Abstract
Background and Objectives
The purpose of this study is to identify the prevalence of progressive dilation in patients with acute myocardial infarction (AMI) combined with heart failure (HF) and determine the prognostic significance and associated factors with a geometric change of an infarcted heart.
Subjects and Methods
A total of 1310 AMI patients with HF (63.9±12.5 years, 70% male) between November 2005 and April 2011 underwent echocardiography at admission and one year later. Left ventricular (LV) remodeling is defined as 20% progression, and left atria (LA) remodeling is 10% compared with the initial volume index.
Results
The prevalence of both LA and LV remodeling was 13.9%; LV only was 9.3%, LA only 22.8% and non-remodeling was 55.1%, respectively. In the non-remodeling group, Killip class II was more frequent (83.9%, p<0.001) whereas in other remodeling groups, Killip class III was more frequent. Initial wall motion score index, ejection fraction, maximal cardiac enzyme, high sensitive C-reactive protein, B type natriuretic peptide, and triglyceride serum levels were significantly associated with heart remodeling. All causes of death occurred in 168 cases (12.8%) during the follow-up period. Mortality was the highest in the LV and LA remodeling group (20.9%) and the lowest in the non-remodeling group (11.4%). During the period of follow-up, the cumulative survival rate was significantly lower in the groups of LA and LV remodeling than in others (log rank p=0.006).
Conclusion
Total mortality was significantly increased in patients AMI with geometrically progressive LA and LV dilatation.
Keywords: Myocardial infarction, Ventricular remodeling, Heart failure, Prognosis
Introduction
The remodeling process after acute myocardial infarction (AMI) is clinically characterized by progressive cavity dilatation. Left ventricular (LV) remodeling is a globally heterogeneous process, involving both infarcted and non-infarcted myocardium, which results from infarction expansion in the acute phase, whereas late cavity dilation is the result of the eccentric hypertrophy process. LV remodeling after AMI is stimulated by the interaction of a number of factors, playing a key role in the pathology of post-infarction ventricular dysfunction.1-3) When reacting to aggression, the genetic, structural, and biochemical changes arising from that process will result in the deterioration of the functional ability of the heart in the long run. It was reported that patients who develop LV dilatation following AMI have significantly reduced survival rates.4),5)
Left atrial (LA) volume is considered to be a presentation of the diastolic burden, and increased LA volume usually reflects elevated ventricular filling pressure. During ventricular diastole, the left atrium is directly exposed to LV pressure through the open mitral valve. As an adaptation to the decreased ventricular compliance following myocardial infarction (MI), LA pressure rises, increasing LA wall tension and stretching the atrial myocardium. Therefore, LA remodeling tends to be a result of adaptation to allow for the preservation of LV function and reflect increased LA pressure.6),7) A previous report showed that LA volume is a independent predictor of mortality and smaller LA volumes are associated with a good prognosis after MI.8) However, the effect of both LV and LA remodeling in the post-MI period was not clear. The purpose of the present study was, therefore, to assess the prevalence of LV and LA remodeling in patients with AMI combined with heart failure (HF) during the first one year after MI, and to define the long-term clinical prognostic significance and associated factors with a geometric change of an infarcted heart.
Subjects and Methods
Study population and grouping
A total of 1310 AMI patients with HF (63.9±12.5 years, 70% male) enrolled retrospectively from Chonnam University Hospital between November 2005 and April 2011 underwent echocardiography at admission and one year later. We excluded Killip class IV, due to inappropriate image quality on admission period.
The subjects were divided into four groups according to the remodeling of LV and/or LA during one year. LV remodeling is defined as 20% progression and LA remodeling 10%, compared with initial volume index. Group I was with progressive LA and LV dilatation (n=182, 64.2±12.3 years, 128 males), group II with only LV remodeling without LA (n=120, 65.7±11.9 years, 78 males), group III with LA remodeling without LV (n=286, 63.3±12.3 years, 195 males), and group IV without LA and LV dilatation (n=722, 62.9±12.5 years, 519 males). Hospital records collected on admission were registered. Information on events and mortality were obtained from hospital records and phone calls. This study protocol was approved by the Institutional Review Board or ethics committee at Chonnam National University Hospital (No=2010-05-092).
Definition of hypertension, diabetes, dyslipidemia, myocardial infarction, and heart failure
Subjects were considered as having hypertension if their blood pressure was ≥140/≥90 mm Hg as Joint National Committee VII9) or if they were undergoing treatment for hypertension. The American Diabetes Association criteria10) were used to define diabetes (DM). We considered a subject as having DM when the fasting plasma glucose levels were ≥126 mg/dL in 2 consecutive assessments or if they were on treatment for DM. Dyslipidemia was diagnosed according to the 2004 update of the National Cholesterol Education Program guidelines.11) According to these guidelines, high levels of low density lipoprotein-cholesterol ≥160 mg/dL, low high density lipoprotein-cholesterol ≤40 mg/dL, and high triglycerides ≥150 mg/dL were included.12)
The presence of ST-segment elevation MI was determined by >30 minutes of continuous chest pain, a new ST-segment elevation ≥2 mm on at least two contiguous electrocardiographic leads, creatine kinase (CK)-MB or troponin (Tn) >3 times normal.13) Infarct-related arteries were identified using a combination of electrocardiographic findings, LV wall motion abnormalities on two-dimensional echocardiography, and coronary angiography.
