study. Neuropsychologia 38:426–440. [DOI] [PubMed] [Google Scholar]
- Hofer A, Siedentopf CM, Ischebeck A, Rettenbacher MA, Verius M, Felber S, Fleischhacker WW (2007): Sex differences in brain activation patterns during processing of positively and negatively valenced emotional words. Psychological Med 37:109–119. [DOI] [PubMed] [Google Scholar]
- Hogan MJ (2004): The cerebellum in thought and action: A fronto‐cerebellar aging hypothesis. New Ideas Psychol 22:97–125. [Google Scholar]
- Honey GD, Bullmore ET, Sharma T (2000): Prolonged reaction time to a verbal working memory task predicts increased power of posterior parietal cortical activation. Neuroimage 12:495–503. [DOI] [PubMed] [Google Scholar]
- Imaizumi S, Mori K, Kiritani S, Kawashima R, Sugiura M, Fukuda H, Itoh K, Kato T, Nakamura A, Hatano K, Kojima S, Nakamura K (1997): Vocal identification of speaker and emotion activates different brain regions. Neuroreport 8:2809–2812. [DOI] [PubMed] [Google Scholar]
- Ito M (1997): Cerebellar microcomplexes In: Schmahmann JD, editor.The Cerebellum and Cognition.San Diego:Academic Press; pp475–487. [Google Scholar]
- Ivry R (1996): The representation of temporal information in perception and mother control. Current Opin Neurobiol 6:851–857. [DOI] [PubMed] [Google Scholar]
- Ivry R (1997): Cerebellar timing systems In: Schmahmann JD, editor.The Cerebellum and Cognition.San Diego:Academic Press; pp555–573. [Google Scholar]
- Ivry RB, Keele SW (1989): Timing functions of the cerebellum. J Cogn Neurosci 3:355–366. [DOI] [PubMed] [Google Scholar]
- Ivry R, Spencer RM, Zelaznik HN, Diedrichsen J (2002): The cerebellum and event timing. Ann NY Acad Sci 978:302–317. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Dirnberger G, Fuller R, Frith CD (2000): The role of the dorsolateral prefrontal cortex in random number generation: A study with positron emission tomography. Neuroimage 12:713–725. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Loose R, Lutz K, Specht K, Shah NJ (2000): Cortical activations during paced finger‐tapping applying visual and auditory pacing stimuli. Cogn Brain Res 10(1–2):51–66. [DOI] [PubMed] [Google Scholar]
- Jantzen KJ, Oullier O, Marshall M, Steinberg FL, Kelso JAS (2007): A parametric fMRI investigation of context effects in sensorimotor timing and coordination. Neuropsychologia 45:673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen KJ, Steinberg FL, Kelso JAS (2005): Functional MRI reveals the existence of modality and coordination‐dependent timing networks. Neuroimage 25:1031–1042. [DOI] [PubMed] [Google Scholar]
- Jollant F, Lawrence NS, Giampietro V, Brammer MJ, Fullana MA, Drapier D, Courtet P, Philips ML (2008): Orbitofrontal cortex response to angry faces in men with histories of suicide attempts. Am J Psychiatry 165:740–748. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Koeppe RA, Swh E, Reuter‐Lorenz PA, Marshuetz C, Willis CR (1998): The role of parietal cortex in verbal working memory. J Neurosci 18:5026–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justus T, Ravizza SM, Fiez JA, Ivry RB (2005): Reduced phonological similarity effects in patients with damage to the cerebellum. Brain Lang 95:304–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabanov A, Blom Ö, Forsman L, Ullén F (2009): The dorsal auditory pathway is involved in performance of both visual and auditory rhythms. Neuroimage 44:480–488. [DOI] [PubMed] [Google Scholar]
- Kirschen MP, Chen SHA, Desmond JE (2010): Modality specific cerebro‐cerebellar activations in verbal working memory: An fMRI study. Behav Neurol 23:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschen MP, Chen SHA, Schraedley‐Desmond P, Desmond JE (2005): Load‐ and practice‐dependent increases in cerebro‐cerebellar activation in verbal working memory: An fMRI study. Neuroimage 24:462–472. [DOI] [PubMed] [Google Scholar]
