Abstract
Purpose
In radical prostatectomy (RP) procedures, sparing the neurovascular bundles adjacent to the posterolateral aspect of the prostatic fascia has often been suggested as a possible risk factor for positive surgical margins. Here we aimed to quantify the probability of extracapsular extension (ECE) at the posterolateral side of the prostate to aid in nerve-sparing decision making.
Materials and Methods
We evaluated 472 patients who underwent RP between July 2007 and January 2012. All patients underwent preoperative magnetic resonance imaging (MRI) with diffusion-weighted imaging and apparent diffusion coefficient mapping. We analyzed 944 side-specific prostate lobes with preoperative variables. To quantify the risk of side-specific posterolateral ECE after RP, we developed a risk-stratification scoring system through logistic regression analysis.
Results
Overall, 20.6% of 944 prostate lobes had ECE. In the multivariate analysis, prostate-specific antigen (PSA), biopsy Gleason score ≥7, percentage of side-specific cores with tumor, and posterolateral ECE on MRI were independent predictive factors of posterolateral ECE. On internal and external validation to calculate the predicted risk, the Hosmer-Lemeshow goodness-of-fit test showed good calibration (p=0.396).
Conclusions
PSA, biopsy Gleason score, percentage of side-specific cores with tumor, and posterolateral ECE on MRI are independent predictors of posterolateral ECE. The scoring system derived from this study will provide objective parameters for use when deciding if the neurovascular bundle can be safely spared.
Keywords: Magnetic resonance imaging, Prostatectomy, Prostatic neoplasms
INTRODUCTION
In 2012, Jung et al. [1] announced that an estimated 11,016 new cases of prostate cancer-9.3% of the total cancers in men-would be diagnosed in Korea and that approximately 1,540 men (3.4%) were expected to die from this disease [2]. Radical prostatectomy (RP) is a major treatment for clinically localized prostate cancer. Since Quinlan et al. [3] detailed the anatomy of the pelvic cavity and nerve-sparing RP, the maintenance of potency after RP has greatly improved [4-6]. However, sparing the neurovascular bundles (NVBs) adjacent to the posterolateral aspect of the prostatic fascia has often been suggested as a possible risk factor for positive surgical margins (PSMs) [7].
A PSM has been shown to be associated with higher rates of biochemical recurrence and disease progression [8-10]. In most studies, NVB sparing had no significant impact on PSMs [11-15]. Nonetheless, in several robotic prostatectomy series, increased PSM rates in pathological T3 tumors were found to be related to nerve sparing [16-19].
Many nomograms and guidelines for nerve-sparing RP have been reported previously [20-23]. The designs of these earlier studies were mostly based on the likelihood of extracapsular extension (ECE). ECE is associated with a greater risk of a PSM. In general, NVB sparing is not recommended if the chance of ECE is high. However, ECE is often not in the region of the NVB [23].
Our current study was specifically aimed at quantifying the probability of ECE at the posterolateral side of the prostate. We developed a risk-stratification scoring system for the prediction of posterolateral ECE to help in nerve-sparing decision making.
MATERIALS AND METHODS
1. Study subjects
From July 2007 to January 2012, 1,083 men underwent RP by a single surgeon at the Asan Medical Center. Patients who received neoadjuvant therapy (n=66) and referred patients without detailed information (n=545) were excluded. The following patient characteristics were evaluated: age, body mass index (BMI), pretreatment prostate-specific antigen (PSA), results of side-specific digital rectal exam (DRE), prostate volume by transrectal ultrasonography (TRUS), side-specific data from TRUS-guided prostate needle biopsies, side-specific pathological findings, and side-specific magnetic resonance imaging (MRI) findings.
Extended 12-core biopsies (6 from each side at the prostatic apex, middle, and base) were preoperatively performed in all patients. Preoperative evaluation included prostate MRI (Philips Achieva 3.0-T TX or Philips Ingenia 3.0-T, Philips Healthcare, Andover, MA, USA) and bone scanning in most patients. Prostate MRI with sagittal, coronal, and axial T2-weighted imaging; diffusion-weighted imaging; and apparent diffusion coefficient (ADC) maps was performed in all patients. All images were analyzed prospectively by expert genitourinary radiologists. Criteria for the presence of cancer on T2-weighted images included round, ovoid, or irregular areas of low signal intensity without a corresponding high T1 signal intensity. Restricted diffusion on diffusion-weighted images and ADC maps on MRI were also positive cancer findings.
2. Pathological analysis
For side-specific analysis, 944 prostate lobes (right and left) were evaluated. Both the prostate apex and the base were examined by the cone method with sagittal sectioning of the tissue sample, and the remaining specimen was sectioned transversely to the major axis after routine fixation at 3- to 5-mm intervals. Each slice was prepared for microscopic examination on whole-mount slides or was divided and split between two slides. Sections were formalin fixed and processed for paraffin embedding with routine protocols. All prostate blocks were site-specifically labeled. All pathology slides were reviewed by a single uropathologist. Pathological staging was performed by using the 2002 TNM classification and the differentiation was assigned according to the Gleason scheme.
