Abstract
Most patients with severe motor and intellectual disabilities (SMID) have restricted mobility capability and have been bedridden for long periods because of paralysis of the extremities caused by abnormal muscular tonicity due to cerebral palsy and developmental disabilities, and such patients are associated with a high risk for the complications of deep vein thrombosis (DVT). Here, we report 8 patients (34.8%) with DVT among 23 patients with SMID during prolonged bed rest. However, we did not detect thrombosis in the soleal veins, finding it mostly in the superficial femoral and common femoral veins. Regarding laboratory data for the coagulation system, there were no cases with D-dimer above 5 µg/ml. Concerning sudden death in patients with SMID, we have to be very careful of the possibility of pulmonary thromboembolism due to DVT. Therefore, we should consider the particularities of an underdeveloped vascular system from underlying diseases for the evaluation of DVT in patients with SMID. A detailed study of DVT as a vascular complication is very important for smooth medical care of SMID and compression Doppler ultrasonography of the lower extremities, as noninvasive examination, is very helpful. (*English translation of Jpn J Phlebol 2012; 23: 17-24)
Keywords: severe motor and intellectual disabilities (SMID), deep vein thrombosis (DVT), pulmonary thromboembolism, ultrasonography
Introduction
To ensure the smooth provision of medical care for patients with severe motor and intellectual disabilities (SMID), it is very important that we deal with complications of the circulatory and vascular systems in addition to complications of the respiratory system. Most patients with SMID have motor paralysis of the extremities and restricted mobility due to abnormalities in muscle tone associated with cerebral palsy, among others;1,2) they are also associated with a high rate of complications of the vascular system, especially deep vein thrombosis (DVT).2,3) With the remarkable technological and pharmacological advances in neonatal care, the number of infants with severe brain impairment and respiratory problems has increased, particularly in extremely low-birth-weight infants. Among them, in those with profoundly severe motor and intellectual disabilities (p-SMID) who require particularly careful medical care and who have undergone tracheostomy and been placed under mechanical ventilation management, there are important tasks in home medical care.2,4,5) In patients with SMID confined to bed and with decreased mobility of the lower extremities, there are prolonged bed rest and a higher risk of the complication of DVT.2) It has been reported that respiratory tract infections represent the most common cause of mortality in SMID, for which the rate of sudden death is over 4.2%.6) DVT can have an asymptomatic clinical course, but many cases of DVT develop pulmonary thromboembolism (PTE), possibly causing sudden death.7–10) In this study, we closely investigated DVT in patients with SMID.
Materials and Methods
1. Patients
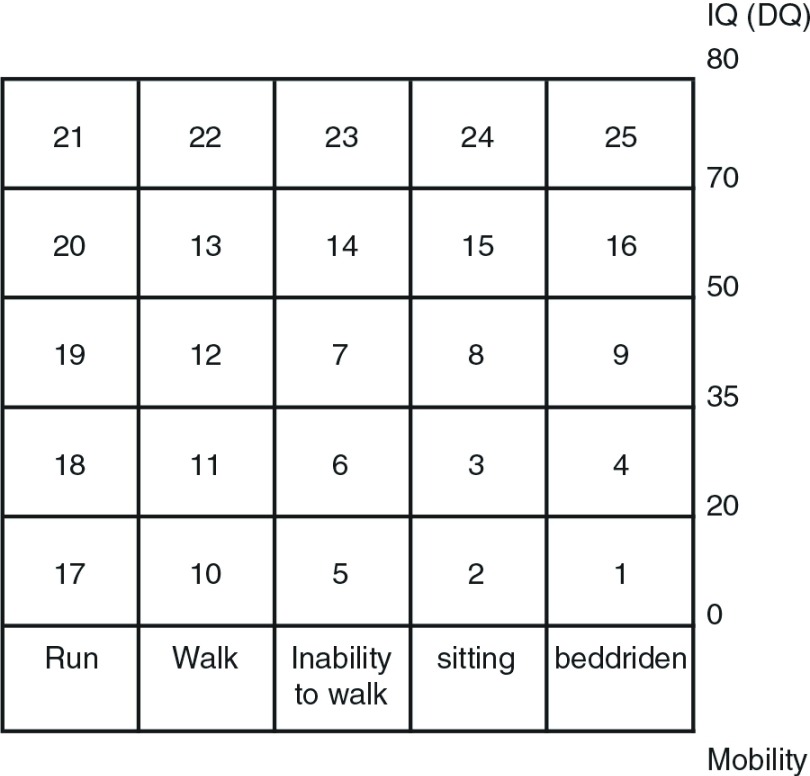
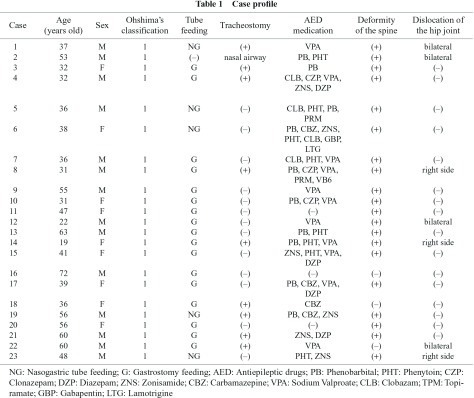
Twenty three patients with SMID who were classified in grade 1 of Ohshima’s classification criteria for SMID (Fig. 1)11) having physical and intellectual disabilities, including the inability to maintain a sitting position and being bedridden, were eligible for this study; they included those under intensive medical treatment with long-term hospitalization for SMID in the wards of the National Hospital Organization Yanai Medical Center (Table 1).
