Abstract
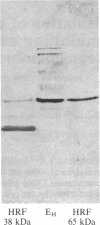
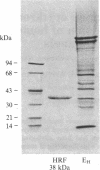
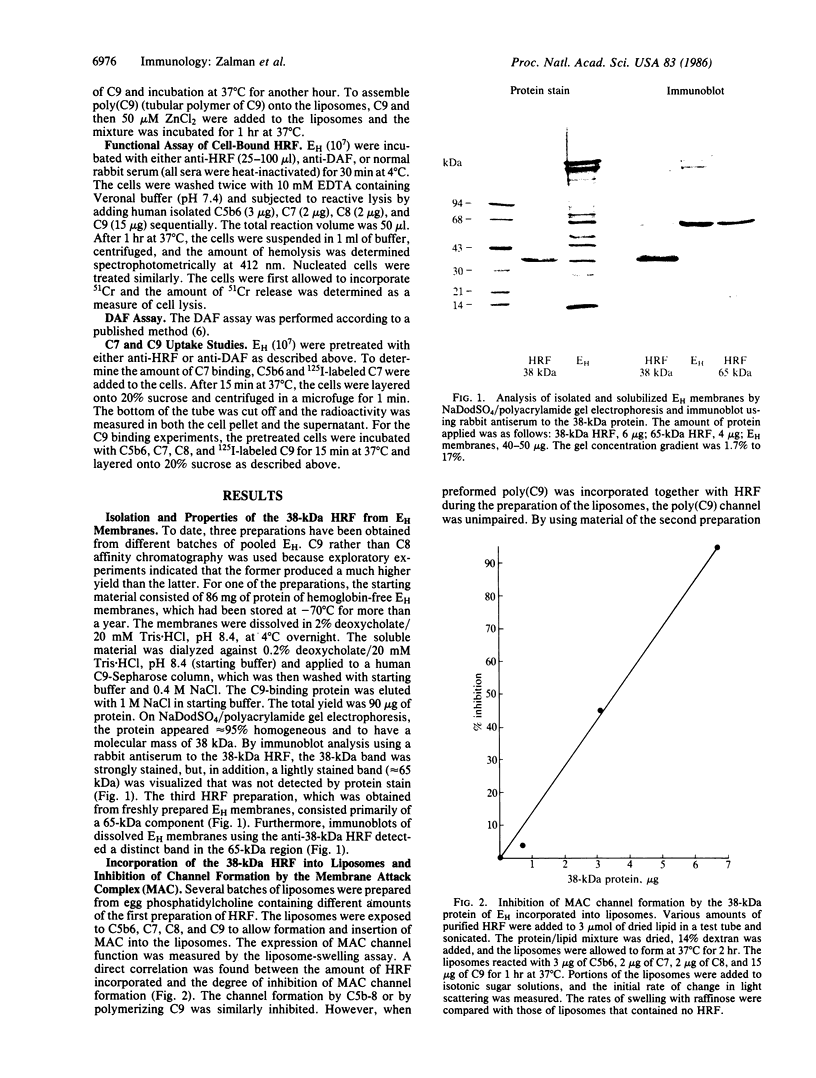
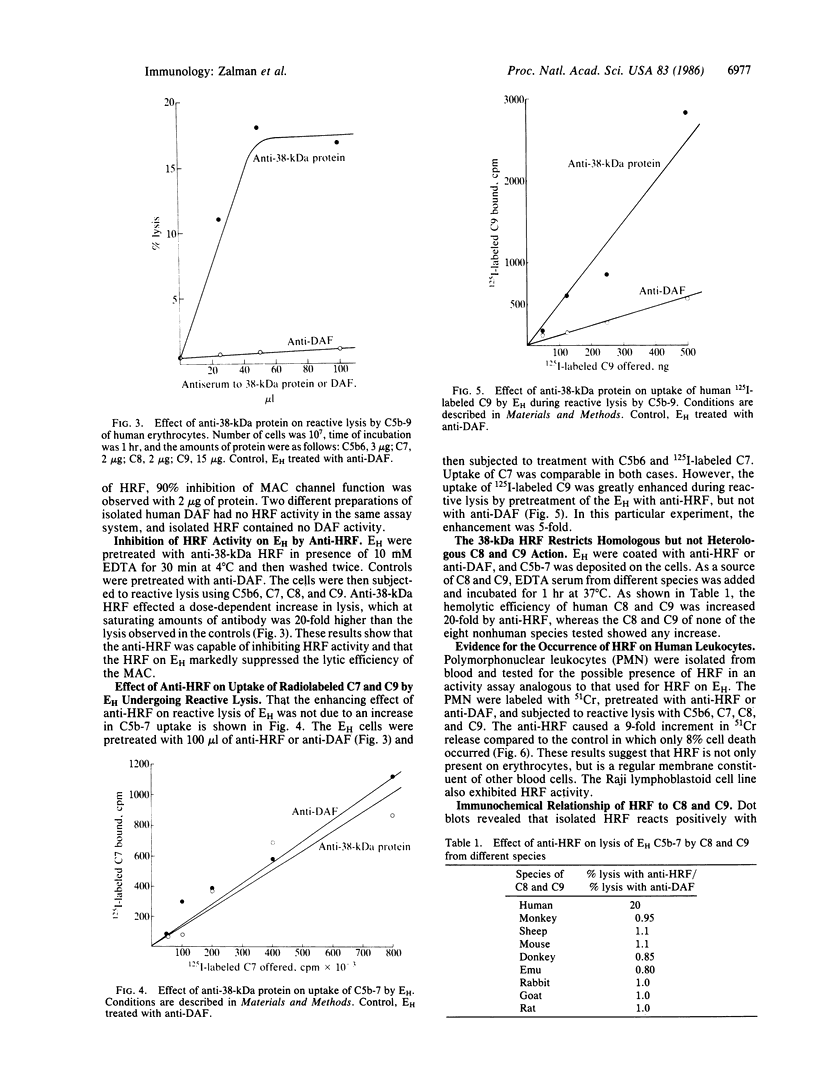
Erythrocytes are poorly lysed by homologous complement, whereas they are readily lysed by heterologous complement. This phenomenon had been attributed to an interference by the cell surface with the action of complement components C8 and C9. To isolate the responsible membrane constituent, detergent-solubilized human erythrocyte (EH) membranes were subjected to affinity chromatography by using human C9-Sepharose. The isolated protein had a mass of 38 kDa and, incorporated into liposomes, was highly effective in inhibiting complement-mediated channel expression, including the C5b-8, membrane attack complex, and tubular polymer of C9 channels. Antibody produced to the 38-kDa protein caused a 20-fold increase in reactive lysis of EH by isolated C5b6, C7, C8, and C9. The antibody did not enhance C5b-7 uptake, but it affected C9 binding to the target cell membrane. Antibody to human decay-accelerating factor, used as a control, had no effect on reactive lysis of EH. Anti-38-kDa protein did not enhance the action on EH of C8 and C9 from other species, indicating that the action of this regulatory protein is species specific. It was therefore termed homologous restriction factor (HRF). Blood cells other than erythrocytes, such as polymorphonuclear leukocytes, also exhibited cell-surface HRF activity. In immunoblots of freshly isolated EH membranes, anti-38-kDa HRF detected primarily a 65-kDa protein, suggesting that the 38-kDa protein constitutes an active fragment of membrane HRF. Because of the specific binding reaction observed between HRF and C8 or C9, HRF was tested with anti-human C8 and anti-human C9. A limited immunochemical relationship of HRF to C8 and C9 could be established and solid-phase anti-C9 proved an efficient tool for the isolation of HRF from solubilized EH membranes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biesecker G., Müller-Eberhard H. J. The ninth component of human complement: purification and physicochemical characterization. J Immunol. 1980 Mar;124(3):1291–1296. [PubMed] [Google Scholar]