Acute HF is defined as the rapid onset of symptoms and signs secondary to abnormal cardiac function. The symptoms include shortness of breath, decreased exercise ability, orthopnea, profound fatigue, and dizziness. The frequent signs were edema (no site specificity), ankle edema, palpitation, an irregular pulse, and abdominal edema. The Killip classification is based on clinical signs and chest X-ray findings, and has been validated in HF after AMI. Killip stage I is no HF. There were no clinical signs of cardiac decompensation. Killip classification stage II is defined as HF. Diagnostic criteria include rales, S3 gallop and pulmonary venous hypertension, pulmonary congestion with wet rales in the lower half of the lung field. Stage III is severe HF with pulmonary edema and rales throughout the lung field fields. Stage IV is cardiogenic shock. Signs include hypotension and evidence of peripheral vasoconstriction, such as oliguria, cyanosis and diaphoresis.14) According to this classification, we chose patients with MI conditioned with Killip stage II and III. Although Killip IV is advanced HF, we excluded patients with Killip IV due to insufficient imaging quality. A family history of premature coronary artery disease (CAD) is defined as CAD before the age of 55 in a first-degree male relative, or before the age of 60 in a first-degree female relative. Hospital records of patients were reviewed to obtain information on clinical demographics.
Laboratory tests
A routine laboratory study was performed upon admission. Cardiac enzyme CK-MB and Tn-I were followed up serially, and we choose the maximal values for data analysis. Blood samples to assess the serum lipid profile and glucose were obtained the morning following admission. High sensitive C-reactive protein (hs-CRP) was measured by immunoturbidimetric CRP-Latex (II) high sensitive assay using an Olympus 5431 auto analyzer (Olympus America Inc., Melville, NY, USA). Serum N-terminal-pro B-type-natriuretic peptide (NT-pro BNP) was measured using an eletrochemiluminescence sandwich immunoassay method with an Elecsys 2010 analyzer (Roche Diagnostics, Mannheim, Germany). The analytic range of the NT-pro BNP assay extends from 5 to 35000 pg/mL.
Measurement of left ventricular and left atrial dilatation
Two-dimensional, M-mode echocardiography and Doppler ultrasound examination were performed with an echocardiography (VIVID7, GE Healthcare, Milwaukee, WI, USA). Image-Point at the time of initial admission on day 1 or 2 and at one year after MI was assessed. LV volume and ejection fraction (EF) were measured using Simpson's formula.15) The LV and LA volume indices were obtained by dividing the volume of the body surface area. The mean values of three measurements of the technically best cardiac cycles were taken from each examination performed by two independent inter-observers. Intra-observer and inter-observer variabilities of Simpson's method were 4±5% and 5±4% (absolute difference divided by the mean value of measurement). In each patient, the wall motion score index was derived. The LV was divided according to a 17-segment model.16) For each segment, wall motion was scored from 1 (normal) to 4 (dyskinetic). The LA maximum volume was also measured by the biplane area-length method indexed to body surface area. An increase in LV end-diastolic volume index >20% between the initial and one-year follow-up was considered as an LV remodeling pattern, whereas17) an increase in LA volume index >10% between the initial and one-year follow-up was defined as an LA remodeling pattern.18)
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) for Windows, version 15.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses. For each parameter, mean, median, and standard deviation were calculated. The statistical significance between means for different groups was calculated by analysis of variance. Statistical significance between frequencies was calculated using chi square tests. A p of less than 0.05 was required to reject the null hypothesis. The event-free survival rates were calculates using the Kaplan-Meier analysis, and the event rates were compared using the log-rank test. We used the multivariate regression analysis method to seek the independent predictor. The variables that were significant in the univariate analysis were entered into multivariate methods.
Results
Baseline clinical characteristics
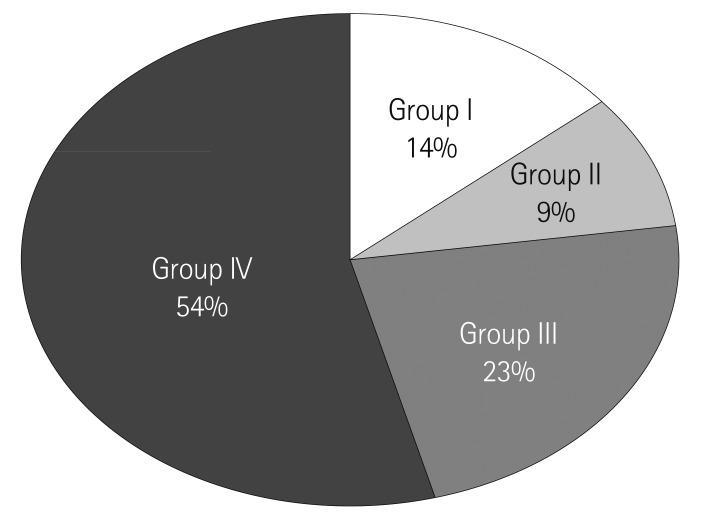
The prevalence of remodeling was presented in Fig. 1. Group I with progressive LA and LV dilatation was 13.9% (n=182, 64.2±12.3 years, 128 males), group II with LV remodeling without LA dilatation was 9.3% (n=120, 65.7±11.9 years, 78 males), group III with LA remodeling without LV dilatation was 22.8% (n=286, 63.3±12.3 years, 195 males), and group IV without LA and LV dilatation was 55.1% (n=722, 62.9±12.5 years, 519 males), respectively.
