Abstract
Background and Objectives
The aim of this study was to examine the histolopathogical effects among the biolimus, zotarolimus, and everolimus eluting stent (EES) in the porcine coronary restenosis model.
Subjects and Methods
Pigs were randomized into three groups in which the coronary arteries (15 pigs, 10 coronaries in each group) had either a biolimus A9 eluting stent (BES, n=10), zotarolimus eluting stent (ZES, n=10) or an EES (n=10). Histopathologic analysis was performed at 28 days after stenting.
Results
There were no significant differences in the injury score among the three groups. There was a significant difference in the internal elastic lamina, lumen area, neointima area, percent area stenosis, and the fibrin and inflammation score among the three groups (4.3±0.53 mm2, 2.5±0.93 mm2, 1.8±1.03 mm2, 40.7±20.80%, 1.7±0.41, 1.4±0.72 in the BES group vs. 5.1±0.55 mm2, 2.3±1.14 mm2, 2.8±1.00 mm2, 55.4±21.23%, 2.0±0.39, 1.6±0.76 in the ZES group vs. 4.4±0.53 mm2, 1.7±1.22 mm2, 2.8±1.23 mm2, 64.0±26.00%, 1.8±0.76, 2.1±0.90 in the EES group, respectively). BES is more effective in inhibiting neointimal hyperplasia compared to ZES and EES (p<0.0001). According to the fibrin and inflammation score, BES and EES are more effective in decreasing the fibrin deposition compared to ZES (p<0.001). Moreover, BES and ZES are more effective in reducing the inflammatory reaction compared to EES (p<0.001).
Conclusion
The result demonstrates that BES shows better histopathological characteristics than ZES and EES at one month after stenting in the porcine coronary restenosis model.
Keywords: Drug-eluting stents, Percutaneous coronary intervention, Coronary restenosis, Inflammation
Introduction
Currently, percutaneous coronary intervention (PCI) with the use of antiproliferative agents coated stents, such as the -limus and -taxol families, is the treatment of choice for patients with acute myocardial infarction.1) The introduction of drug eluting stents (DES) has provided improved clinical outcomes compared to the use of bare metal stents (BMS), by reducing neointimal hyperplasia and target vessel revascularizations.2),3)
The clinical results of the sirolimus eluting stent (SES) as compared to BMS have provided clues for improved limus drug development.4-6) Biolimus A9, zotarolimus, and everolimus, a potent antiproliferative drug of the -limus families, has been shown to reduce clinical restenosis in clinical trials.7-9)
The objective of the present study was to compare the histological effects among the biolimus, zotarolimus, and everolimus eluting stents in the porcine coronary restenosis model.
Subjects and Methods
Animal preparation and stent implantation
The animal study was approved by the Ethics Committee of Chonnam National University Medical School and Chonnam National University Hospital (CNU IACUC-H-2010-18), and conformed to the Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Study animals were castrated male pigs weighing 20-25 kg. To prevent acute thrombosis after stenting, premedication with aspirin 100 mg and clopidogrel 75 mg per day was applied for 5 days before the procedure. On the day of the procedure, pigs were anesthetized with zolazepam and tiletamine (2.5 mg/kg, Zoletil50®, Virbac, Caros, France), xylazine (3 mg/kg, Rompun®, Bayer AG, Leverkusen, Germany) and azaperone (6 mg/kg, Stresnil®, Janssen-Cilag, Neuss, Germany). They received supplemental oxygen continuously through oxygen masks. Subcutaneous 2% lidocaine at the cut-down site was administered, the left carotid artery was surgically exposed, and a 7 Fr sheath was inserted.
Continuous hemodynamic and surface electrocardiographic monitoring was maintained throughout the procedure. Then, 5000 units of heparin was administered intravenously as a bolus prior to the procedure, the target coronary artery was engaged using standard 7 Fr guide catheters, and control angiograms of both coronary arteries were performed using a nonionic contrast agent in two orthogonal views.
The stent was deployed by inflating the balloon and the resulting stent-to-artery ratio was 1.3 : 1. Coronary angiograms were obtained immediately after stent implantation. Thereafter, all equipment was removed and the carotid artery was ligated.
Four weeks after stenting, the animals underwent follow-up angiography in the same orthogonal views as before death with 20 mL of a potassium chloride intracoronary injection.