Fig. 1.
Ohshima’s classification shows the degree of intellectual and physical disabilities, consisting of IQ (DQ) and mobility. SMID: severe motor and intellectual disabilities; IQ: intelligence quotient; DQ: developmental quotient.
All of these patients had severely decreased mobility of the lower extremities and many required tube feeding, tracheostomy, mechanical ventilation, and nutritional management; there were 22 cases of tube feeding (5 cases of nasogastric tube, 17 cases of gastrostomy), 9 cases of tracheostomy (3 cases of mechanically ventilated patients), one case of nasal airway, and 20 cases (87.0%) of epilepsy.
2. Methods
We evaluated DVT in deep veins of the lower extremities by venous sonography using a GE Healthcare LOGIQ-S6 or LOGIQ-e (GE Healthcare Japan) and an 8–12 MHz variable linear probe in the 23 patients.
For the diagnosis of DVT by venous sonography, we confirmed a collapsed vein with the creation of vessels by B-mode ultrasound transverse imaging and compression of the probe, and on the basis of blocking of the blood flow by color Doppler ultrasonography with or without respiratory variation on a pulse Doppler method arbitrarily.
In addition, we specifically examined blood coagulation test results, including D-dimer (refined measurement of latex agglutination method, criterion value under 1.0 µg/ml), which has high diagnostic specificity for DVT as a hematological assessment.12,13) Furthermore, in terms of ethical aspects, we carried out our research with anonymous clinical data under close supervision after approval by the medical ethics committee of our hospital.
Results
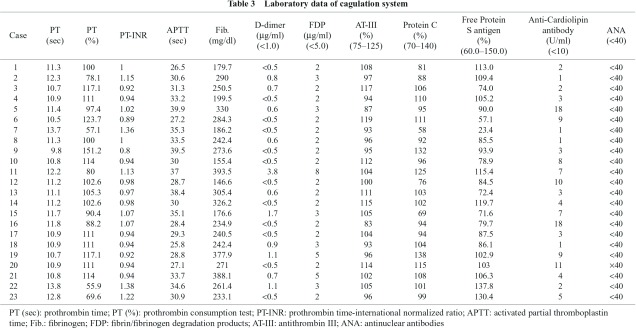
The background of the 23 patients with SMID is summarized in Table 1. The patients included 14 males and 9 females with a mean age of 43.5 years (from 19 to 72 years old). They had no symptoms in all cases, but 8 of the 23 cases (34.8%) showed DVT in the lower extremities asymptomatically (mean age of 39.4 years, 5 males and 3 females) (Table 2, Fig. 1). In the sites of DVT formation, there were 2 cases in left superficial femoral vein, one case in left posterior tibial vein + left posterior tibial vein communicating branch, 2 cases in left common femoral vein, one case in right common femoral vein, one case in bilateral posterior vein communicating branch, and one case in left posterior communicating branch (Table 2). In the posture and limb position, there were no differences between the 8 cases of DVT and the 15 cases of non-DVT in mobility, and all cases in the present study had deformities and contracture, with 20 of 23 cases having moderate to severe deformities of the spine (scoliosis) in the thoracolumbar region, and 7 of 23 cases having dislocation of the hip (4 cases in bilateral sides, 3 cases in the right side) (Table 1). However, there were no differences between the DVT group and the non-DVT group in terms of orthopedic findings, and all cases had general narrowing of the vessels of the lower extremities, with or without effects of immature blood vessels or maldevelopment; we also could not find DVT of the soleal veins.