Fig. 1.
The prevalence of left ventricle (LV) and left atrium (LA) remodeling. Group I: LV and LA remodeling, Group II: LV remodeling, Group III: LA remodeling, Group IV: no remodeling.
The baseline characteristics are as summarized in Table 1. The age and gender were not significant differences among groups. Weight and body mass index were higher in the remodeling groups than the non-remodeling group. The prevalence of hypertension was higher in remodeling groups than group IV (p<0.05). The proportion of Killip class III was more frequent in groups I, II, and III, whereas Killip class II was frequent in group IV (p<0.05). There was no difference in the groups with respect to the initial vital sign or percentage of ST-segment elevation MI or prior MI history.
Table 1.
Baseline clinical characteristics
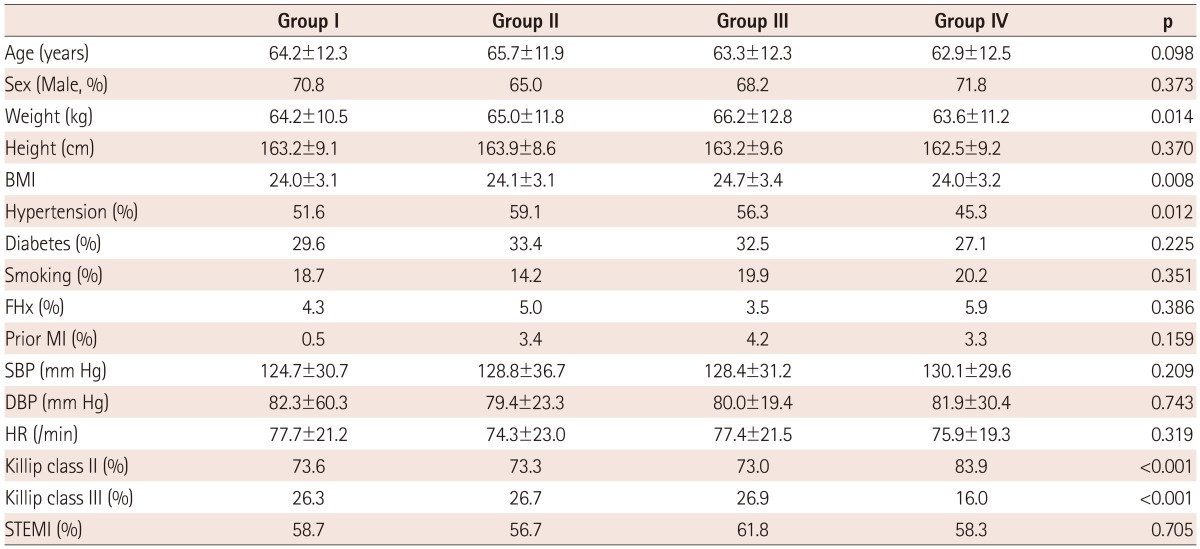
Group I: LV and LA remodeling, Group II: LV remodeling, Group III: LA remodeling, Group IV: no remodeling, BMI: body mass index, FHx: family history, MI: myocardial infarction, SBP: systolic blood pressure, DBP: diastolic blood pressure, HR: heart rate, STEMI: ST-segment elevation myocardial infarction, LA: left atrium, LV: left ventricle
Comparison of biochemical parameters, echocardiographic parameters, angiographic parameters, and medication according to left ventricular and left atrial remodeling
The levels of triglyceride, maximal CK-MB, Tn-I, and hs-CRP were more significantly increased in groups I and II than in groups III and IV (p<0.05) (Table 2). NT-pro BNP was highly increased in groups I, II, and III, whereas it presented a mild increase in group IV. LV end-diastolic and systolic dimension and volume were increased in groups I and II over other groups at admission. LA dimension and volume were increased in groups I and III over other groups. EF was the lowest in group I and wall motion score was the highest in group I. There were no significant differences among groups in diastolic parameters, such as mitral inflow pattern or e/e' velocity. In coronary angiography, the types of infarct-related artery, multi vessel involvement, and reperfusion time were not different among groups. However, no-reflow phenomenon after percutaneous coronary intervention (PCI) was frequently observed in group I (Table 3).
Table 2.