The hearts were removed, and the coronary arteries were pressure-perfusion fixed at 110 mm Hg in 10% neutral buffered formalin overnight. Arteries were step-sectioned, processed routinely for light microscopy, and stained for histological analysis.
Study groups
The pigs were randomly divided into 3 groups (Table 1): group 1 {biolimus A9-eluting stents (BES), BioMatrix®, Biosensors Interventional Technologies Pte Ltd., Singapore, 3.0×18 mm, n=10}, group 2 {zotarolimus-eluting stents (ZES), Endeavor Resolute®, Medtronic CardioVascular, Minneapolis, MN, USA, 3.0×18 mm, n=10}, and group 3 {everolimus-eluting stents (EES), Promus®, Boston Scientific, Natick, MA, USA, 3.0×18 mm, n=10}.
Table 1.
Coronary artery morphometric measurements in stented porcine coronary arteries
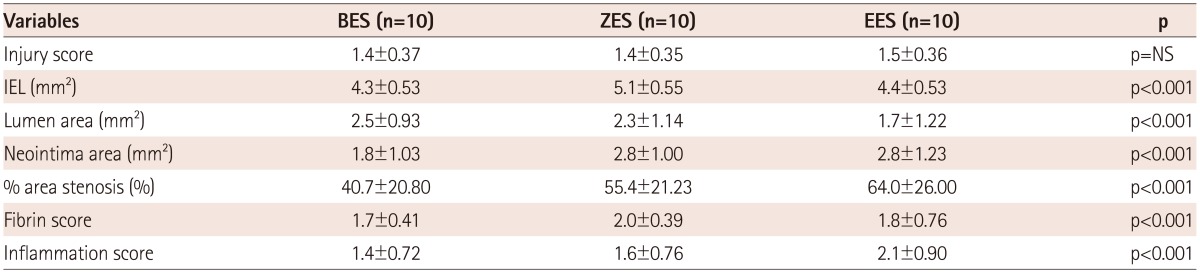
BES: biolimus A9-eluting stents, ZES: zotarolimus-eluting stents, EES: everolimus-eluting stent, IEL: internal elastic lamina, NS: not significant
A total of 15 pigs were used in this study (15 pigs, 30 coronary arteries, 10 coronary arteries in each group). A BES, a ZES, and an EES were implanted in the left anterior descending artery and left circumflex artery in a randomized manner in each pig.
Histopathology and immunohistochemistry analysis
Histopathologic evaluations of each artery were performed by an experienced cardiovascular pathologist. The specimens were embedded, and sections of 50 to 100 µm in thickness were obtained at about 1 mm distances apart, and stained with Hematoxylin-Eosin (Fig. 1) and Carstairs' (Fig. 2) for histological analysis. Measurements of the histopathologic sections were performed using a calibrated microscope, digital video imaging system, and microcomputer program (Visus 2000 Visual Image Analysis System, IMT Tech, CA, USA). Borders were manually traced for the lumen area, the area circumscribed by the internal elastic lamina (IEL), and the innermost border of the external elastic lamina (external elastic lamina area). Morphometric analysis of the neointimal area for a given vessel was calculated as the measured IEL area minus the lumen area. The measurements were made on five cross-sections from the proximal and distal ends, and the three midpoints of each stented segment. Histopathologic stenosis was calculated as 100×{1-(lesion lumen area/lesion IEL area)}.10) Immunohistochemistry (IHC) was conducted through standard procedures, as previously described.11) Anti-smooth muscle actin monoclonal antibody (Sigma Aldrich, St. Louis, MO, USA) was used. IHC specimens were analyzed by fluorescence microscopy and digital photography. All histology and IHC results were interpreted by two independent pathologists in a blind manner.