Additionally, there were no differences in the thickness of the lower extremities in all cases. In coagulation test, values of D-dimer (refined measurement of latex agglutination method, criterion value under 1.0 µg/ml) as a useful marker for the diagnosis of DVT in hematological assessment12,13) were above 0.5 µg/ml in 11 of 23 cases, but were never above 5 µg/ml (Table 3). There were no marked abnormalities in the values of protein C, protein S, anti-thrombin III, and anti-cardiolipin antibody. In 8 cases of DVT in the lower extremities, we initially applied anticoagulant therapy (heparin and warfarin or warfarin alone), and carefully followed up the cases, regulating the warfarin dosage at PT-INR values around 2.
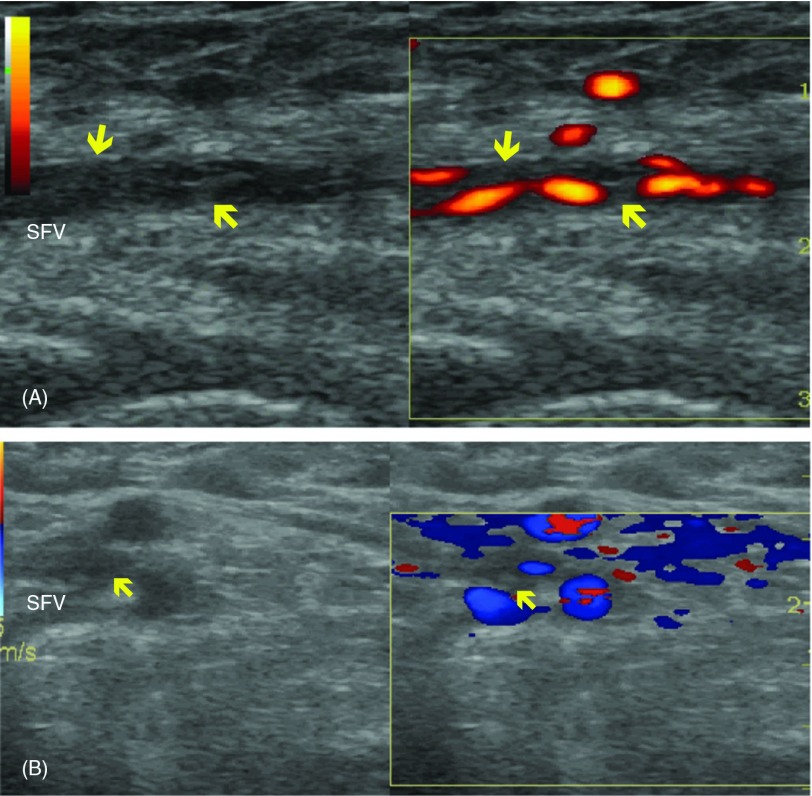
Fig. 2.
Longitudinal (A) and transverse (B) scans of the superficial femoral vein of case 15 by ultrasonography. Thromboses (arrow) are shown in the vein. The vessel is narrowed and the diameter of the vessel is 2.7 mm. SFV: superficial femoral vein.
