Abstract
Purpose
To investigate the incidence and predictive factors associated with the development of chronic kidney disease (CKD) in patients undergoing curative surgery for renal cell carcinoma.
Materials and Methods
From 2003 to 2010, we retrospectively investigated 108 patients undergoing partial nephrectomy or radical nephrectomy (RN) for renal tumors with a preoperative glomerular filtration rate (GFR)≥60. The GFR was calculated by use of the four-variable modification of diet in renal disease (MDRD) formula. CKD was defined as an estimated GFR (eGFR) less than 60 mL/min per 1.73 m2. Demographic and clinicopathologic parameters were evaluated by using the chi-square and Student t-tests and multivariate regression analysis to determine the variables independently associated with the development of postoperative CKD.
Results
Of the 108 patients without preoperative CKD, CKD developed in 43 patients (39.8%). In the analysis of clinical factors between patients with and those without CKD development, gender, body mass index, diabetes mellitus, hypertension, and tumor size were not significant clinical factors. Statistical significance for CKD development was found for age of 60 years or greater (p=0.013), decreased preoperative eGFR (p<0.001), and RN group (p<0.001). In the multivariate analysis, decreased preoperative eGFR (p=0.001) and RN group (p=0.002) were significant independent predictors.
Conclusions
The results of our study show that decreased preoperative renal function and RN were significant independent predictors of postoperative CKD. In patients who had a relatively decreased preoperative eGFR, especially when estimated by use of the MDRD formula, nephron-sparing surgery should be considered for the treatment of small renal tumors.
Keywords: Chronic kidney disease, Glomerular filtration rate, Renal cell carcinoma
INTRODUCTION
The widespread availability of imaging modalities has led to more frequent early detection of small renal tumors. Most of these relatively small tumors are localized within the kidney, which means that most of these patients rarely die of disease after tumor treatment [1].
Recent studies also suggest that chronic kidney disease (CKD) is associated with an increased incidence of renal insufficiency in long-term follow-up [2,3]. This is because a decreased glomerular filtration rate (GFR) is known to be associated with an increased risk of cardiovascular events, hospitalization, and death [4,5].
Considering overall cancer survival and postoperative renal function, partial nephrectomy (PN) might be regarded as a standard treatment for small renal tumors sized 4 cm or less in patients with a normal contralateral kidney [6,7]. However, controversies remain regarding the predictive factors for the development of CKD in patients undergoing PN or radical nephrectomy (RN) for the treatment of renal tumors. Whereas some investigators have reported that older age and diabetes are prognostic factors for the development of CKD [8], others have reported that male gender and older age, and not diabetes mellitus, are prognostic factors [9].
Knowing these predictive factors may help us to choose the proper surgical procedure in patients considering treatment for renal tumors. Thus, in the present study, we investigated the predictive factors associated with the development of CKD in patients undergoing PN or RN for the treatment of renal tumors.
MATERIALS AND METHODS
From 2003 to 2010, we retrospectively reviewed the medical records of 135 patients with renal cell carcinoma who underwent open surgical procedures. Clinical data including age, gender, body mass index (BMI), estimated GFR (eGFR), type of surgical procedure, renal tumor size, history of diabetes mellitus, and history of hypertension were obtained. After excluding 27 patients (20.0%) with preoperative CKD, data from 108 patients, including 29 patients who underwent PN, were analyzed. The eGFR (mL/min per 1.73 m2) was calculated by using the abbreviated four-variable modification of diet in renal disease (MDRD) study equation: GFR=186.3×(Pcr)-1.154×(age)-0.203×(0.742 if female)×(1.210 if black) [10].
CKD was defined as eGFR less than 60 mL/min per 1.73 m2 according to CKD stage 3 (moderate decrease of GFR: 30-59 mL/min per 1.73 m2) [11]. The eGFR was measured every 6 months until 3 years postoperatively.
Because preoperative eGFR was a qualitative variable as well as a categorical variable, by explicating an ordinal scale, we transformed the preoperative eGFR scale into a quantitative variable by expressing the values as 1, 2, and 3. When the preoperative eGFR value increased by one unit (i.e., 60-69 or 70-79 or ≥80), the odds ratio of postoperative eGFR<60 compared with eGFR≥60 was 0.192.
Age was analyzed as less than 60 years versus 60 years or greater according to the National Kidney Foundation Practice Guidelines for Chronic Kidney Disease [12].