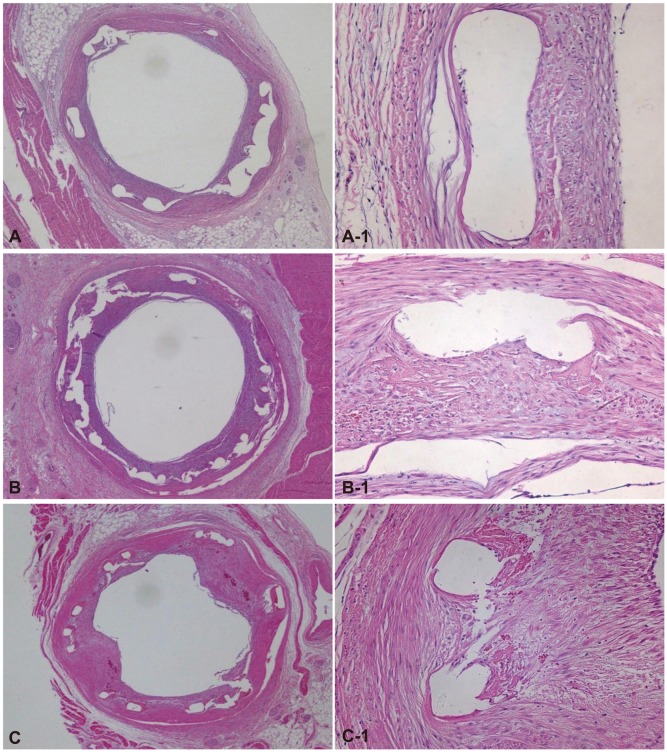
Fig. 1.
Representative images of H&E staining after 4 weeks of stenting. Specimen BES implanted (A: ×20, A-1: ×200), ZES implanted (B: ×20, B-1: ×200), and EES implanted (C: ×20, C-1: ×200). Inflammatory reaction was more severe in the EES stented artery compared to BES and ZES. BES: biolimus A9-eluting stents, ZES: zotarolimus-eluting stents, EES: everolimus-eluting stents.
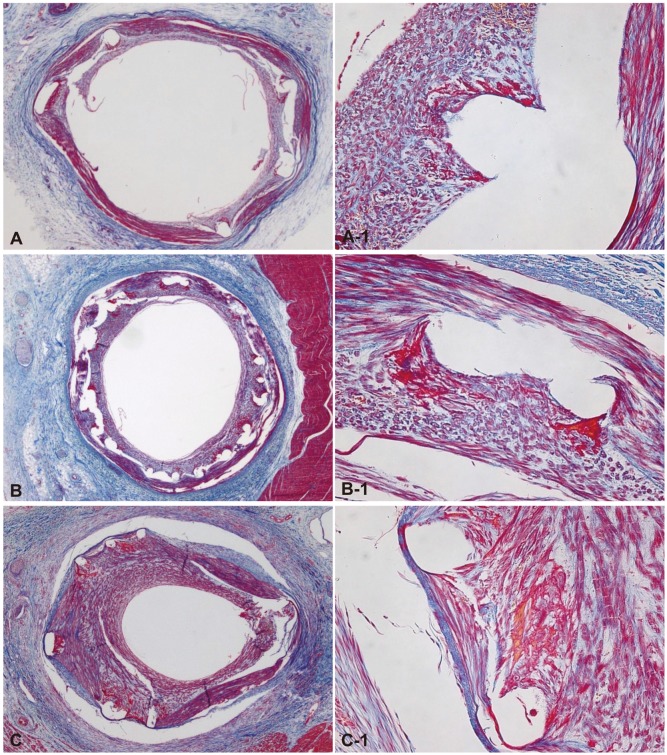
Fig. 2.
The Carstair fibrin stain of the low-power fields (magnitude, ×20, ×200) of fibrin infiltration in BES implanted (A and A-1), ZES implanted (B and B-1), and EES implanted (C and C-1). Fibrin deposition surrounding the stent struts was higher in ZES than in BES and EES cases. BES: biolimus A9-eluting stents, ZES: zotarolimus-eluting stents, EES: everolimus-eluting stents.
Evaluation of the arterial injury
The arterial injury at each strut site was determined by the anatomic structures penetrated by each strut. A numeric value was assigned, as previously described by Schwartz et al.:10) 0=no injury; 1=break in the internal elastic membrane; 2=perforation of the media; 3=perforation of the external elastic membrane to the adventitia. The average injury score for each segment was calculated by dividing the sum of the injury scores by the total number of struts at the examined section.
Evaluation of inflammation scores, neointimal reaction, and fibrin score
With regard to the inflammation score for each individual strut, the grading was as follows: 0=no inflammatory cells surrounding the strut; 1=light, noncircumferential lymphohistiocytic infiltrate surrounding the strut; 2=localized, moderate to dense cellular aggregate surrounding the strut noncircumferentially; 3=circumferential dense lymphohistiocytic cell infiltration of the strut. The inflammation score for each cross section was calculated by dividing the sum of the individual inflammation scores by the total number of struts at the examined section.12) Ordinal data for fibrin were collected on each stent section using a scale of 0 to 3, as previously reported.13)
Statistical analysis
Statistical analysis was performed with the aid of commercially available software {Statistical Package for the Social Sciences (SPSS) Version 15, SPSS Inc., Chicago, IL, USA}. The data were presented as mean value±SD. An unpaired Student's t-test was used for the comparison of each of the stent groups. Analysis of variance was used to make comparisons of the three stents groups. A value of p<0.05 was considered statistically significant.