Comparison of laboratory findings
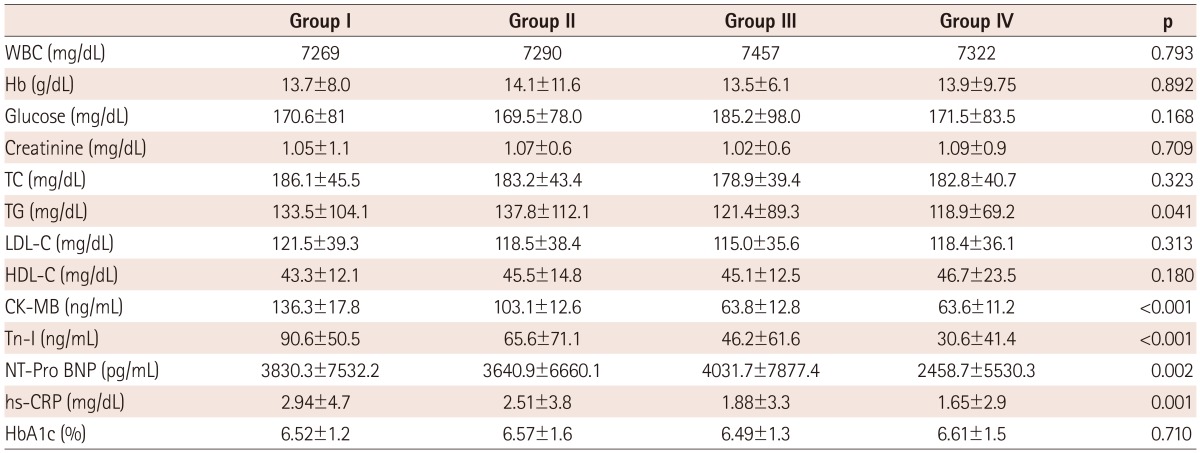
Group I: LV and LA remodeling, Group II: LV remodeling, Group III: LA remodeling, Group IV: no remodeling, WBC: white blood cell, Hb: hemoglobin, TC: total cholesterol, TG: triglyceride, LDL-C: low density lipoprotein-cholesterol, HDL-C: high density lipoprotein-cholesterol, CK-MB: creatinine kinase MB, Tn-I: troponin I, NT-pro BNP: N-terminal pro B natriuretic peptide, hs-CRP: high sensitivity C-reactive protein, HbA1c: glycosylated hemoglobin, LA: left atrium, LV: left ventricle
Table 3.
Comparison of echocardiographic and coronary angiographic findings
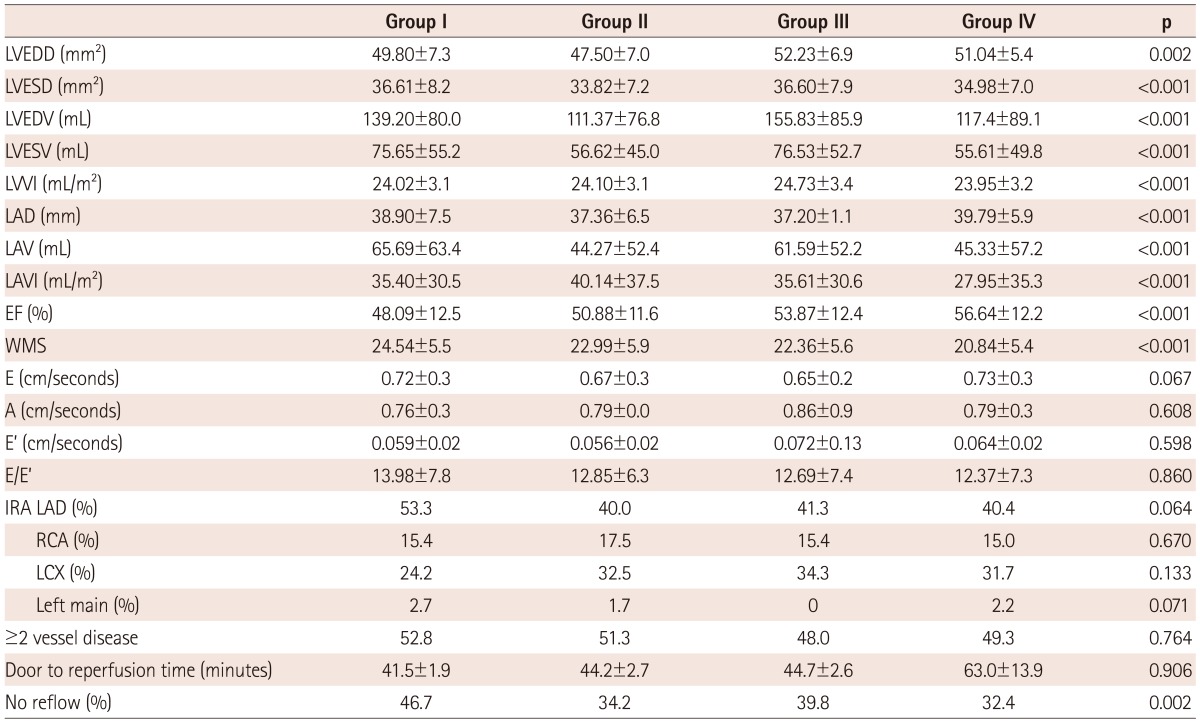
Group I: LV and LA remodeling, Group II: LV remodeling, Group III: LA remodeling, Group IV: no remodeling, LVEDD: left ventricle end diastolic dimension, LVESD: left ventricle end systolic dimension, LVEDV: left ventricle end diastolic volume, LVESV: left ventricle end systolic volume, LVVI: left ventricular volume index, LAD: left atrial dimension, LAV: left atrial volume, LAVI: left atrial volume index, EF: ejection fraction, WMS: wall motion score, E: mitral inflow velocity, A: atrial contraction velocity, E': systolic velocity of mitral septal annulus, IRA: infarct related artery, LAD: left anterior descending artery, RCA: right coronary artery, LCX: left circumflex artery, min: minute, LA: left atrium, LV: left ventricle
Most of the patients enrolled in this study used aspirin and clopidogrel. There were no significant differences among groups for using beta-blockers, angiotensin converting enzyme inhibiter (ACEI), or aldosterone receptor blocker (ARB), known to affect remodeling. Although the species of statin were various, statin usage rates were similar among groups. However, diuretics were used less in group IV than in other remodeling groups, quite significantly (Table 4).