Discussion
There are three major factors contributing to vein thrombosis: stagnated blood flow, intravascular endothelial damage, and hypercoagulable state, as proposed by Rudolf C. Virchow in 1865.14,15) There are certain main risk factors for pulmonary thromboembolism (PTE), specifically, protein C deficiency, protein S deficiency, antithrombin deficiency, and homocystinemia as congenital risk factors, and operation, obesity, bed rest, malignancy (Trousseau syndrome), trauma, fracture, insertion of a central intravenous catheter, congestive heart failure, chronic pulmonary diseases, cerebrovascular disorders, antiphospholipid antibody syndrome, and drugs (estrogen, oral contraceptive, steroid, etc.), among others, as acquired risk factors.15) Over 90% of cases of embolus of PTE involve thrombus of a vein in the extremities or pelvis,9,15) and in DVT of veins in the pelvis and lower extremities, patterns of the disease are divided into a central type (iliac and femoral type) from the popliteal vein centrally and a peripheral type from the popliteal vein distally (crural type). The crural type accounts for the largest proportion of DVT,15–18) and the main initial region of DVT in the lower extremities is the soleal vein.15,19) Clinical manifestations of acute PTE range from no symptoms to sudden death depending on the severity and size of the thrombus occluding the pulmonic vascular bed, the cardiopulmonary reserve capacity of individual patients, and the presence or absence of pulmonary infarction.14) Studies examining autopsy results of PTE cases showed residual thrombi of the lower deep veins in almost all cases, and the prevention of DVT in the lower extremities is receiving attention as a way of preventing PTE.9,10)
As risk factors associated with the pathogenesis of DVT,15,16,18,19) there are surgery, fractures of pelvic, femoral, and tibial bones, angiographic procedure, critical medical illness (cerebrovascular diseases, heart failure, and myocardial infarction, etc.), and drugs as transient risk factors, and prolonged bed rest, advanced age, malignancy, obesity, and hypercoagulable status, among others, as persistent risk factors. Patients with SMID are seriously restricted to mobility due to motor impairment and, among them, patients with grade 1 of Ohshima’s classification who are incapable of maintaining a sitting position require critical care such as respiratory and nutritional management, especially1,2,20,21) when undergoing long-term tube feeding from disuse atrophy in association with a bedridden state and difficulty in swallowing. As a result, they may have an extremely high risk for DVT from prolonged immobility.2) In the Japanese Guidelines for the Prevention of Venous Thromboembolism (revised edition, 2009),15) it has been reported that the clinical conditions of prolonged bed rest, paralysis of the lower extremities, and spinal injury, among other things, are risk factors for DVT, and in the present study, we found a significant rate of DVT of 34.8% (8/23 cases) in patients in a bedridden state, with severely decreased mobility of the lower extremities and tube-feeding, and receiving respiratory care by tracheostomy and/or mechanical ventilation. However, in the region of thrombus formation with DVT, mostly the superficial femoral vein and common femoral vein were involved, and we did not detect DVT in the soleal vein, which was previously reported as the initial site of DVT.19,22) Most patients with SMID have communication difficulties and we thus need to observe potential clinical symptoms of DVT carefully. In terms of the clinical manifestations of especially the peripheral type of DVT, pain is common, although conversely, there are often no symptoms. Therefore, it is very difficult to understand the pathogenesis of DVT accurately.
To diagnose DVT, phlebography of the lower extremities is the most reliable standard test for confirming the diagnosis of DVT, even today, but it is only a candidate approach in cases not diagnosed by other imaging methods because of an intensely invasive procedure.15,16,22) This is because it cannot be performed easily on patients with SMID owing to the troublesome transfer to a clinical laboratory. On the other hand, venous sonography of the lower extremities is generally used via a compression procedure as a non-invasive test, and we could obtain precise imaging findings more easily at the bedside using portable ultrasound instruments.15,22,23) It is a very useful method for the evaluation of DVT in patients with SMID with restricted motility. In our present investigation, we could not detect thrombus in the soleal vein as the initial site of DVT in the deep veins of the lower extremities, but did in left common femoral vein, left superficial femoral vein, right common femoral vein, right superficial femoral vein, and bilateral posterior tibial vein communicating branch. Whether or not we failed to visualize thrombus in the soleal vein by venous sonography in the lower extremities and only detected it in the common femoral and superficial veins centrally from the popliteal vein, there were no associations with significantly elevated levels of D-dimer. Thus, it is considered that thrombus formation is causally related to disturbance of mobility due to a prolonged bedridden state related to cerebral palsy from young childhood, especially due to paralysis of the lower extremities other than vascular endothelial damage, which leads to contracture deformities of the lower extremities and narrowing of the vessels by vascular underdevelopment and immaturity.26,27) Consequently, it has been suggested that thrombus forms in the conduct veins centrally in patients with SMID.