Results
Analysis after stenting
Two stents were placed for two coronary arteries per swine. A total of thirty stents, including ten BES, ten ZES, and ten EES, were placed in the proximal left anterior descending and proximal circumflex artery for fifteen swine. The mortality rate in this study was zero. There was no significant difference in the stent-to-artery ratio among the three stent groups.
Histopathological and immunohistochemistry analysis
To exam the characteristics of smooth muscle cells (SMC) in neointima tissue, the stented coronary artery was stained by anti-SMC antibody. Vascular SMC were major components of neointima formation after stenting in all groups (Fig. 3).
Fig. 3.
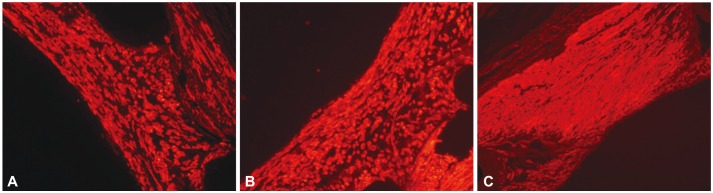
Representative images of immunohistochemistry using anti-smooth muscle actin monoclonal antibody in the neointima tissue. Immunofluorescence staining showing an expression of α-smooth muscle actin (bright red positive cells, ×200, A: BES, B: ZES, C: EES). BES: biolimus A9-eluting stents, ZES: zotarolimus-eluting stents, EES: everolimus-eluting stents.
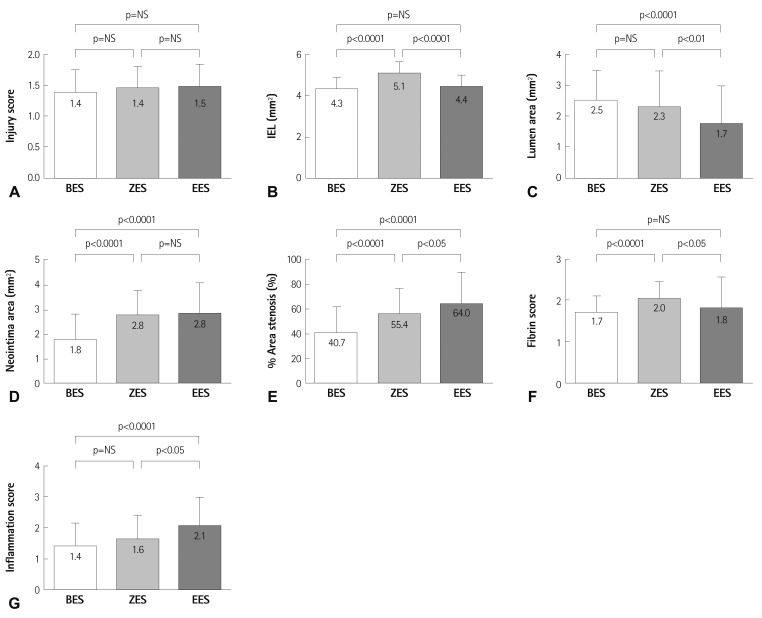
There was no statistically significant difference in the injury score among the three groups (1.4±0.37 in the BES group vs. 1.4±0.35 in the ZES group vs. 1.5±0.36 in the EES group, p=not significant). There was a statistically significant difference in the IEL among the three groups (4.3±0.53 mm2 in the BES group vs. 5.1±0.55 mm2 in the ZES group vs. 4.4±0.53 mm2 in the EES group, p<0.0001). There was a statistically significant difference in the lumen area among the three groups (2.5±0.93 mm2 in the BES group vs. 2.3±1.14 mm2 in the ZES group vs. 1.7±1.22 mm2 in the EES group, p<0.001). There was a statistically significant difference in the neointima area among the three groups (1.8±1.03 mm2 in the BES group vs. 2.8±1.00 mm2 in the ZES group vs. 2.8±1.23 mm2 in the EES group, p<0.0001). There was a statistically significant difference in percentage area stenosis among the three groups (40.7±20.80% in the BES group vs. 55.4±21.23% in the ZES group vs. 64.0±26.00% in the EES group, p<0.0001).