Table 4.
Comparison of medication for treatment
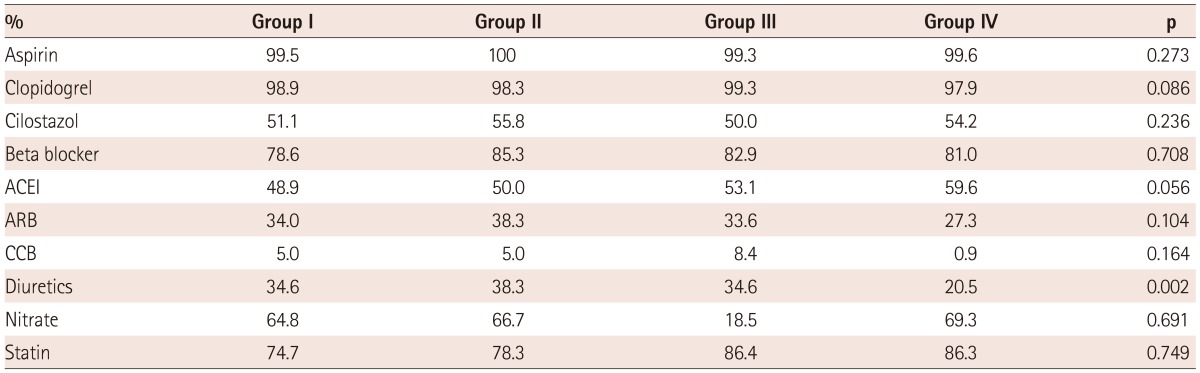
Group I: LV and LA remodeling, Group II: LV remodeling, Group III: LA remodeling, Group IV: No remodeling, ACEI: angiotensin converting enzyme inhibiter, ARB: aldosterone receptor blocker, CCB: calcium channel blocker, LA: left atrium, LV: left ventricle
Mortality and long-term prognosis
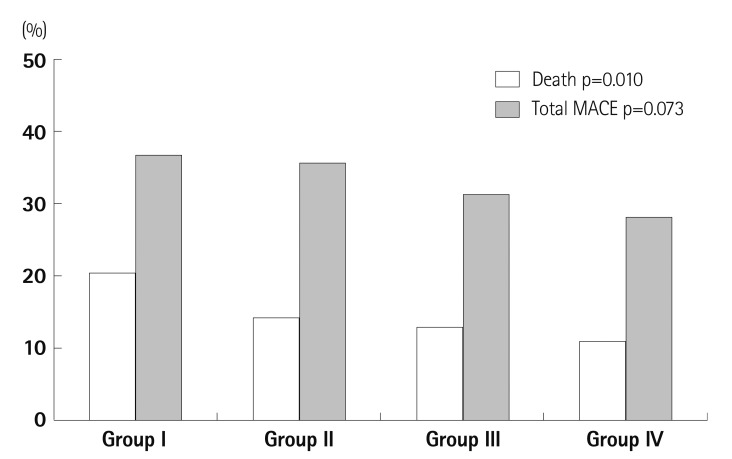
All causes of death occurred in 168 cases (12.8%) in follow-up periods (mean 1240±680, maximum 2210 days). Mortality was highest in the LA and LV remodeling group (20.9%) and lowest in the non-remodeling group (11.4%). Although the development of adverse cardiac events included in cerebrovascular events, re-MI, and re-admission rates for HF tends to frequent in remodeled groups, there was no statistical significance among the groups during follow-up (mean 945±746 days) (Fig. 2).
Fig. 2.
Mortality and major adverse cardiac events in four groups. Group I: LV and LA remodeling, Group II: LV remodeling, Group III: LA remodeling, Group IV: no remodeling. LV: left ventricle, LA: left atrium, MACE: major adverse cardiac event.
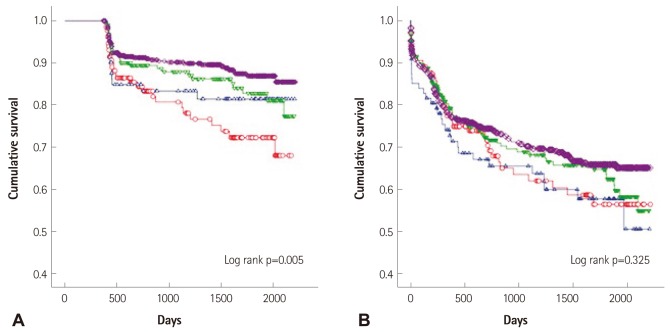
During the period of follow-up, the cumulative survival rate was significantly lower in the group of LV and LA remodeling than in others, but cardiac events were not significant (Fig. 3).
Fig. 3.
Comparison of all-cause mortality and cardiac events. A: survival curve according to left ventricle (LV) and left atrium (LA) remodeling. B: major adverse cardiac events according to LV and LA remodeling. Group I: LV and LA remodeling; open red circle; ○, Group II: LV remodeling; open blue triangle; ▵, Group III: LA remodeling; open reverse green triangle; ▿, Group IV: no remodeling; open purple diamond; ⋄.