3. Statistical methods
In our two study groups, the clinical and pathological characteristics are expressed as frequencies and means. Differences were assessed by t-tests for continuous variables and chi-square tests for categorical variables. To develop the prediction model, multivariable analysis was performed with the use of logistic regression using Generalized Estimating Equations that accounted for the clustering of lobe pairs. A logistic regression model was repeated for each of 1,000 bootstrap resamplings. A 50% relative frequency of selection of bootstrap resampling was the criterion for inclusion of predictors in the final logistic model. Points associated with each category of each risk factor were computed by bootstrap bias-corrected regression coefficients. To evaluate this points system, the C-statistic was used to measure discrimination and the Hosmer-Lemeshow test was used to assess calibration. The scoring system was externally validated on an independent cohort of 376 prostate lobes.
RESULTS
A total of 944 lobes were analyzed. ECE was observed in 194 prostate lobes (20.6%). Table 1 summarizes patient characteristics, subdivided by the side-specific posterolateral ECE status. There were no significant differences between the two groups with regard to age, BMI, or prostate volume. The patient group with posterolateral ECE, however, had a higher PSA level and clinical stage than did the patient group without posterolateral ECE (p<0.001 and p=0.001, respectively). In the TRUS-guided prostate biopsy findings, the patient group with posterolateral ECE had a higher Gleason score (GS) and percentage of side-specific cores with a tumor than did the patient group without posterolateral ECE (all p<0.001). In the side-specific MRI findings, a higher proportion of positive findings of ECE was shown in the patient group with posterolateral ECE (p<0.001). Robot-assisted laparoscopic prostatectomy was performed in 359 patients (76.1%) and NVBs were preserved in 765 lobes (81.0%). Overall, PSMs were identified in 80 lobes (8.5%) and posterolateral PSMs were seen in 51 lobes (5.4%). When ECE was present in the lobe, a posterolateral area was the most common PSM site (16.5%).
TABLE 1.
Patient characteristics subdivided by side-specific posterolateral ECE status
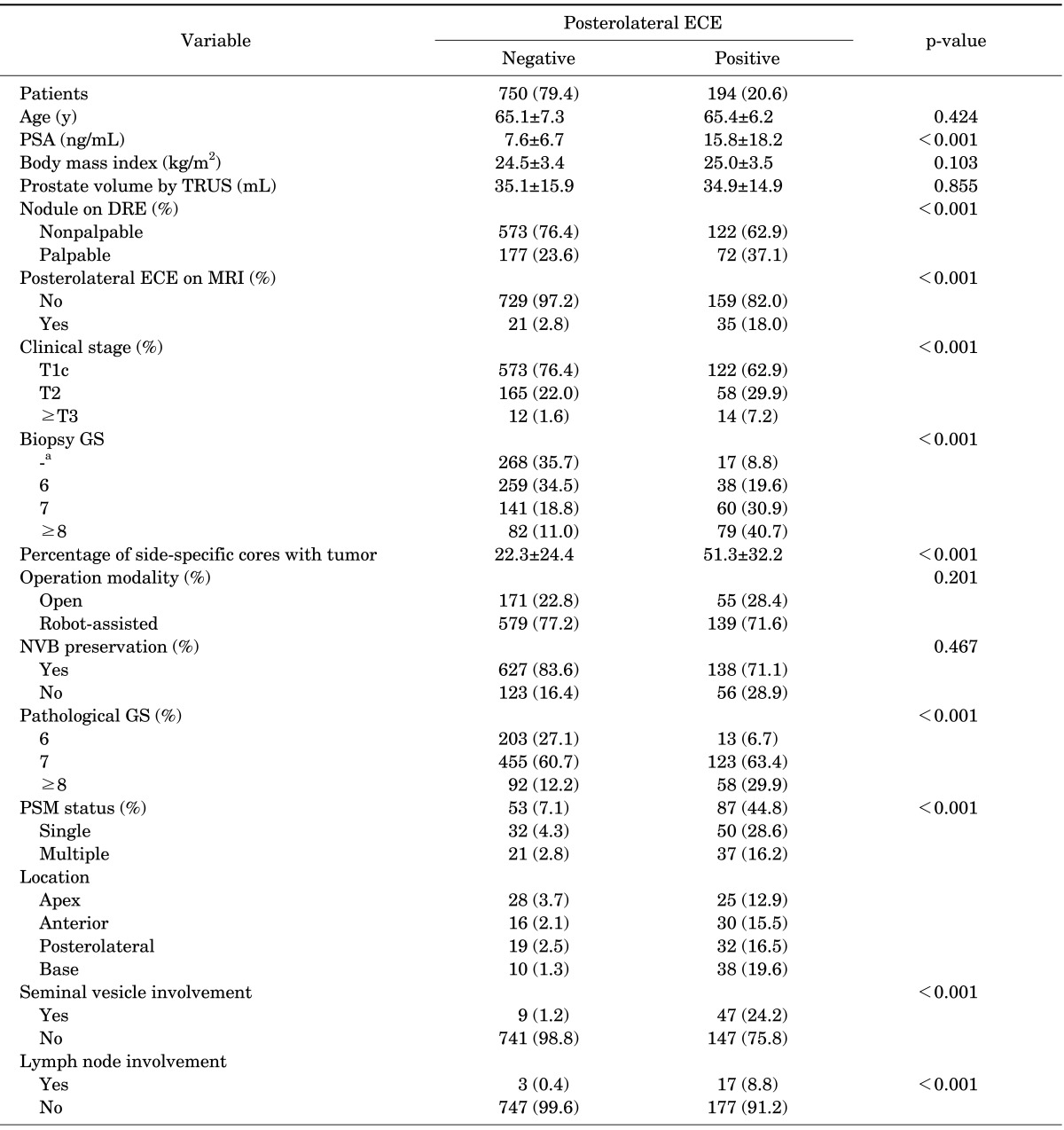
Values are presented as number (%) or mean±standard deviation.