The chi-square and Student t-tests were used for comparative analysis, and multivariate regression analysis was used for the variables associated with the development of postoperative CKD. All statistical analysis was done by using IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA), and differences were considered statistically significant when the p-value was <0.05.
RESULTS
The patients' median age was 62.5 years, and 71 patients (65.7%) were men. The mean preoperative eGFR was 89.7±18.4 mL/min per 1.73 m2. Of the 108 patients without preoperative CKD, CKD developed in 43 (39.8%).
In the analysis of clinical factors between patients with and those without CKD development, gender, BMI, diabetes mellitus, hypertension, and tumor size were not significant clinical factors. Statistical significance for CKD development was found in patients aged 60 years or older (p=0.013), in patients with decreased preoperative eGFR (p<0.001), and in the RN group (p<0.001) (Table 1). In the multivariate analysis, decreased preoperative eGFR (p=0.001) and RN group (p=0.002) were significant independent predictors (Table 2).
TABLE 1.
Patient characteristics
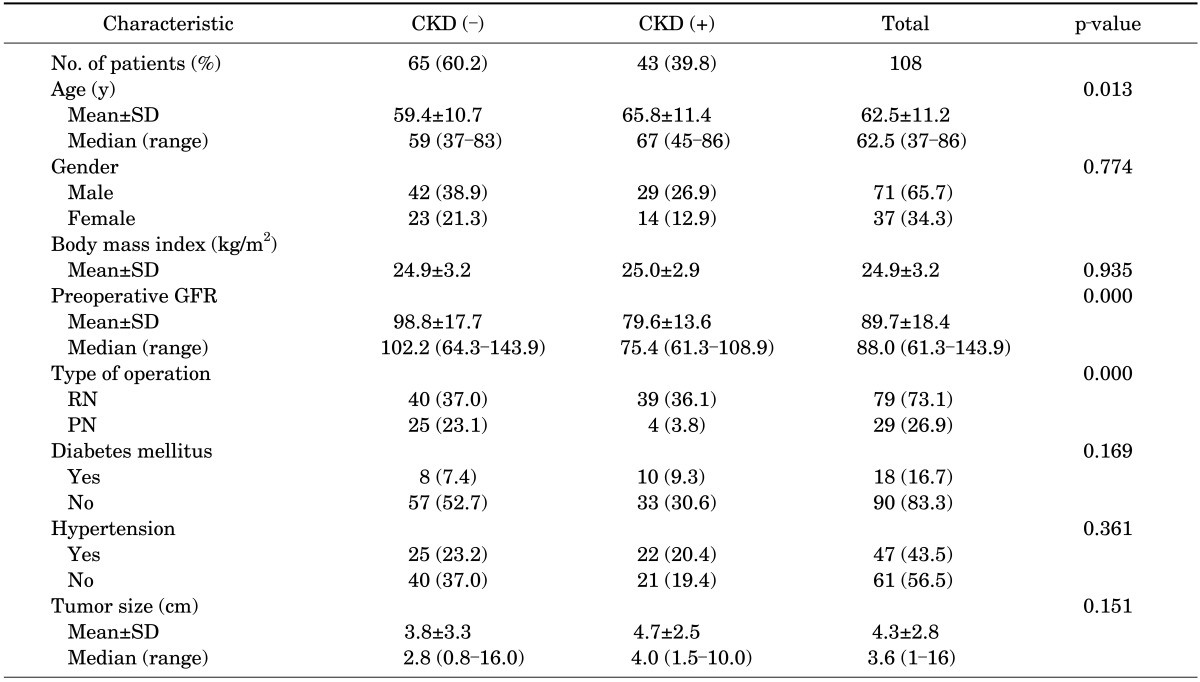
Values are presented as number (%) unless otherwise indicated.
CKD, chronic kidney disease; SD, standard deviation; GFR, glomerular filtration rate; RN, radical nephrectomy; PN, partial nephrectomy.
TABLE 2.