As DVT is associated with the malformation of blood vessels, there is an association with Klippel-Trenaunay syndrome and Proteus syndrome; in particular, Klippel-Trenaunay syndrome (KTS) is characterized by mainly port-wine stain capillary malformation, varicose veins, and bony and soft tissue hypertrophy involving an extremity. It is also commonly associated with hypoplasia or aplasia of the veins of the lower limbs, the varicosities of which expand remarkably with age;25,26) KTS has also been reported to have the potential complications of DVT and to bring on PTE frequently,24–26) so future studies should examine this issue in detail. Heparin and warfarin as anticoagulant therapy have been used in the treatment and management of DVT,15,28,29) and warfarin monotherapy for DVT at the onset of treatment has been associated with a high relapse rate, so it has been considered essential to apply combined therapy of heparin and warfarin in the treatment of DVT.15) Our treatment of DVT involved combination therapy of warfarin and low-molecular-weight heparin. In the Japanese Guidelines for Prevention of Venous Thromboembolism (revised edition, 2009),15) prothrombin time is described as acting as a general indicator of warfarin dosage, and the dosage is regulated with reference to the international normalized ratio (INR). That is, warfarin at a daily dose of 5 mg has been administered for the first two days of therapy, and the PT-INR levels are controlled in the range of 1.5 to 2.5 (desired value 2.0). In cases with irreversible risk factors, warfarin is administered for 3 months, and in cases having relapse and persistent risk factors, warfarin is considered to be given chronically. Accordingly, anticoagulant therapy for over 3 months to manage DVT in patients with SMID in a prolonged bedridden state and with restricted mobility due to underlying diseases such as cerebral palsy should be required, along with the assessment of DVT regularly for long periods by venous ultrasonography in the lower extremities. This is because DVT mostly remains asymptomatic and has been detected only at the onset of PTE.
Additionally, a sudden death rate of 4.2% has been reported among the causes of death in patients with SMID, after pneumonia, respiratory failure, heart failure, and asphyxia.6) Right ventricular hypertrophy was found in only five cases of seven autopsies for the clinical consideration of sudden death in patients with SMID,30) but it cannot be ruled out that DVT might have developed into PTE because these cases were not specifically examined the involvement of the pulmonary arteries. In future, it will be very important to assess and investigate DVT as a cardiovascular complication in order to ensure quality of life and to provide detailed medical support to patients with SMID; this should involve ultrasonography of the lower extremities, as a noninvasive examination that is very helpful for checking for DVT.
Acknowledgment
We thank Dr. Makoto Kajiwara, director of Ehime Prefectural Central Hospital, for valuable advice and cooperation.
Disclosure Statement
Hiromitsu Ohmori and coauthors have no conflicts of interest to disclose.
Footnotes
This article is English translation of Jpn J Phlebol 2012; 23: 17-24.
References
- Hiramoto A. Diagnosis and assessment of severe motor and intellectual disabilities. In: Egusa Y. ed. Manual of Severe Motor and Intellectual Disabilities Care, 2nd ed. Tokyo: Ishiyaku Shuppan, 2005: 18-27. (in Japanese) [Google Scholar]
- Ochi F, Ohmori H, Nakano T, et al. Asymptomatic deep vein thrombosis in children with severe motor and intellectual disabilities. J Jpn Pediatr Soc 2010; 114: 1909-14. (in Japanese) [Google Scholar]
- Radecki RT, Gaebler-Spira D. Deep vein thrombosis in the disabled pediatric population. Arch Phys Med Rehabil 1994; 75: 248-50. [DOI] [PubMed] [Google Scholar]
- Ohmori H. High risk neonate: with special reference to medical support of long-term hospitalized children in NICU. The Japanese Journal of Child Nursing 2002; 25: 1093-8. (in Japanese) [Google Scholar]
- Ohmori H, Yokoo K. A consideration for home medical support to long-term hospitalized children in NICU. Neonatal Care 2002; 15: 635-41. (in Japanese) [Google Scholar]
- Arima M. Prognosis in patients with severe motor and intellectual disabilities. In: Yasuhiko Egusa Y. ed. Manual of Severe Motor and Intellectual Disabilities Care, 2nd ed. Tokyo: Ishiyaku Shuppan, 2005: 35-9. (in Japanese) [Google Scholar]