ECE, extracapsular extension; PSA, prostate-specific antigen; TRUS, transrectal ultrasonography; DRE, digital rectal exam; MRI, magnetic resonance imaging; GS, Gleason score; NVB, neurovascularbundle; PSM, positive surgical margin.
a:Tumor was not present in the corresponding lobe onprostate biopsy.
Table 2 lists the predictive factors identified by multivariate logistic regression analysis for posterolateral ECE after RP. We found that PSA (odds ratio [OR], 1.039; p<0.001), biopsy GS≥7 (OR, 2.057; p=0.050), side-specific cores with tumor (OR, 1.018; p<0.001), and posterolateral ECE on MRI (OR, 3.047; p=0.001) were independent predictors of posterolateral ECE.
TABLE 2.
Multivariate logistic analysis for the prediction of posterolateral ECE
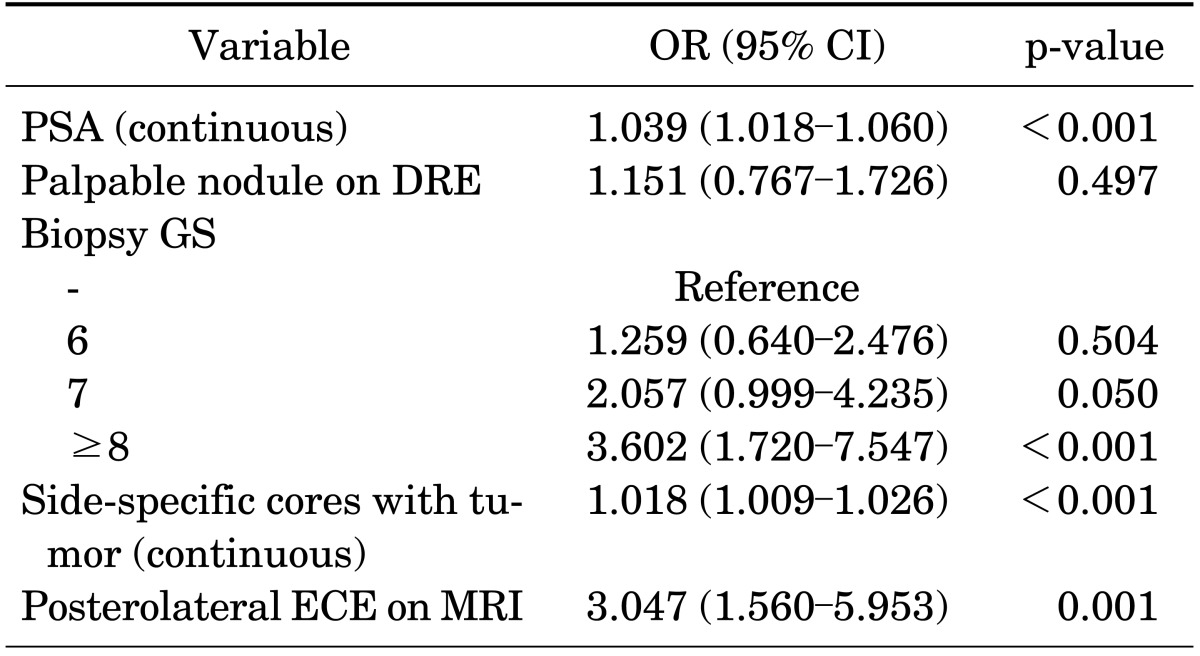
ECE, extracapsular extension; OR, odds ratio; CI, confidence interval; PSA, prostate-specific antigen; DRE, digital rectal exam; GS, Gleason score; MRI, magnetic resonance imaging.
To quantify the risk of side-specific posterolateral ECE after RP, we developed a risk-stratification scoring system based on a logistic regression model (Table 3). We selected the preoperative variables with p<0.05 in the univariate analysis. The model included PSA, biopsy GS, percentage of side-specific cores with tumor, and posterolateral ECE on MRI. All factors had 0 to 4 points each. The predicted risk estimation by the scoring system is shown in Table 4. A total score ranged from 0 to 14 points and the risk of posterolateral ECE ranged from 4.1% to 91.7%.