- Kirschen MP, Davis‐Ratner MS, Milner MW, Chen SHA, Schraedley‐Desmond P, Fisher PG, Desmond JE (2008): Verbal memory impairments in children after cerebellar tumor resection. Behav Neurol 20:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Lavond DG, Thompson RF (1988): The effects of lesions of the cerebellar cortex on retention of the classically conditioned eyeblink response when stimulation of the lateral reticular nucleus is used as a conditioned stimulus. Behav Neural Biol 49:293–301. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Torriero S, Salerno S, Gerfo EL, Caltagirone C (2007): Repetitive TMS of cerebellum interferes with millisecond time processing. Exp Brain Res 179:291–299. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Schulze K, Sammler D, Fritz T, Muller K, Gruber O (2009): Functional architecture of verbal and tonal working memory: An fMRI study. Hum Brain Mapp 30:859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Morishita M, Osaka N, Osaka M, Fukuyama H, Shibasaki H (2004): Functional roles of the cingulo‐frontal network in performance on working memory. Neuroimage 21:2–14. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL (2009): Segregated fronto‐cerebellar circuits revealed by intrinsic functional connectivity. Cerebral Cortex 19:2485–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam MM (1999): Neuroanatomic overlap of working memory and spatial attention networks: A functional MRI comparison within subjects. Neuroimage 10:695–704. [DOI] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT (2005): A comparison of label‐based review and activation likelihood estimation in the Stroop task. Hum Brain Mapp 25:6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE (1997): Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia 35:1437–1444. [DOI] [PubMed] [Google Scholar]
- Lee G, Meador K, Loring D, Allison J, Brown W, Paul L, Pillai J, Lavin T (2004): Neural substrates of emotion as revealed by functional magnetic resonance imaging. Cogn Behav Neurol 17:9–17. [DOI] [PubMed] [Google Scholar]
- Leiner H, Leiner A, Dow R (1991): The human cerebro‐cerebellar system: Its computing, cognitive, and language skills. Behav Brain Res 44:113–128. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM (2001): Event‐related fMRI study of response inhibition. Hum Brain Mapp 12:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurito JT, Kareken DA, Lowe MJ, Chen SHA, Mathews VP (2000): Comparison of rhyming and word generation with FMRI. Hum Brain Mapp 10:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitsch HJ, Vandekerckhove MM, Lanfermann H, Russ MO (2003): Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories. Cortex 39(4–5):643–665. [DOI] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE (2010a): Functional topography of the cerebellum in verbal working memory. Neuropsychol Rev 20:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE (2010b): The contributions of cerebro‐cerebellar circuitry to executive verbal working memory. Cortex 46:880–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschke M, Schugens M, Kindsvater K, Drepper J, Kolb FP, Diener HC, Daum I, Timmann D (2002): Fear conditioned changed of heart rate in patients with medial cerebellar lesions. J Neurol Neurosurg Psychiatry 72:116–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Clark GA, Lavond DG, Thompson RF (1982): Initial localization of the memory trace for a basic form of learning. Proc Natl Acad Sci 79:2731–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF (1984): Cerebellum: Essential involvement in the classically conditioned eyelid response. Science 223:296–299. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Petersen SE, Watson JM, Ojemann JG (2003): A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia 41:293–303. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (1994): Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science 266:458–461. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (2000): Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res Rev 31(2–3):236–250. [DOI] [PubMed] [Google Scholar]
- Najib A, Lorberbaum JP, Kose S, Bohning DE, George MS (2004): Regional brain activity in women grieving a romantic relationship breakup. Am J Psychiatry 161:2245–2256. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ (2002): A PET study of stimulus‐ and task‐induced semantic processing. Neuroimage 15:927–935. [DOI] [PubMed] [Google Scholar]