- Yamada N, Nakamura M, Ishikura K, et al. Epidemiological characteristics of acute pulmonary thromboembolism in Japan. Int Angiol 2003; 22: 50-4. [PubMed] [Google Scholar]
- Ota M, Nakamura M, Yamada N, et al. Prognostic significance of early diagnosis in acute pulmonary thromboembolism with circulatory failure. Heart Vessels 2002; 17: 7-11. [DOI] [PubMed] [Google Scholar]
- Ro A, Kageyama N, Fukunaga T. Correlation between deep vein thrombosis and acute pulmonary thromboembolism through autopsy standpoints. Medicina 2009; 46: 715-7. (in Japanese) [Google Scholar]
- Nakamura Y, Yutani C, Imakita M, et al. Pathophysiology of clinicopathological aspect of venous thrombosis and pulmonary thromboembolism. Jpn J Phlebol 1996; 7: 17-22. (in Japanese) [Google Scholar]
- Ohshima K. Fundamental issues for severe motor and intellectual disabilities. Japanese Journal of Public Health 1971; 35: 648-55. (in Japanese) [Google Scholar]
- Bozic M, Blinc A, Stegnar M. D-dimer, other markers of haemostasis activation and soluble adhesion molecules in patients with different clinical probabilities of deep vein thrombosis. Thromb Res 2002; 108: 107-14. [DOI] [PubMed] [Google Scholar]
- Stein PD, Hull RD, Patel KC, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med 2004; 140: 589-602. [DOI] [PubMed] [Google Scholar]
- Meissner MH, Strandness DE. The epidemiology and natural history of acute deep vein thrombosis. In: Gloviczki P, et al. eds. Handbook of Venous Disorders. New York: Arnold, 2001: 38-48. [Google Scholar]
- Japanese Guideline for Prevention of Venous Thromboembolism. revised edition in 2009. (in Japanese)
- Browse NL, Burnand KG, Irvine AT, et al. Deep vein thrombosis: pathology and diagnosis. In: Browse NL, et al. eds. Diseases of the Veins. London: Arnold, 1999: 249-91. [Google Scholar]
- Hill SL, Holtzman GI, Martin D, et al. The origin of lower extremity deep vein thrombi in acute venous thrombosis. Am J Surg 1997; 173: 485-90. [DOI] [PubMed] [Google Scholar]
- Meissner MH, Wakefield TW, Ascher E, et al. Acute venous disease: venous thrombosis and venous trauma. J Vasc Surg 2007; 46 Suppl S: 25S-53S. [DOI] [PubMed] [Google Scholar]
- Ohgi S, Tachibana M, Ikebuchi M, et al. Pulmonary embolism in patients with isolated soleal vein thrombosis. Angiology 1998; 49: 759-64. [DOI] [PubMed] [Google Scholar]
- Ohmori H, Ebihara T, Ochi F, et al. Bedside placement of small-bowel feeding tube in patients with severe motor and intellectual disabilities. Japanese Journal of Pediatric Gastroenterology, Hepatology and Nutrition 2009; 23: 24-30. (in Japanese) [Google Scholar]
- Ochi F, Ohmori H, Ebihara T. Selenium deficiency in children with severe motor and intellectual disabilities. Japanese Journal of Pediatric Gastroenterology, Hepatology and Nutrition 2010; 23: 141-6. (in Japanese) [Google Scholar]
- Ohgi S. Diagnosis and treatment for pulmonary embolic sources in the venous system of lower limbs. Jpn J Phlebol 1998; 9: 263-70. (in Japanese) [Google Scholar]
- Meissner MH, Moneta G, Burnand K, et al. The hemodynamics and diagnosis of venous disease. J Vasc Surg 2007; 46 Suppl S: 4S-24S. [DOI] [PubMed] [Google Scholar]
- Huiras EE, Barnes CJ, Eichenfield LF, et al. Pulmonary thromboembolism associated with Klippel-Trenaunay syndrome. Pediatrics 2005; 116: e596-600. [DOI] [PubMed] [Google Scholar]
- Lee A, Driscoll D, Gloviczki P, et al. Evaluation and management of pain in patients with Klippel-Trenaunay syndrome: a review. Pediatrics 2005; 115: 744-9. [DOI] [PubMed] [Google Scholar]
- Timur AA, Driscoll DJ, Wang Q. Biomedicine and diseases: the Klippel-Trenaunay syndrome, vascular anomalies and vascular morphogenesis. Cell Mol Life Sci 2005; 62: 1434-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AE. Maldevelopments of the vascular system: clinical conundrums. In: Nugent J, O’Connor M. eds. Development of the Vascular System, Ciba Foundation Symposium 100. London: Pitman, 1983: 222-43. [DOI] [PubMed] [Google Scholar]
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133: 381S-453S. [DOI] [PubMed] [Google Scholar]
- Cardiovascular Disease Educational and Research Trust; Cyprus Cardiovascular Disease Educational and Research Trust; European Venous Forum et al. Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence). Int Angiol 2006; 25: 101-61. [PubMed] [Google Scholar]
- Yoshida R, Ishizaki A, Sato J, et al. A clinical study of sudden death in the severely handicapped persons. No To Hattatsu 1995; 27: 466-72. (in Japanese) [PubMed] [Google Scholar]