TABLE 3.
Scoring system for the prediction of posterolateral ECE
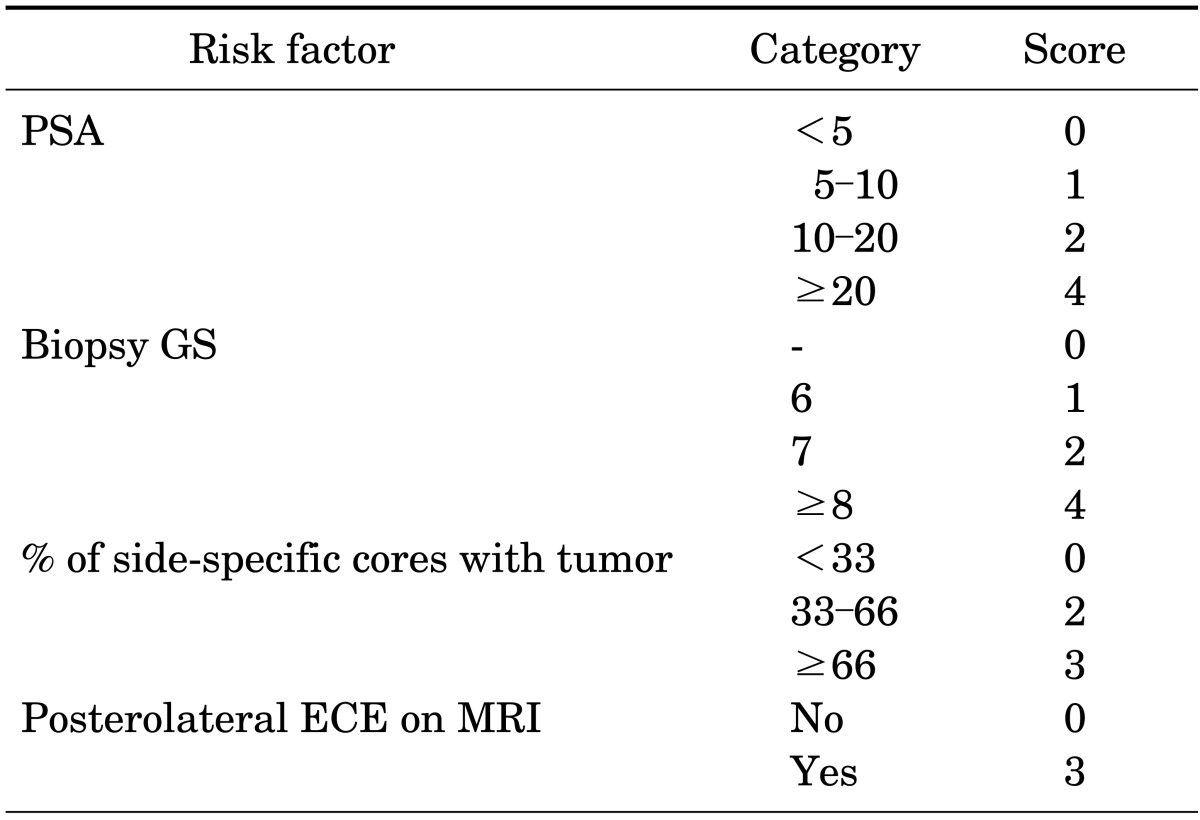
ECE, extracapsular extension; PSA, prostate-specific antigen; GS, Gleason score; MRI, magnetic resonance imaging.
TABLE 4.
Predicted risk estimation by the scoring system
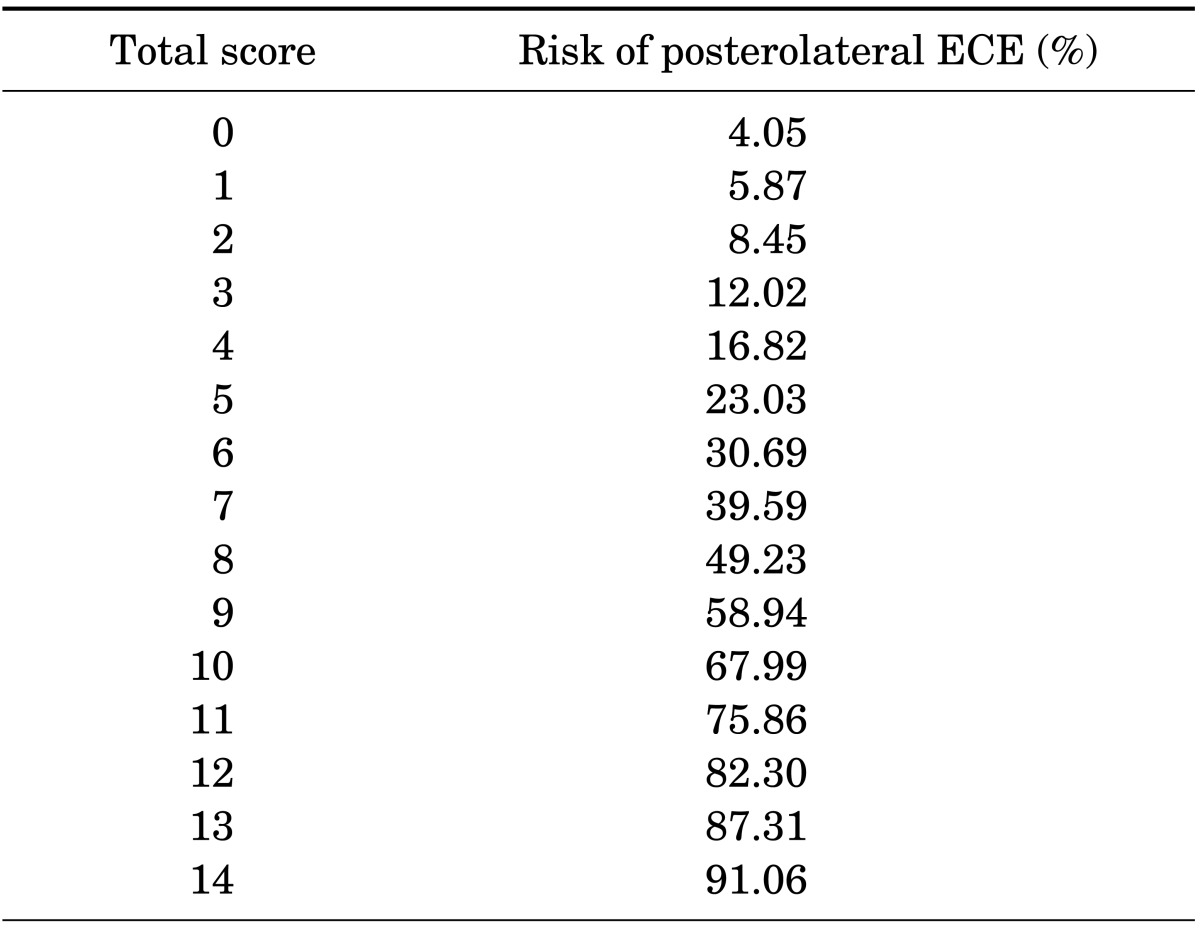
ECE, extracapsular extension.
To assess the accuracy of predicting posterolateral ECE, we performed internal and external validation and calculated the area under the ROC curve. Fig. 1 and Table 5 show the