There was a statistically significant difference in the fibrin score among the three groups (1.7±0.41 in the BES group vs. 2.0±0.39 in the ZES group vs. 1.8±0.76 in the EES, p<0.001). There was a statistically significant difference in the inflammation score among the three groups (1.4±0.72 in the BES group vs. 1.6±0.76 in the ZES group vs. 2.1±0.90 in the EES group, p<0.001) (Table 1, Fig. 4).
Fig. 4.
Injury score (A), internal elastic lamina (B), lumen area (C), neointima area (D), % area stenosis (E) fibrin score (F) and inflammation score (G), in the BES, ZES, and EES groups. BES: biolimus A9-eluting stents, ZES: zotarolimus-eluting stents, EES: everolimus-eluting stents.
Discussion
This study was conducted in order to compare the histopathological differentiation of the BES, ZES, and EES in the porcine coronary restenosis model. Our study demonstrated that BES was more effective in reducing neointima proliferation compared to ZES and EES. In the fibrin score, which indicates delayed arterial healing, ZES was inferior to BES and EES. BES and ZES were more effective in inhibiting the inflammatory reaction compared to EES, according to the inflammation score. The results demonstrated that BES displayed improved histopathological characteristics in the three limus families.
Biolimus A9-eluting stents, EES, and ZES were compared to BMS and/or other generation DES in several clinical trials. Two types of second generation stents, ZES and EES, have shown promising results in clinical trials and registries compared with BMS, SES, and paclitaxel eluting stent (PES).8),14),15)
Major adverse cardiac events, death, and myocardial infarction were lower for ZES vs. SES and PES in patients with diabetes mellitus.16) In a comparison study between ZES and EES using optical coherence tomography, neointima proliferation was greater in the ZES group than in the EES group at both 3 and 12 months.17) Other clinical trials comparing ZES and EES, however, have found that both stents demonstrated comparable levels of safety and efficacy.18),19)
One year clinical results after 3rd generation BES implantation were as safe and efficacious as those after 2nd generation EES implantation.20) Both stents displayed an excellent low rate of target lesion revascularization and an extremely low rate of stent thrombosis.21) In a 4-year long-term follow-up, BES has shown improved safety and efficacy compared with SES.22)
In our previous study using BES, BES appeared to be reliable in terms of inflammation at overlapping segments, as well as at nonoverlapping segments.23) In clinical research, BES displayed a lower rate of the composite of major adverse cardiac events in patients with ST-elevation myocardial infarction undergoing primary PCI compared with BMS.24)
The major difference between 2nd and 3rd generation stents is the biodegradable polymer used. The polymers are potentially linked with neointima hyperplasia, inflammation, and late stent thrombosis.25),26) The polymer-free BES demonstrated the equivalent early and superior late inhibition of neointima hyperplasia compared with SES in a porcine model.27)
The biolimus-eluting stent with biodegradable polymer (BES) was developed as a third generation DES elutes biolimus A9 from a bioabsorbable polylactic acid (PLA) polymer.28),29) BES releases biolimus A9 into the artery wall while the PLA polymer is absorbed by the contacted coronary vessel tissues. Therefore, this study suggests that the biodegradable polymer of BES achieved superior histopathologic results compared to the permanent polymer of ZES and EES in the porcine coronary restenosis model.
Study limitations
Our study had some limitations. First, we used normal porcine coronary arteries without atherosclerotic lesions, unlike in human clinical situations with pre-existing atherosclerosis. Second, we examined the inflammatory reaction based on H&E stain. IHC techniques were the standard for such studies.30) Third, we did not perform long-term follow-up experiments, such as over 6 months using minipigs.
In conclusion, this study shows that BES is more effective in inhibiting neointima hyperplasia compared to ZES and EES. According to fibrin and inflammation score, BES and EES are more effective in decreasing fibrin deposition compared to ZES. Moreover, BES and ZES are more effective in reducing the inflammatory reaction compared to EES. The result demonstrates that BES shows superior histopathological characteristics in BES, ZES, and EES at one month after stenting in the porcine coronary restenosis model.