Independent predictors of mortality
There were several factors affecting death during follow-up. We found the independent predictors of mortality from multivariate regression using positive factors from univariate analysis. Age, elevated NT-pro BNP, no reflow after PCI, and the presence of LV & LA remodeling were (odd ratio; 3.688, confidence interval; 1.309-10.387, p=0.014) significant independent predictors of long-term mortality (Table 5).
Table 5.
Predictors of mortality by multivariate analysis
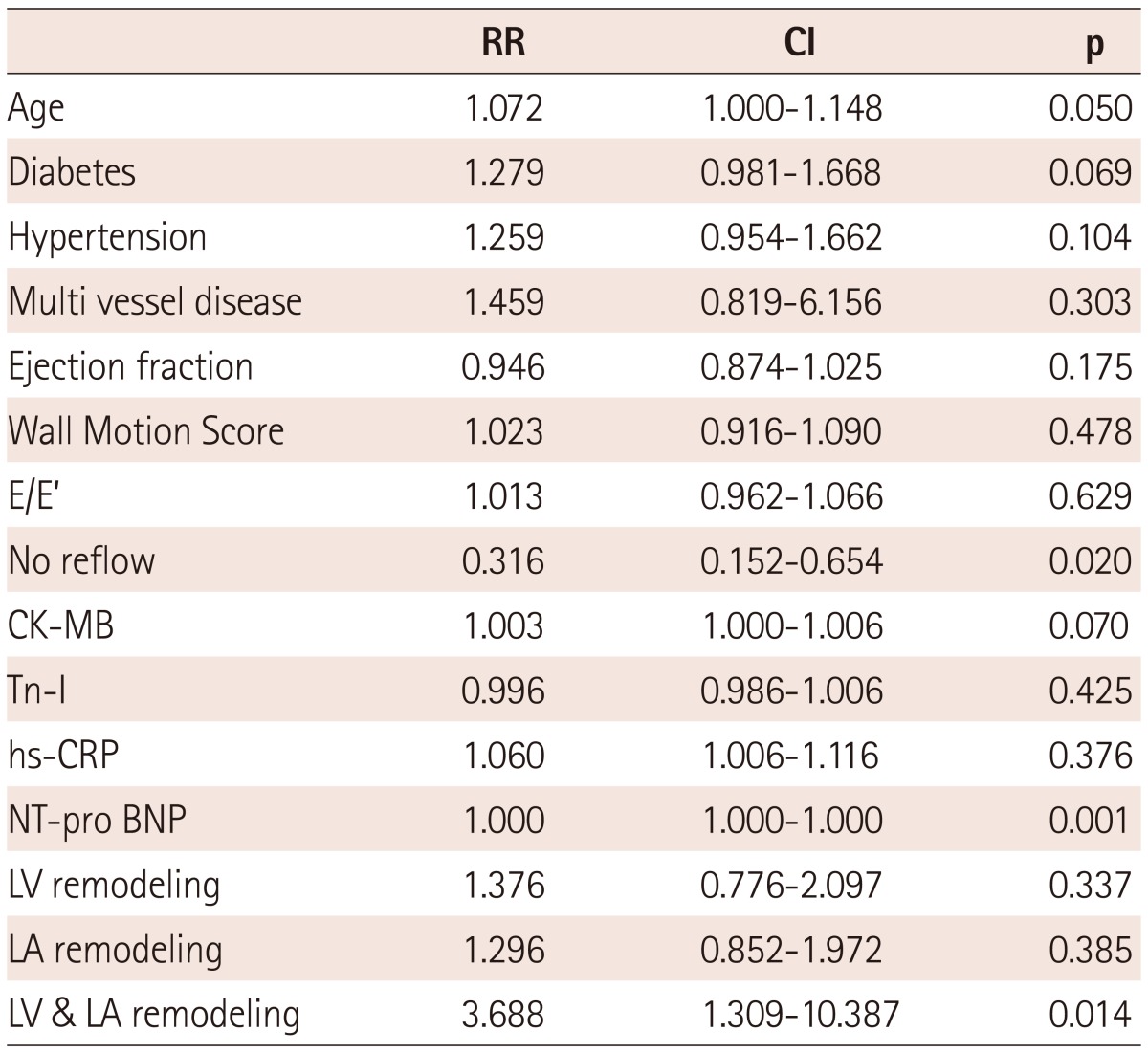
RR: relation risk, CI: confidence interval, CK-MB: creatinine kinase MB, Tn-I: troponine I, hs-CRP: high sensitivity C-reactive protein, NT-pro BNP: N type pro brain type natriuretic peptide, LV: left ventricle, LA: left atrium
Discussion
The major finding of this study is that the progressive dilation of both LA and LV resulted in a poor prognosis, especially mortality in MI patients with HF. Conversely, the preservation of LA and LV size after MI was the best prognosis among the remodeling groups. Initial and follow-up noninvasive measurements of LA and LV size provides long-term prognostic information in patients with AMI. Therefore, it may be important to identify patients at risk of LV and LA remodeling to prevent remodeling after AMI.