discrimination ability and calibration ability of the scoring system. Figs. 2 and 3 show the calibration curve of the scoring system.
FIG. 1.
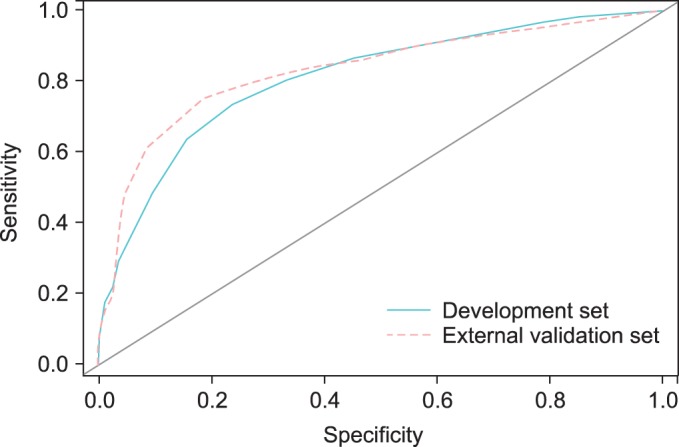
Receiver operating characteristic curves for the development set (AUC=0.810) and external validation set (AUC=0.830). AUC, area under the curve.
TABLE 5.
Discrimination and calibration ability of the scoring system
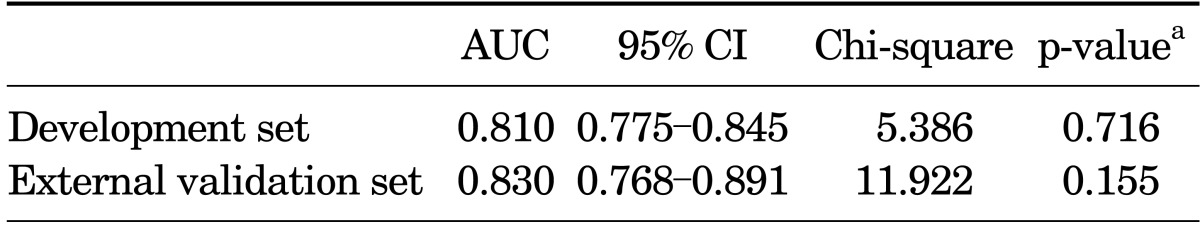
AUC, area under the curve; CI, confidence interval.
a:Hosmer and Lemeshow test.
FIG. 2.