Acknowledgments
This study was supported by a grant of the Korea Healthcare Technology R&D Project (A084869), Ministry of Health and Welfare, Republic of Korea, Cardiovascular Research Center, Chonnam National University Hospital and Regeneromics Research Center, Chonnam National University.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Kushner FG, Hand M, Smith SC, Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Clinical outcomes with drug-eluting and bare metal stents in patients with ST-segment elevation myocardial infarction: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2013;62:496–504. doi: 10.1016/j.jacc.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Min GS, Lee JH, Park JH, et al. Long-term safety and efficacy of sirolimus-and Paclitaxel-eluting stents in patients with acute myocardial infarction: four-year observational study. Korean Circ J. 2012;42:266–273. doi: 10.4070/kcj.2012.42.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangalore S, Kumar S, Fusaro M, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012;125:2873–2891. doi: 10.1161/CIRCULATIONAHA.112.097014. [DOI] [PubMed] [Google Scholar]
- 5.Jiménez-Quevedo P, Hernando L, Gómez-Hospital JA, et al. Sirolimuseluting stent versus bare metal stent in diabetic patients: the final fiveyear follow-up of the DIABETES trial. EuroIntervention. 2013;9:328–335. doi: 10.4244/EIJV9I3A54. [DOI] [PubMed] [Google Scholar]
- 6.De Luca G, Dirksen MT, Spaulding C, et al. Meta-analysis comparing efficacy and safety of first generation drug-eluting stents to bare-metal stents in patients with diabetes mellitus undergoing primary percutaneous coronary intervention. Am J Cardiol. 2013;111:1295–1304. doi: 10.1016/j.amjcard.2013.01.281. [DOI] [PubMed] [Google Scholar]
- 7.de Souza CF, El Mouallem AM, Brito Junior FS, et al. Safety and efficacy of biolimus-eluting stent with biodegradable polymer: insights from EINSTEIN (Evaluation of Next-generation drug-eluting STEnt IN patients with coronary artery disease) Registry. Einstein (Sao Paulo) 2013;11:350–356. doi: 10.1590/S1679-45082013000300015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kandzari DE, Leon MB, Meredith I, Fajadet J, Wijns W, Mauri L. Final 5-year outcomes from the endeavor zotarolimus-eluting stent clinical trial program: comparison of safety and efficacy with first-generation drug-eluting and bare-metal stents. JACC Cardiovasc Interv. 2013;6:504–512. doi: 10.1016/j.jcin.2012.12.125. [DOI] [PubMed] [Google Scholar]
- 9.Sabate M, Cequier A, Iñiguez A, et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012;380:1482–1490. doi: 10.1016/S0140-6736(12)61223-9. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992;19:267–274. doi: 10.1016/0735-1097(92)90476-4. [DOI] [PubMed] [Google Scholar]
- 11.Jonas M, Edelman ER, Groothuis A, Baker AB, Seifert P, Rogers C. Vascular neointimal formation and signaling pathway activation in response to stent injury in insulin-resistant and diabetic animals. Circ Res. 2005;97:725–733. doi: 10.1161/01.RES.0000183730.52908.C6. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz RS, Edelman E, Virmani R, et al. Drug-eluting stents in preclinical studies: updated consensus recommendations for preclinical evaluation. Circ Cardiovasc Interv. 2008;1:143–153. doi: 10.1161/CIRCINTERVENTIONS.108.789974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolodgie FD, John M, Khurana C, et al. Sustained reduction of in-stent neointimal growth with the use of a novel systemic nanoparticle paclitaxel. Circulation. 2002;106:1195–1198. doi: 10.1161/01.cir.0000032141.31476.15. [DOI] [PubMed] [Google Scholar]
- 14.Onuma Y, Miquel-Hebert K, Serruys PW SPIRIT II Investigators. Five-year long-term clinical follow-up of the XIENCE V everolimus-eluting coronary stent system in the treatment of patients with de novo coronary artery disease: the SPIRIT II trial. EuroIntervention. 2013;8:1047–1051. doi: 10.4244/EIJV8I9A161. [DOI] [PubMed] [Google Scholar]