The remodeling pattern after MI is not precisely known. It might possibly be a time dependent progression, but not a linear sequence. In our MI population with HF, cardiac remodeling was frequently observed in patients with hypertension, elevated cardiac enzyme, and elevated serum inflammatory markers. These factors are already reported to increase LV filling pressure and, therefore, affect LV and LA function. Traditionally, ventricular remodeling was regarded to continue for several months until the distending forces were counterbalanced by collagen scarring.19),20) The underlying cellular mechanism includes progressive myocyte lengthening without a proportional increase in the myocyte cross-sectional area.21),22) Failure to normalize increased wall stresses results in progressive dilatation, recruitment of border zone myocardium into the scar, and deterioration in contractile function. This balance is determined by infarct size, location, and trans-murality, the extent of myocardial stunning, the patency of the infarct-related artery, and local tropic factors.23) The biomarkers of myocardial damage, such as cardiac Tn-I and T, CK, and CK-MB appear to be useful in predicting late ventricular dilation. In our study, cardiac enzyme and serum inflammatory markers were significantly elevated in remodeled groups. Markedly increased cardiac enzyme suggests a large infarction. Previous data showed dyslipidemia, a low EF, and high wall motion score affecting independently progressive LV dilation after 6 month in AMI patients.24) One report showed dyssynchrony affects the remodeling process.25) We obtained a somewhat similar result from our 6-month remodeling data. There were initially decreased EF and elevated wall motion scores in this post MI 12-month remodeled group, whereas lipid level was not significantly different, except in the elevated triglyceride in the remodeled group. This may be affected by the lipid lowering drug during the follow-up period. Studies have showed that anti-inflammation treatment ameliorated LV remodeling and improved cardiac performance. Statins could affect the expression of inflammatory cytokines.26-28) Therefore, treatment of dyslipidemia with statins may be somewhat helpful in reducing progressive LV dilatation. In this study, we used the statin of more than 70% in each of the groups. Although the frequencies of statin treatment tended to be lower in the remodeling groups (groups I, II, and III) than in group VI, this difference was not statistically significant. Results may differ according to the species of statin. Because the usage rates of medication such as beta blockers, ACEI, or ARB were similar among groups, there were other factors affecting remodeling in these patients. However, in the significant difference of diuretics, this may be correlated with clinical symptoms according to groups.
After an AMI, ventricular structural and functional alterations can lead to ventricular relaxation abnormalities resulting in a worsening of diastolic dysfunction and LA remodeling.29) In the case of LV dysfunction, adequate cardiac output is maintained by the rearranging of storage, conduction, and pumping functions of the LA. LA remodeling represents the impaired diastolic function beyond systolic dysfunction after MI. LA size is an important predictor of prognosis both in the general population and in patients with heart disease, including LV dysfunction, mitral regurgitation, or atrial fibrillation.30) However, progressive LA morphology was not significantly affected from the initial diastolic Doppler parameter, such as mitral inflow or e/e'. It might be a result of a sensitive Doppler parameter affected by acute loading condition, whereas LA volume was a relatively stable parameter predicting chronic geometric change and presenting an unfavorable prognosis. LA remodeling was not a minor problem added to LV remodeling. A combination of LV and LA dilation had a serious mortality outcome in this study.
A limitation of our study is that we carried out data analysis retrospectively. Therefore, we could not conduct intervention or modulation of risk factors. Yet, it is not known how long LA and LV remodeling continues in patients with MI. Studies with longer follow-up periods are required to determine this period. Also to be considered is the fact that this process is not homogenous and the difference of susceptible indicators for processing LV or LA remodeling in AMI patients exists. Several correlated factors might have critical effects on the remodeling process, which includes infarct-related artery patency, species of drugs, and combined morbidity during clinical follow-up periods. This is necessary for comparing the associated factors according to remodeling patterns. Patients with diagnosed remodeling or under high risk of developing remodeling must undergo intervention in an aggressive fashion in order to prevent, attenuate, or even revert the process. Another consideration is the definition of HF in these patients. We used the definition of Killip classification, as even though Killip class is based on clinical signs and chest X-ray findings, it has been validated in HF after AMI, as previously mentioned.
Conclusively, a serial echocardiographic examination may be beneficial for identifying high-risk post-MI patients and AMI patients with LA, and LV remodeling should be carefully monitored clinically. This may prove helpful in preventing or reversing the remodeling.