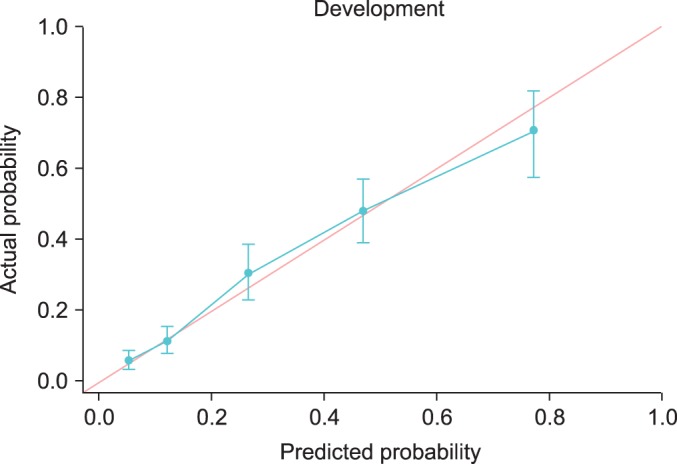
Calibration curves for the development set.
FIG. 3.
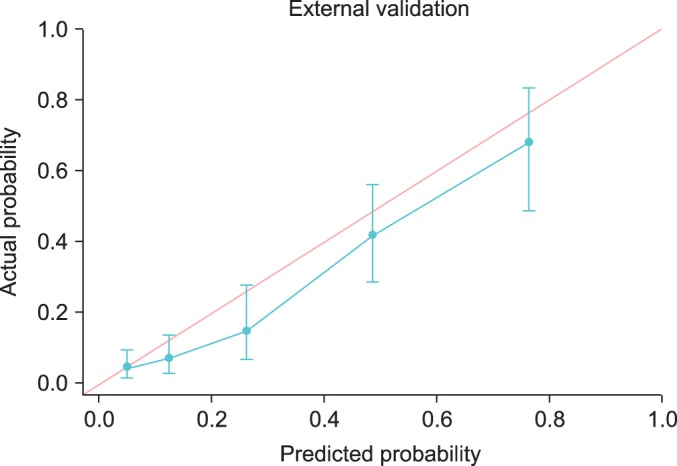
Calibration curves for the external validation set.
DISCUSSION
Patient selection for nerve-sparing RP is important to best balance functional and oncological outcomes. A PSM after RP is defined as cancer at the inked surface of the specimen. This result can either be due to surgical incision into the capsule of an organ-confined prostate cancer or be associated with ECE beyond the limits of surgical resection. In particular, a posterolateral PSM in nerve-sparing RP is a critical issue because of its anatomical location. PSMs were most commonly seen at the apex in this study. However, in patients with pT3a tumors, PSMs were most commonly located at the posterolateral site. Although a recent multicenter study found no significant difference in the risk of biochemical recurrence according to location of the PSM [24], a careful dissection of the posterolateral prostate after RP is required if there is any suspicion of ECE. In general, nerve sparing is not recommended when ECE is suspected.
Many algorithms and nomograms have been developed to predict the risk of ECE. Shah et al. [21] introduced the New York University nerve-sparing algorithm, which is based on biopsy GS, tumor volume on biopsy, and perineural invasion. This algorithm was developed by using the likelihood of ECE. Tsuzuki et al. [23] also developed an algorithm for the prediction of ECE in the region of the NVB. This model combines the biopsy GS, preoperative PSA, palpable nodules on DRE, percentage of side-specific cores with tumor, and average percentage involvement of each positive core. Tewari et al. [22] reported a risk-stratified approach to nerve sparing in robot-assisted laparoscopic prostatectomy. This Cornell algorithm included the clinical T stage, biopsy Gleason grade, preoperative PSA, percentage of tumor in any biopsy core, and MRI findings. However, it is still unclear whether all men with ECE should undergo excision of the NVB. Patients at high risk of ECE are presumably at higher risk of harboring preexisting occult metastasis [25], and the ECE is often not in the region of the NVB [23]. In this study, however, we focused on quantifying the probability of posterolateral ECE at the NVB, rather than overall ECE. Significant independent predictors of posterolateral ECE on nerve sparing were PSA, biopsy GS≥7, percentage of side-specific cores with tumor, and posterolateral ECE on MRI. The scoring system was constructed on the basis of a logistic regression model.
According to the scoring system, the predicted risk of ECE is 91.1% when all factors are positive and 4.1% when all factors are negative. For example, the risk of ECE is predicted to be 49.2% when a prostate lobe has 8 points.
In this study, we found that MRI findings were important factors for the prediction of ECE and for planning nerve-sparing RP. Positive findings of posterolateral ECE on MRI was one of the independent risk factors (OR, 3.047; p=0.001) for posterolateral PSMs, and it had the second highest score (3 points) for the predictive model. McClure et al. [26] reported that preoperative prostate MRI data modified the decision to use a nerve-sparing technique during robot-assisted laparoscopic prostatectomy in 27% of patients. Furthermore, in patients whose surgical plan was changed to a nerve-sparing technique, there were no PSMs on the side of the prostate. In that study, the nerve-sparing technique was reserved for patients without intermediate or high probability of ECE. Several studies also examined the prediction of ECE on MRI [26-28]. In general, the diagnostic criteria for determining ECE on MRI included an irregular capsular bulge, periprostatic fat infiltration, obliteration of the rectoprostatic angle, and asymmetry or direct involvement of the NVB. Findings in studies in which MRI was used for prostate cancer staging and prediction of ECE have yielded a range of values for accuracy (56.88%), sensitivity (51.89%), and specificity (67.87%). Reader expertise, MRI equipment, and study protocols may have influenced this variability.