- O'Hare ED, Lu LH, Houston SM, Bookheimer SY, Sowell ER (2008): Neurodevelopmental changes in verbal working memory load‐dependency: An fMRI investigation. NeuroImage 42:1678–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly JX, Mesulam MM, Nobre AC (2008): The cerebellum predicts the timing of perceptual events. J Neurosci 28:2252–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann JG, Buckner RL, Akbudak E, Snyder AZ, Ollinger JM, McKinstry RC, Rosen BR, Petersen SE, Raichle ME, Conturo TE (1998): Functional MRI studies of word‐stem completion: Reliability across laboratories and comparison to blood flow imaging with PET. Hum Brain Mapp 6:203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso S, Johnson DL, Andreasen NC, O'Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD (1999): Cerebral blood flow changes associated with attribution of emotional valence to pleasant, unpleasant, and neutral visual stimuli in a PET study of normal subjects. Am J Psychiatry 156:1618–1629. [DOI] [PubMed] [Google Scholar]
- Park JY, Gu BM, Kang DH, Shin YW, Choi CH, Lee JM, Kwon JS (2010).Integration of cross‐modal emotional information in the human brain: An fMRI study. Cortex 46:161–169. [DOI] [PubMed] [Google Scholar]
- Peretz I, Zatorre R (2005): Brain organization for music processing. Ann Rev Psychol 56:89–114. [DOI] [PubMed] [Google Scholar]
- Petacchi A, Laird AR, Fox PT, Bower JM (2005): Cerebellum and auditory function: An ALE meta‐analysis of functional neuroimaging studies. Hum Brain Mapp 25:118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope P, Wing AM, Praamstra P, Miall RC (2005): Force related activations in rhythmic sequence production. Neuroimage 27:909–918. [DOI] [PubMed] [Google Scholar]
- Rao SM, Bobholz JA, Hammeke TA, Rosen AC, Woodley SJ, Cunningham JM, Cox RW, Stein EA, Binder JR (1997).Functional MRI evidence for subcortical participation in conceptual reasoning skills. Neuroreport 8:1987–1993. [DOI] [PubMed] [Google Scholar]
- Rauschecker AM, Pringle A, Watkins KE (2008): Changes in neural activity associated with learning to articulate novel auditory pseudowords by covert repetition. Hum Brain Mapp 29:1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Takino R, Tamada T, Iwata NK, Nielsen M (1999): Neural representation of a rhythm depends on its interval ratio. J Neurosci 19:10074–10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall U, Johnston P, Lagopoulos J, Juptner M, Jentzen W, Thienel R, Dittmann‐Balcar A, Bender S, Ward PB (2003): Functional brain maps of Tower of London performance: A positron emission tomography and functional magnetic resonance imaging study. Neuroimage 20:1154–1161. [DOI] [PubMed] [Google Scholar]
- Scheuerecker J, Ufer S, Zipse M, Frodl T, Koutsouleris N, Zetzsche T, Wiesmann M, Albrecht J, Brückmann H, Schmitt G, Möller HJ, Meisenzahl EM (2008): Cerebral changes and cognitive dysfunctions in medication‐free schizophrenia—An fMRI study. J Psychiatric Res 42:469–476. [DOI] [PubMed] [Google Scholar]
- Schlösser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, Dewey SL, Brodie JD (1998): Functional magnetic resonance imaging of human brain activity in a verbal fluency task. J Neurol Neurosurg Psychiatry 64:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD (1998): Dysmetria of thought: Clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn Sci 2:362–371. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD (2004): Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci 16:367–378. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC (2000):MRI Atlas of the Human Cerebellum.USA:Academic Press. [DOI] [PubMed] [Google Scholar]
- Schulze K, Zysset S, Mueller K, Friederici AD, Koelsch S (2011): Neuroarchitecture of verbal and tonal working memory in nonmusicians and musicians. Hum Brain Mapp 32:771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, Lauber E, Awh E, Jonides J, Smith EE, Koeppe RA (1996): PET evidence for an amodal verbal working memory system. NeuroImage 3:79–88. [DOI] [PubMed] [Google Scholar]
- Schutter D, Honk J (2005): The cerebellum on the rise in human emotion. Cerebellum 4:290–294. [DOI] [PubMed] [Google Scholar]
- Schweizer TA, Alexander MP, Cusimano M, Stuss DT (2007): Fast and efficient visuotemporal attention requires the cerebellum. Neuropsychologia 45:3068–3074. [DOI] [PubMed] [Google Scholar]
- Seger CA, Desmond JE, Glover GH, Gabrieli JDE (2000): Functional magnetic resonance imaging evidence for right‐hemisphere involvement in processing unusual semantic relationships. Neuropsychology 14:361–369. [DOI] [PubMed] [Google Scholar]
- Seki A, Okada T, Koeda T, Sadato N (2004): Phonemic manipulation in Japanese: An fMRI study. Cogn Brain Res 20:261–272. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Ongur D, Akbudak E, Conturo E, Ollinger JM, Snyder AZ, Gusnard DA, Raichle ME (2000): The emotional modulation of cognitive processing: An fMRI study. J Cogn Neurosci 12:157–170. [DOI] [PubMed] [Google Scholar]