- Brickner A., Sodetz J. M. Functional domains of the alpha subunit of the eighth component of human complement: identification and characterization of a distinct binding site for the gamma chain. Biochemistry. 1985 Aug 13;24(17):4603–4607. doi: 10.1021/bi00338a019. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. M. Inhibition of complement by a substance isolated from human erythrocytes. II. Studies on the site and mechanism of action. Immunochemistry. 1969 May;6(3):405–419. doi: 10.1016/0019-2791(69)90297-3. [DOI] [PubMed] [Google Scholar]
- Hu V. W., Nicholson-Weller A. Enhanced complement-mediated lysis of type III paroxysmal nocturnal hemoglobinuria erythrocytes involves increased C9 binding and polymerization. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5520–5524. doi: 10.1073/pnas.82.16.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänsch G. M., Hammer C. H., Vanguri P., Shin M. L. Homologous species restriction in lysis of erythrocytes by terminal complement proteins. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5118–5121. doi: 10.1073/pnas.78.8.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klob W. P., Müller-Eberhard H. J. The membrane attack mechanism of complement: the three polypeptide chain structure of the eigth component (C8). J Exp Med. 1976 May 1;143(5):1131–1139. doi: 10.1084/jem.143.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Specificity of diffusion channels produced by lambda phage receptor protein of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):167–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Silber R., Nussenzweig V. Amelioration of lytic abnormalities of paroxysmal nocturnal hemoglobinuria with decay-accelerating factor. Proc Natl Acad Sci U S A. 1985 May;82(9):2980–2984. doi: 10.1073/pnas.82.9.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Nicholson-Weller A., March J. P., Rosenfeld S. I., Austen K. F. Affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria are deficient in the complement regulatory protein, decay accelerating factor. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5066–5070. doi: 10.1073/pnas.80.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5430–5434. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. J., Wiedmer T., Sims P. J., Rosse W. F. Characterization of the complement sensitivity of paroxysmal nocturnal hemoglobinuria erythrocytes. J Clin Invest. 1985 Jun;75(6):2074–2084. doi: 10.1172/JCI111927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Esser A. F., Biesecker G., Müller-Eberhard H. J. Membrane attack complex of complement: a structural analysis of its assembly. J Exp Med. 1980 Feb 1;151(2):301–313. doi: 10.1084/jem.151.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R., Kolb W. P., Esser A. F., Müller-Eberhard H. J. Structural similarities between C6 and C7 of human complement. J Immunol. 1979 Sep;123(3):1071–1077. [PubMed] [Google Scholar]