Our study had several limitations. First, although we attempted to carefully select our study population, the problems inherent in a retrospective study are unavoidable and may have affected the results. Second, we cannot show any evidence that applying this model truly decreases posterolateral PSM after RP. Last, four different uroradiologists analyzed the MRI findings and, although many MRI criteria are used when staging prostate cancer, interobserver variability was not considered.
The present study, however, is of value because our study is the first report of a risk-stratification scoring system for ECE. In addition, no other studies have, to our knowledge, used nomograms or scoring systems that included MRI findings as factors. Our study has an advantage because it was a large single-center series. Finally, the current work is the first study of predicting ECE in Korean prostate cancer patients. We believe that the scoring system derived from this study will help to predict the individual risk of posterolateral ECE and will help surgeons to decide whether to perform nerve-sparing RP. Nonetheless, future efforts to decrease the PSM in nerve-sparing RP require more specific predictors and better imaging and surgical techniques.
CONCLUSIONS
We found that PSA, biopsy GS≥7, percentage of side-specific cores with tumor, and posterolateral ECE on MRI are independent predictors of posterolateral ECE. We developed a scoring system to predict the risk of side-specific posterolateral ECE. The use of the scoring system derived from this study will provide objective parameters when determining whether the NVB can be safely spared.
Footnotes
The authors have nothing to disclose.
References
- 1.Jung KW, Park S, Won YJ, Kong HJ, Lee JY, Seo HG, et al. Prediction of cancer incidence and mortality in Korea, 2012. Cancer Res Treat. 2012;44:25–31. doi: 10.4143/crt.2012.44.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chi BH, Chang IH. Prostate cancer: recent trends in Korea. Urol Int. 2010;85:88–93. doi: 10.1159/000318677. [DOI] [PubMed] [Google Scholar]
- 3.Quinlan DM, Epstein JI, Carter BS, Walsh PC. Sexual function following radical prostatectomy: influence of preservation of neurovascular bundles. J Urol. 1991;145:998–1002. doi: 10.1016/s0022-5347(17)38512-9. [DOI] [PubMed] [Google Scholar]
- 4.Berryhill R, Jr, Jhaveri J, Yadav R, Leung R, Rao S, El-Hakim A, et al. Robotic prostatectomy: a review of outcomes compared with laparoscopic and open approaches. Urology. 2008;72:15–23. doi: 10.1016/j.urology.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 5.Ficarra V, Novara G, Artibani W, Cestari A, Galfano A, Graefen M, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037–1063. doi: 10.1016/j.eururo.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Raina R, Agarwal A, Zippe CD. Management of erectile dysfunction after radical prostatectomy. Urology. 2005;66:923–929. doi: 10.1016/j.urology.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 7.Catalona WJ, Bigg SW. Nerve-sparing radical prostatectomy: evaluation of results after 250 patients. J Urol. 1990;143:538–543. doi: 10.1016/s0022-5347(17)40013-9. [DOI] [PubMed] [Google Scholar]
- 8.Blute ML, Bostwick DG, Bergstralh EJ, Slezak JM, Martin SK, Amling CL, et al. Anatomic site-specific positive margins in organ-confined prostate cancer and its impact on outcome after radical prostatectomy. Urology. 1997;50:733–739. doi: 10.1016/S0090-4295(97)00450-0. [DOI] [PubMed] [Google Scholar]
- 9.Eastham JA, Kuroiwa K, Ohori M, Serio AM, Gorbonos A, Maru N, et al. Prognostic significance of location of positive margins in radical prostatectomy specimens. Urology. 2007;70:965–969. doi: 10.1016/j.urology.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 10.Karakiewicz PI, Eastham JA, Graefen M, Cagiannos I, Stricker PD, Klein E, et al. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology. 2005;66:1245–1250. doi: 10.1016/j.urology.2005.06.108. [DOI] [PubMed] [Google Scholar]
- 11.Katz R, Salomon L, Hoznek A, de la Taille A, Antiphon P, Abbou CC. Positive surgical margins in laparoscopic radical prostatectomy: the impact of apical dissection, bladder neck remodeling and nerve preservation. J Urol. 2003;169:2049–2052. doi: 10.1097/01.ju.0000065822.15012.b7. [DOI] [PubMed] [Google Scholar]