- Smith A, Taylor E, Lidzba K, Rubia K (2003): A right hemispheric frontocerebellar network for time discrimination of several hundreds of milliseconds. Neuroimage 20:344–350. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson G, Calhoun VD (2007): Functional neural circuits for mental timekeeping. Hum Brain Mapp 28:394–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD (2009): Functional topography in the human cerebellum: A meta‐analysis of neuroimaging studies. Neuroimage 44:489–501. [DOI] [PubMed] [Google Scholar]
- Sweet LH, Paskavitz JF, Haley AP, Gunstad JJ, Mulligan RC, Nyalakanti PK, Cohen RA (2008): Imaging phonological similarity effects on verbal working memory. Neuropsychologia 46:1114–1123. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Koeda M, Oda K, Matsuda T, Matsushima E, Matsuura M, Asai K, Okubo Y (2004): An fMRI study of differential neural response to affective pictures in schizophrenia. Neuroimage 22:1247–1254. [DOI] [PubMed] [Google Scholar]
- Tavano A, Grasso R, Gagliardi C, Triulzi F, Bresolin N, Fabbro F, Borgatti R (2007).Disorders of cognitive and affective development in cerebellar malformations. Brain 130:2646–2660. [DOI] [PubMed] [Google Scholar]
- Thaut MH, Demartin M, Sanes JN (2008): Brain networks for integrative rhythm formation. PLoS ONE 3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, Birmaher V, Casey BJ (1999).A developmental functional MRI study of spatial working memory. NeuroImage 10:327–338. [DOI] [PubMed] [Google Scholar]
- Tieleman A, Seurinck R, Deblaere K, Vandemaele P, Vingerhoets G, Achten E (2005): Stimulus pacing affects the activation of the medial temporal lobe during a semantic classification task: An fMRI study. Neuroimage 26:565–572. [DOI] [PubMed] [Google Scholar]
- Timmann D, Drepper J, Frings M, Maschke M, Richter S, Gerwig M, Kolb FP (2010): The human cerebellum contributes to motor, emotional and cognitive associative learning. A review. Cortex 46:845–857. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Caparelli EC, Chang L, Ernst T (2005): fMRI‐acoustic noise alters brain activation during working memory tasks. Neuroimage 27:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Chang L, Caparelli EC, Ernst T (2007): Different activation patterns for working memory load and visual attention load. Brain Res 1132:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy JI, Faro SH, Mohamed FB, Pinsk M, Pinus A (2000): Functional localization of a ‘time keeper’ function separate from attentional resources and task strategy. Neuroimage 11:228–242. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Davalos DB, Rojas DC (2006): Effect of task difficulty on the functional anatomy of temporal processing. Neuroimage 32:307–315. [DOI] [PubMed] [Google Scholar]
- Turkeltaub P, Eden G, Jones K, Zeffiro T (2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. NeuroImage 16:765–780. [DOI] [PubMed] [Google Scholar]
- Ueda K, Okamoto Y, Okada G, Yamashita H, Hori T, Yamawaki S (2003).Brain activity during expectancy of emotional stimuli: An fMRI study. Neuroreport 14:51–55. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ (2005): Functional neuroanatomy of working memory in adults with attention‐deficit/hyperactivity disorder. Biol Psychiatry 57:439–447. [DOI] [PubMed] [Google Scholar]
- Watter S, Geffen GM, Geffen LB (2001): The n‐back as a dual‐task: P300 morphology under divided attention. Psychophysiology 38:998–1003. [DOI] [PubMed] [Google Scholar]
- Woodward TS, Cairo TA, Ruff CC, Takane Y, Hunter MA, Ngan ETC (2006): Functional connectivity reveals load dependent neural systems underlying encoding and maintenance in verbal working memory. Neuroscience 139:317–325. [DOI] [PubMed] [Google Scholar]
- Xiang H, Lin C, Ma X, Zhang Z, Bower JM, Weng X, Gao JH (2003): Involvement of the cerebellum in semantic discrimination: An fMRI study. Hum Brain Mapp 18:208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh YY, Kuo BC, Liu HL (2007): The neural correlates of attention orienting in visuospatial working memory for detecting feature and conjunction changes. Brain Res 1130:146–157. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E (1994): Neural mechanisms underlying melodic perception and memory for pitch. J Neurosci 14:1908–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]


