Abstract
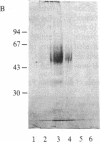
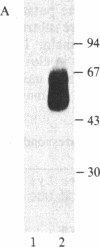
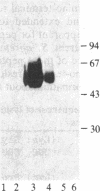
The murine Fc receptor for IgG (Fc gamma R) was purified to homogeneity by immunoaffinity chromatography from detergent lysates of the macrophage cell line J774. Microsequencing of intact protein yielded a single amino-terminal sequence, which was confirmed and extended to 20 residues by the isolation of an overlapping peptide. The isolation of additional proteolytic fragments obtained by using Staphylococcus aureus V8 protease, cyanogen bromide, and lysine C proteinase, facilitated sequence analysis of a total of 119 amino acid residues. Codon usage charts were used to construct oligonucleotide probes based on the amino acid sequences of three nonoverlapping peptides. These probes were used to screen a cDNA library derived from the WEHI-3B myelomonocytic cell line, and a single cDNA clone (pFc24) to which all three probes hybridized was isolated. This clone, containing a 1.02-kilobase cDNA insert, has been characterized by restriction mapping and partial DNA sequencing, and it has been shown to encode the Fc gamma R. The sequence at the 5' end of the clone contained the coding information for the amino-terminal sequence of the Fc gamma R as well as a putative 13-amino acid signal sequence. The 3' end of the clone encoded a peptide identified in purified receptor preparations. Thus, the presence of coding information at the 5' and 3' ends of this clone suggests that full-length Fc receptor cDNA spans greater than 1 kilobase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bich-Thuy L. T., Samarut C., Rabourdin-Combe C., Revillard J. P. The suppression activity of Fc gamma receptors is not related to their T-cell origin. Cell Immunol. 1982 Apr;68(2):252–260. doi: 10.1016/0008-8749(82)90110-1. [DOI] [PubMed] [Google Scholar]
- Campbell D. G., Williams A. F., Bayley P. M., Reid K. B. Structural similarities between Thy-1 antigen from rat brain and immunoglobulin. Nature. 1979 Nov 15;282(5736):341–342. doi: 10.1038/282341a0. [DOI] [PubMed] [Google Scholar]
- Fisher M. M., Nagy B., Bazin H., Underdown B. J. Biliary transport of IgA: role of secretory component. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2008–2012. doi: 10.1073/pnas.76.4.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- Garger S. J., Griffith O. M., Grill L. K. Rapid purification of plasmid DNA by a single centrifugation in a two-step cesium chloride-ethidium bromide gradient. Biochem Biophys Res Commun. 1983 Dec 28;117(3):835–842. doi: 10.1016/0006-291x(83)91672-8. [DOI] [PubMed] [Google Scholar]
- Gisler R. H., Fridman W. H. Suppression of in vitro antibody synthesis by immunoglobulin-binding factor. J Exp Med. 1975 Aug 1;142(2):507–517. doi: 10.1084/jem.142.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M., Metcalf D., Gough J., Grail D., Dunn A. R. Structure and expression of the mRNA for murine granulocyte-macrophage colony stimulating factor. EMBO J. 1985 Mar;4(3):645–653. doi: 10.1002/j.1460-2075.1985.tb03678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. A., Plutner H., Mellman I. Biosynthesis and intracellular transport of the mouse macrophage Fc receptor. J Biol Chem. 1985 Aug 15;260(17):9867–9874. [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hibbs M. L., Hogarth P. M., McKenzie I. F. The mouse Ly-17 locus identifies a polymorphism of the Fc receptor. Immunogenetics. 1985;22(4):335–348. doi: 10.1007/BF00430917. [DOI] [PubMed] [Google Scholar]
- Hogarth P. M., Edwards J., McKenzie I. F., Goding J. W., Liew F. Y. Monoclonal antibodies to the murine Ly-2.1 cell surface antigen. Immunology. 1982 May;46(1):135–144. [PMC free article] [PubMed] [Google Scholar]
- Holmes K. L., Palfree R. G., Hammerling U., Morse H. C., 3rd Alleles of the Ly-17 alloantigen define polymorphisms of the murine IgG Fc receptor. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7706–7710. doi: 10.1073/pnas.82.22.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hewick R. M., Dreyer W. J., Hood L. E. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 1983;91:399–413. doi: 10.1016/s0076-6879(83)91038-8. [DOI] [PubMed] [Google Scholar]
- Kiyono H., Mosteller-Barnum L. M., Pitts A. M., Williamson S. I., Michalek S. M., McGhee J. R. Isotype-specific immunoregulation. IgA-binding factors produced by Fc alpha receptor-positive T cell hybridomas regulate IgA responses. J Exp Med. 1985 Apr 1;161(4):731–747. doi: 10.1084/jem.161.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Albrandt K., Mendel E., Kulczycki A., Jr, Orida N. K. Identification of an IgE-binding protein by molecular cloning. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4100–4104. doi: 10.1073/pnas.82.12.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwy I., Brezin C., Neauport-Sautes C., Theze J., Fridman W. H. Isotype regulation of antibody production: T-cell hybrids can be selectively induced to produce IgG1 and IgG2 subclass-specific suppressive immunoglobulin-binding factors. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2323–2327. doi: 10.1073/pnas.80.8.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens C. L., Huff T. F., Jardieu P., Trounstine M. L., Coffman R. L., Ishizaka K., Moore K. W. cDNA clones encoding IgE-binding factors from a rat-mouse T-cell hybridoma. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2460–2464. doi: 10.1073/pnas.82.8.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Friedlander M., Blobel G. The receptor for transepithelial transport of IgA and IgM contains multiple immunoglobulin-like domains. Nature. 1984 Mar 1;308(5954):37–43. doi: 10.1038/308037a0. [DOI] [PubMed] [Google Scholar]
- Pure E., Durie C. J., Summerill C. K., Unkeless J. C. Identification of soluble Fc receptors in mouse serum and the conditioned medium of stimulated B cells. J Exp Med. 1984 Dec 1;160(6):1836–1849. doi: 10.1084/jem.160.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O., Poulik M. D. Initiation of protein synthesis at an unusual position in an immunoglobulin gene? Science. 1972 Jan 14;175(4018):187–189. doi: 10.1126/science.175.4018.187. [DOI] [PubMed] [Google Scholar]
- Toh H., Ono M., Miyata T. Retroviral gag and DNA endonuclease coding sequences in IgE-binding factor gene. 1985 Nov 28-Dec 4Nature. 318(6044):388–389. doi: 10.1038/318388a0. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C., Fleit H., Mellman I. S. Structural Aspects and Heterogeneity of Immunoglobulin Fc Receptors. Adv Immunol. 1981;31:247–270. doi: 10.1016/s0065-2776(08)60922-0. [DOI] [PubMed] [Google Scholar]
- Walker I. D., Murray B. J., Kirszbaum L., Chambers G. W., Deacon N. J., McKenzie I. F. The amino-terminal sequences of Ly-2 and Ly-3. Immunogenetics. 1986;23(1):60–63. doi: 10.1007/BF00376523. [DOI] [PubMed] [Google Scholar]
- Yodoi J., Ishizaka K. Lymphocytes bearing Fc receptors for IgE. IV. Formation of IgE-binding factor by rat T lymphocytes. J Immunol. 1980 Mar;124(3):1322–1329. [PubMed] [Google Scholar]