- 12.Nelles JL, Freedland SJ, Presti JC, Jr, Terris MK, Aronson WJ, Amling CL, et al. Impact of nerve sparing on surgical margins and biochemical recurrence: results from the SEARCH database. Prostate Cancer Prostatic Dis. 2009;12:172–176. doi: 10.1038/pcan.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palisaar RJ, Noldus J, Graefen M, Erbersdobler A, Haese A, Huland H. Influence of nerve-sparing (NS) procedure during radical prostatectomy (RP) on margin status and biochemical failure. Eur Urol. 2005;47:176–184. doi: 10.1016/j.eururo.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Sofer M, Hamilton-Nelson KL, Civantos F, Soloway MS. Positive surgical margins after radical retropubic prostatectomy: the influence of site and number on progression. J Urol. 2002;167:2453–2456. [PubMed] [Google Scholar]
- 15.Ward JF, Zincke H, Bergstralh EJ, Slezak JM, Myers RP, Blute ML. The impact of surgical approach (nerve bundle preservation versus wide local excision) on surgical margins and biochemical recurrence following radical prostatectomy. J Urol. 2004;172(4 Pt 1):1328–1332. doi: 10.1097/01.ju.0000138681.64035.dc. [DOI] [PubMed] [Google Scholar]
- 16.Liss M, Osann K, Ornstein D. Positive surgical margins during robotic radical prostatectomy: a contemporary analysis of risk factors. BJU Int. 2008;102:603–608. doi: 10.1111/j.1464-410X.2008.07672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potdevin L, Ercolani M, Jeong J, Kim IY. Functional and oncologic outcomes comparing interfascial and intrafascial nerve sparing in robot-assisted laparoscopic radical prostatectomies. J Endourol. 2009;23:1479–1484. doi: 10.1089/end.2009.0369. [DOI] [PubMed] [Google Scholar]
- 18.Shikanov S, Woo J, Al-Ahmadie H, Katz MH, Zagaja GP, Shalhav AL, et al. Extrafascial versus interfascial nerve-sparing technique for robotic-assisted laparoscopic prostatectomy: comparison of functional outcomes and positive surgical margins characteristics. Urology. 2009;74:611–616. doi: 10.1016/j.urology.2009.01.092. [DOI] [PubMed] [Google Scholar]
- 19.Smith JA, Jr, Chan RC, Chang SS, Herrell SD, Clark PE, Baumgartner R, et al. A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. J Urol. 2007;178:2385–2389. doi: 10.1016/j.juro.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Graefen M, Haese A, Pichlmeier U, Hammerer PG, Noldus J, Butz K, et al. A validated strategy for side specific prediction of organ confined prostate cancer: a tool to select for nerve sparing radical prostatectomy. J Urol. 2001;165:857–863. [PubMed] [Google Scholar]
- 21.Shah O, Robbins DA, Melamed J, Lepor H. The New York University nerve sparing algorithm decreases the rate of positive surgical margins following radical retropubic prostatectomy. J Urol. 2003;169:2147–2152. doi: 10.1097/01.ju.0000057496.49676.5a. [DOI] [PubMed] [Google Scholar]
- 22.Tewari AK, Srivastava A, Huang MW, Robinson BD, Shevchuk MM, Durand M, et al. Anatomical grades of nerve sparing: a risk-stratified approach to neural-hammock sparing during robot-assisted radical prostatectomy (RARP) BJU Int. 2011;108(6 Pt 2):984–992. doi: 10.1111/j.1464-410X.2011.10565.x. [DOI] [PubMed] [Google Scholar]
- 23.Tsuzuki T, Hernandez DJ, Aydin H, Trock B, Walsh PC, Epstein JI. Prediction of extraprostatic extension in the neurovascular bundle based on prostate needle biopsy pathology, serum prostate specific antigen and digital rectal examination. J Urol. 2005;173:450–453. doi: 10.1097/01.ju.0000151370.82099.1a. [DOI] [PubMed] [Google Scholar]
- 24.Stephenson AJ, Wood DP, Kattan MW, Klein EA, Scardino PT, Eastham JA, et al. Location, extent and number of positive surgical margins do not improve accuracy of predicting prostate cancer recurrence after radical prostatectomy. J Urol. 2009;182:1357–1363. doi: 10.1016/j.juro.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 25.Lepor H, Tareen B. Neurovascular bundle resection: does it improve the margins? Urol Oncol. 2010;28:215–218. doi: 10.1016/j.urolonc.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 26.McClure TD, Margolis DJ, Reiter RE, Sayre JW, Thomas MA, Nagarajan R, et al. Use of MR imaging to determine preservation of the neurovascular bundles at robotic-assisted laparoscopic prostatectomy. Radiology. 2012;262:874–883. doi: 10.1148/radiol.11103504. [DOI] [PubMed] [Google Scholar]
- 27.Bloch BN, Furman-Haran E, Helbich TH, Lenkinski RE, Degani H, Kratzik C, et al. Prostate cancer: accurate determination of extracapsular extension with high-spatial-resolution dynamic contrast-enhanced and T2-weighted MR imaging: initial results. Radiology. 2007;245:176–185. doi: 10.1148/radiol.2451061502. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, Hricak H, Kattan MW, Chen HN, Scardino PT, Kuroiwa K. Prediction of organ-confined prostate cancer: incremental value of MR imaging and MR spectroscopic imaging to staging nomograms. Radiology. 2006;238:597–603. doi: 10.1148/radiol.2382041905. [DOI] [PubMed] [Google Scholar]