- Rosenfeld S. I., Jenkins D. E., Jr, Leddy J. P. Enhanced reactive lysis of paroxysmal nocturnal hemoglobinuria erythrocytes by C5b-9 does not involve increased C7 binding or cell-bound C3b. J Immunol. 1985 Jan;134(1):506–511. [PubMed] [Google Scholar]
- Rosenfeld S. I., Packman C. H., Jenkins D. E., Jr, Countryman J. K., Leddy J. P. Complement lysis of human erythrocytes. III. Differing effectiveness of human and guinea pig C9 on normal and paroxysmal nocturnal hemoglobinuria cells. J Immunol. 1980 Nov;125(5):2063–2068. [PubMed] [Google Scholar]
- Schönermark S., Rauterberg E. W., Shin M. L., Löke S., Roelcke D., Hänsch G. M. Homologous species restriction in lysis of human erythrocytes: a membrane-derived protein with C8-binding capacity functions as an inhibitor. J Immunol. 1986 Mar 1;136(5):1772–1776. [PubMed] [Google Scholar]
- Shin M. L., Hänsch G., Hu V. W., Nicholson-Weller A. Membrane factors responsible for homologous species restriction of complement-mediated lysis: evidence for a factor other than DAF operating at the stage of C8 and C9. J Immunol. 1986 Mar 1;136(5):1777–1782. [PubMed] [Google Scholar]
- Tschopp J. Circular polymerization of the membranolytic ninth component of complement. Dependence on metal ions. J Biol Chem. 1984 Aug 25;259(16):10569–10573. [PubMed] [Google Scholar]
- Yamamoto K. I. Lytic activity of C5-9 complexes for erythrocytes from the species other than sheep: C9 rather than C8-dependent variation in lytic activity. J Immunol. 1977 Oct;119(4):1482–1485. [PubMed] [Google Scholar]
- Zalman L. S., Brothers M. A., Chiu F. J., Müller-Eberhard H. J. Mechanism of cytotoxicity of human large granular lymphocytes: relationship of the cytotoxic lymphocyte protein to the ninth component (C9) of human complement. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5262–5266. doi: 10.1073/pnas.83.14.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalman L. S., Wisnieski B. J. Mechanism of insertion of diphtheria toxin: peptide entry and pore size determinations. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3341–3345. doi: 10.1073/pnas.81.11.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]