- 15.Yamasaki M, Tsujino I, Lima-Filho MO, et al. Comparison of vascular response to the everolimus-eluting stent versus the paclitaxel-eluting stent: intravascular ultrasound results from the SPIRIT III trial. EuroIntervention. 2012;8:724–731. doi: 10.4244/EIJV8I6A112. [DOI] [PubMed] [Google Scholar]
- 16.Vardi M, Burke DA, Bangalore S, et al. Long term efficacy and safety of zotarolimus-eluting stent in patients with diabetes mellitus: Pooled 5-year results from the ENDEAVOR III and IV trials. Catheter Cardiovasc Interv. 2013 doi: 10.1002/ccd.25045. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Kim SJ, Lee H, Cho JM, et al. Comparison of zotarolimus-eluting stent and everolimus-eluting stent for vascular healing response: serial 3-month and 12-month optical coherence tomography study. Coron Artery Dis. 2013;24:431–439. doi: 10.1097/MCA.0b013e328362b2e7. [DOI] [PubMed] [Google Scholar]
- 18.Park KW, Lee JM, Kang SH, et al. Safety and efficacy of second-generation everolimus-eluting Xience V stents versus zotarolimus-eluting resolute stents in real-world practice: patient-related and stent-related outcomes from the multicenter prospective EXCELLENT and RESOLUTE-Korea registries. J Am Coll Cardiol. 2013;61:536–544. doi: 10.1016/j.jacc.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Hannan EL, Zhong Y, Wu C, et al. Everolimus-eluting stents and zotarolimus-eluting stents for percutaneous coronary interventions: two-year outcomes in New York State. Catheter Cardiovasc Interv. 2013;81:1097–1105. doi: 10.1002/ccd.24512. [DOI] [PubMed] [Google Scholar]
- 20.Smits PC, Hofma S, Togni M, et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet. 2013;381:651–660. doi: 10.1016/S0140-6736(12)61852-2. [DOI] [PubMed] [Google Scholar]
- 21.Natsuaki M, Kozuma K, Morimoto T, et al. Biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent: a randomized, controlled, noninferiority trial. J Am Coll Cardiol. 2013;62:181–190. doi: 10.1016/j.jacc.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 22.Stefanini GG, Byrne RA, Serruys PW, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. 2012;33:1214–1222. doi: 10.1093/eurheartj/ehs086. [DOI] [PubMed] [Google Scholar]
- 23.Park KH, Jeong MH, Kim JM, et al. The impact of triple anti-platelet therapy for endothelialization and inflammatory response at overlapping bioabsorbable polymer coated drug-eluting stents in a porcine coronary model. Int J Cardiol. 2013;168:1853–1858. doi: 10.1016/j.ijcard.2012.12.070. [DOI] [PubMed] [Google Scholar]
- 24.Räber L, Kelbæk H, Ostoijc M, et al. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012;308:777–787. doi: 10.1001/jama.2012.10065. [DOI] [PubMed] [Google Scholar]
- 25.Vorpahl M, Yazdani SK, Nakano M, et al. Pathobiology of stent thrombosis after drug-eluting stent implantation. Curr Pharm Des. 2010;16:4064–4071. doi: 10.2174/138161210794454879. [DOI] [PubMed] [Google Scholar]
- 26.Byrne RA, Joner M, Kastrati A. Polymer coatings and delayed arterial healing following drug-eluting stent implantation. Minerva Cardioangiol. 2009;57:567–584. [PubMed] [Google Scholar]
- 27.Tada N, Virmani R, Grant G, et al. Polymer-free biolimus a9-coated stent demonstrates more sustained intimal inhibition, improved healing, and reduced inflammation compared with a polymer-coated sirolimus-eluting cypher stent in a porcine model. Circ Cardiovasc Interv. 2010;3:174–183. doi: 10.1161/CIRCINTERVENTIONS.109.877522. [DOI] [PubMed] [Google Scholar]
- 28.Grube E, Buellesfeld L. BioMatrix Biolimus A9-eluting coronary stent: a next-generation drug-eluting stent for coronary artery disease. Expert Rev Med Devices. 2006;3:731–741. doi: 10.1586/17434440.3.6.731. [DOI] [PubMed] [Google Scholar]
- 29.Ostojic M, Sagic D, Jung R, et al. The pharmacokinetics of Biolimus A9 after elution from the Nobori stent in patients with coronary artery disease: the NOBORI PK study. Catheter Cardiovasc Interv. 2008;72:901–908. doi: 10.1002/ccd.21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik N, Gunn J, Holt CM, et al. Intravascular stents: a new technique for tissue processing for histology, immunohistochemistry, and transmission electron microscopy. Heart. 1998;80:509–516. doi: 10.1136/hrt.80.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]