Multivariate multiple logistic regression analysis for predicting chronic kidney disease
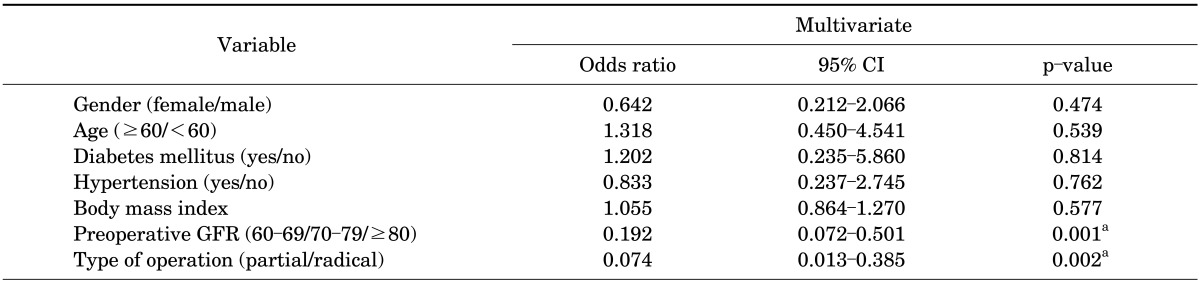
CI, confidence interval; GFR, glomerular filtration rate.
a:Statistically significant with p<0.05.
DISCUSSION
The development of CKD after curative renal surgery has been known to be associated with an increased risk of cardiovascular events, hospitalization, and death [4]. In contrast with patients participating in donor nephrectomy procedures, patients with renal tumors are generally older and have significant comorbidities, such as obesity, diabetes mellitus, and hypertension [13,14]. Because these factors can influence renal function outcomes postoperatively, the surgical procedure for renal tumors should be chosen by taking these factors into account. Currently, with consideration of oncological and functional outcomes, PN is viewed as a standard surgery for small renal tumors sized 4 cm or less. This means that PN contributes to longer patient survival compared with RN. Recent reports have shown that PN for renal tumors sized 4 to 7 cm (T1b) has oncological outcomes equivalent to those of RN [15,16].
Regarding the development of postoperative CKD, our results also showed that nephron-sparing surgery was superior to RN. The incidence of CKD in our study was 36.1% in the RN group and 3.8% in the PN group. This is higher than the rates reported in other series of 9% to 22.4% in the RN group and 0% to 11.6% in the PN group. This difference may be associated with postoperative renal insufficiency defined by serum creatinine values>2 mg/dL in previous studies. However, findings similar to ours were reported in several studies that used the MDRD formula. Barlow et al. [17] reported new-onset renal insufficiency at ≥3 months after surgery in 95 of 133 patients (71.4%) who underwent RN and in 13 of 76 patients (17.1%) who underwent PN with a preoperative GFR of >60 mL/min/1.73 m2. In Huang et al. [18]'s study, the incidence of CKD in patients who underwent RN or PN was 69.7% and 17.4%, respectively, and in Lucas et al. [19]'s study it was 60.1% for RN and 29.3% for PN at the 3-year follow-up, respectively.
In patients with normal renal function who undergo nephrectomy, the use of serum creatinine remains a somewhat imprecise means of determining renal function [20]. There are reports that approximately 25% of patients with a normal serum creatinine level have at least moderate CKD, which means a GFR less than 60 mL/min per 1.73 m2 [18]. The eGFR has been shown to reflect renal function more accurately than does serum creatinine, and the abbreviated MDRD formula has been used in several other reports [18,19]. Recent evidence suggests that this formula accurately predicts eGFR in CKD and underestimates GFR by approximately 9 mL/min per 1.73 m2 in kidney donors [20]. Particularly because of the difficulty of performing renal scans in all patients, the MDRD formula is the best available method for estimating renal function in patients undergoing nephrectomy.
In our study, we used the four-variable MDRD formula, which has been shown to be more precise than the Cockcroft-Gault equation [17], and 20.0% of our patients had a GFR of <60 mL/min per 1.73 m2 before surgery. This result is compatible with previous reports in which 10.6% to 29.9% of patients with renal tumors had CKD with GFR less than 60 mL/min per 1.73 m2 [8,17-19,21].
There are reports that age, diabetes mellitus, hypertension, tumor size, and preoperative renal function could be predictive factors for the development of postoperative renal insufficiency. But, whereas Jeon et al. [8] reported that older age and diabetes are prognostic factors for the development of CKD, Lane et al. [9] reported that male gender and older age are prognostic factors. Our results showed that diabetes and male gender were not prognostic factors; in our study, only preoperative eGFR by use of the four-variable MDRD formula was associated with the development of CKD in the multivariate regression analysis.
Our study had some limitations. The patients were analyzed retrospectively and were from a single institution and the sample size was not large. However, to investigate predictive factors associated with the development of CKD in patients with renal cell carcinoma, we performed eGFR measurements by use of the four-variable MDRD formula within 3 years of surgery in the vast majority of our patients. In the future, to reduce the selection bias on the observed functional outcomes between patients treated with PN and those treated with RN, a prospective study may be needed with a larger sample size and a longer follow-up.