Acknowledgments
This study was supported by a new investigator grant from The Korean Society of Cardiology (2012).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Gaudron P, Eilles CH, Kugler I, Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction. Potential mechanisms and early predictors. Circulation. 1993;87:755–763. doi: 10.1161/01.cir.87.3.755. [DOI] [PubMed] [Google Scholar]
- 2.Giannuzzi P, Temporelli PL, Bosimini E, et al. Heterogenity of left ventricular remodeling after myocardial infarction: results of the Gruppo Italiano per lo Studio della Supravvivenza nell Infarcto Miocardico-3 Echo Substudy. Am Heart J. 2001;141:131–138. doi: 10.1067/mhj.2001.111260. [DOI] [PubMed] [Google Scholar]
- 3.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 4.Gaudron P, Kugler I, Hu K, Bauer W, Eilles C, Ertl G. Time course of cardiac structural, functional and electrical changes in asymptomatic patients after myocardial infarction: their inter-relation and prognostic impact. J Am Coll Cardiol. 2001;38:33–40. doi: 10.1016/s0735-1097(01)01319-5. [DOI] [PubMed] [Google Scholar]
- 5.Bolognese L, Neskovic AN, Parodi G, et al. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation. 2002;106:2351–2357. doi: 10.1161/01.cir.0000036014.90197.fa. [DOI] [PubMed] [Google Scholar]
- 6.Appleton CP, Galloway JM, Gonzalez MS, Gaballa M, Basnight MA. Estimation of left-ventricular filling pressure using two-dimensional and Doppler echocardiography in adult patients with cardiac disease. Additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction. J Am Coll Cardiol. 1993;22:1972–1982. doi: 10.1016/0735-1097(93)90787-2. [DOI] [PubMed] [Google Scholar]
- 7.Basnight MA, Gonzalez MS, Kershenovich SC, Appleton CP. Pulmonary venous flow velocity: relation to hemodynamics, mitral flow velocity and left atrial volume, and ejection fraction. J Am Soc Echocardiogr. 1991;4:547–558. doi: 10.1016/s0894-7317(14)80213-7. [DOI] [PubMed] [Google Scholar]
- 8.Moller JE, Hillis GS, Oh JK, et al. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107:2207–2212. doi: 10.1161/01.CIR.0000066318.21784.43. [DOI] [PubMed] [Google Scholar]
- 9.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2573. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 10.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;27:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 12.Hatzitolios AI, Athyros VG, Karagiannis A, et al. Implementation of strategy for the management of overt dyslipidemia: the IMPROVE-dyslipidemia study. Int J Cardiol. 2009;134:322–329. doi: 10.1016/j.ijcard.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction) Circulation. 2004;110:588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 14.Killip T, 3rd, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20:457–464. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 15.Simonson JS, Schiller NB. Descent of the base of the left ventricle: an echocardiographic index of left ventricular. J Am Soc Echocardiogr. 1989;2:25–35. doi: 10.1016/s0894-7317(89)80026-4. [DOI] [PubMed] [Google Scholar]
- 16.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 17.Brzezińska B, Loboz-Grudzień K, Sokalski L. Patterns of post-MI left ventricular volume changes - clinical implications. Kardiol Pol. 2007;65:1190–1198. [PubMed] [Google Scholar]
- 18.Kuppahally SS, Akoum N, Badger TJ, et al. Echocardiographic left atrial reverse remodeling after catheter ablation of atrial fibrillation is predicted by preablation delayed enhancement of left atrium by magnetic resonance imaging. Am Heart J. 2010;160:877–884. doi: 10.1016/j.ahj.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiyyagura SR, Pinney S. Left ventricular remodeling after myocardial infarction: past, present, and future. Mt Sinai J Med. 2006;73:840–851. [PubMed] [Google Scholar]
- 20.Zimmer HG, Gerdes AM, Lortet S, Mall G. Changes in heart function and cardiac cell size in rats with chronic myocardial infarction. J Mol Cell Cardiol. 1990;22:1231–1243. doi: 10.1016/0022-2828(90)90060-f. [DOI] [PubMed] [Google Scholar]
- 21.Rouleau JL, de Champlain J, Klein M, et al. Activation of neurohumoral systems in postinfarction left ventricular dysfunction. J Am Coll Cardiol. 1993;22:390–398. doi: 10.1016/0735-1097(93)90042-y. [DOI] [PubMed] [Google Scholar]
- 22.Gerdes AM, Kellerman SE, Moore JA, et al. Structural remodeling of cardiac myocytes in patients with ischemic cardiomyopathy. Circulation. 1992;86:426–430. doi: 10.1161/01.cir.86.2.426. [DOI] [PubMed] [Google Scholar]
- 23.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 24.Yoon HJ, Jeong MH, Bae JH, et al. Dyslipidemia, low left ventricular ejection fraction and high wall motion score index are predictors of progressive left ventricular dilatation after acute myocardial infarction. Korean Circ J. 2011;41:124–129. doi: 10.4070/kcj.2011.41.3.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko JS, Jeong MH, Lee MG, et al. Left ventricular dyssynchrony after acute myocardial infarction is a powerful indicator of left ventricular remodeling. Korean Circ J. 2009;39:236–242. doi: 10.4070/kcj.2009.39.6.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yun KH, Shin IS, Shin SN, et al. Effect of previous statin therapy in patients with acute coronary syndrome and percutaneous coronary intervention. Korean Circ J. 2011;41:458–463. doi: 10.4070/kcj.2011.41.8.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eefting FD, Cramer MJ, Stella PRS, Rensing BJ, Doevendans PA. Rational of the REPARATOR study: A randomised trial with serial cardiac MRI follow-up testing the ability of atorvastatin to reduce reperfusion damage after primary PCI for acute MI. Neth Heart J. 2006;14:95–99. [PMC free article] [PubMed] [Google Scholar]
- 28.Hong YJ, Jeong MH, Hyun DW, et al. Prognostic significance of simvastatin therapy in patients with ischemic heart failure who underwent percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol. 2005;95:619–622. doi: 10.1016/j.amjcard.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 29.Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 30.Giannuzzi P, Temporelli PL, Bosimini E, et al. Independent and incremental prognostic value of Doppler-derived mitral deceleration time of early filling in both symptomatic and asymptomatic patients with left ventricular dysfunction. J Am Coll Cardiol. 1996;28:383–390. doi: 10.1016/0735-1097(96)00163-5. [DOI] [PubMed] [Google Scholar]