CONCLUSIONS
The results of our study show that decreased preoperative renal function and RN were significant independent predictors of CKD in patients undergoing curative surgery for renal cell carcinoma. In patients who have a relatively decreased preoperative eGFR, especially when estimated by use of the MDRD formula, nephron-sparing surgery should be considered for the treatment of small renal tumors.
Footnotes
The authors have nothing to disclose.
References
- 1.Patard JJ, Dorey FJ, Cindolo L, Ficarra V, De La Taille A, Tostain J, et al. Symptoms as well as tumor size provide prognostic information on patients with localized renal tumors. J Urol. 2004;172(6 Pt 1):2167–2171. doi: 10.1097/01.ju.0000141137.61330.4d. [DOI] [PubMed] [Google Scholar]
- 2.Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol. 2000;163:730–736. [PubMed] [Google Scholar]
- 3.Lau WK, Blute ML, Weaver AL, Torres VE, Zincke H. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc. 2000;75:1236–1242. doi: 10.4065/75.12.1236. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors: is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55–61. doi: 10.1016/j.juro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKiernan J, Simmons R, Katz J, Russo P. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology. 2002;59:816–820. doi: 10.1016/s0090-4295(02)01501-7. [DOI] [PubMed] [Google Scholar]
- 7.Thompson RH, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED, Cheville JC, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179:468–471. doi: 10.1016/j.juro.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 8.Jeon HG, Jeong IG, Lee JW, Lee SE, Lee E. Prognostic factors for chronic kidney disease after curative surgery in patients with small renal tumors. Urology. 2009;74:1064–1068. doi: 10.1016/j.urology.2009.05.090. [DOI] [PubMed] [Google Scholar]
- 9.Lane BR, Babineau DC, Poggio ED, Weight CJ, Larson BT, Gill IS, et al. Factors predicting renal functional outcome after partial nephrectomy. J Urol. 2008;180:2363–2368. doi: 10.1016/j.juro.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 11.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 13.Sorbellini M, Kattan MW, Snyder ME, Hakimi AA, Sarasohn DM, Russo P. Prognostic nomogram for renal insufficiency after radical or partial nephrectomy. J Urol. 2006;176:472–476. doi: 10.1016/j.juro.2006.03.090. [DOI] [PubMed] [Google Scholar]
- 14.Goldfarb DA, Matin SF, Braun WE, Schreiber MJ, Mastroianni B, Papajcik D, et al. Renal outcome 25 years after donor nephrectomy. J Urol. 2001;166:2043–2047. [PubMed] [Google Scholar]
- 15.Weight CJ, Larson BT, Gao T, Campbell SC, Lane BR, Kaouk JH, et al. Elective partial nephrectomy in patients with clinical T1b renal tumors is associated with improved overall survival. Urology. 2010;76:631–637. doi: 10.1016/j.urology.2009.11.087. [DOI] [PubMed] [Google Scholar]
- 16.Pahernik S, Roos F, Rohrig B, Wiesner C, Thuroff JW. Elective nephron sparing surgery for renal cell carcinoma larger than 4 cm. J Urol. 2008;179:71–74. doi: 10.1016/j.juro.2007.08.165. [DOI] [PubMed] [Google Scholar]
- 17.Barlow LJ, Korets R, Laudano M, Benson M, McKiernan J. Predicting renal functional outcomes after surgery for renal cortical tumours: a multifactorial analysis. BJU Int. 2010;106:489–492. doi: 10.1111/j.1464-410X.2009.09147.x. [DOI] [PubMed] [Google Scholar]
- 18.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucas SM, Stern JM, Adibi M, Zeltser IS, Cadeddu JA, Raj GV. Renal function outcomes in patients treated for renal masses smaller than 4 cm by ablative and extirpative techniques. J Urol. 2008;179:75–79. doi: 10.1016/j.juro.2007.08.156. [DOI] [PubMed] [Google Scholar]
- 20.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol. 2005;16:459–466. doi: 10.1681/ASN.2004060447. [DOI] [PubMed] [Google Scholar]
- 21.Zini L, Patard JJ, Capitanio U, Crepel M, de La Taille A, Tostain J, et al. Cancer-specific and non-cancer-related mortality rates in European patients with T1a and T1b renal cell carcinoma. BJU Int. 2009;103:894–898. doi: 10.1111/j.1464-410X.2008.08252.x. [DOI] [PubMed] [Google Scholar]


