Abstract
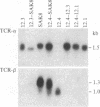
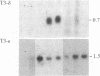
The antigen-specific T-cell receptor (TCR) is composed of variable antigen-recognition chains TCR-alpha and TCR-beta in noncovalent association with the invariant T3 multimer. The TCR-alpha and TCR-beta chains are encoded by gene segments that must be juxtaposed by rearrangement in order to be expressed. To examine whether mechanisms other than gene rearrangement might regulate TCR/T3 gene expression, somatic cell hybrids were formed among closely related murine SL12 T-lymphoma clones that differ in TCR/T3 mRNA levels. In hybrid cells formed between cell clones in which one parent is TCR-beta+ and the other is TCR-beta-, the resultant hybrid cells lack detectable TCR-beta transcripts. Since the protein synthesis inhibitor cycloheximide partially reverses TCR-beta repression in the hybrid cells, we postulate that a labile repressor protein is involved. The amount of mRNA encoding one of the T3 polypeptide chains, T3-delta, is also strongly negatively transregulated in the same hybrid cells in which TCR-beta mRNA expression is repressed. The negative trans-regulation of TCR-beta and T3-delta mRNA expression is relatively specific, since the levels of TCR-alpha mRNA and several thymocyte surface antigens are not repressed in somatic cell hybrids. Our results indicate that rearrangement of the TCR genes alone is not sufficient for TCR-beta expression and that trans-acting factors regulate the amounts of both TCR-beta and T3-delta mRNA in this system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borst J., Alexander S., Elder J., Terhorst C. The T3 complex on human T lymphocytes involves four structurally distinct glycoproteins. J Biol Chem. 1983 Apr 25;258(8):5135–5141. [PubMed] [Google Scholar]
- Chien Y., Becker D. M., Lindsten T., Okamura M., Cohen D. I., Davis M. M. A third type of murine T-cell receptor gene. Nature. 1984 Nov 1;312(5989):31–35. doi: 10.1038/312031a0. [DOI] [PubMed] [Google Scholar]
- Furley A. J., Mizutani S., Weilbaecher K., Dhaliwal H. S., Ford A. M., Chan L. C., Molgaard H. V., Toyonaga B., Mak T., van den Elsen P. Developmentally regulated rearrangement and expression of genes encoding the T cell receptor-T3 complex. Cell. 1986 Jul 4;46(1):75–87. doi: 10.1016/0092-8674(86)90861-5. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Chien Y., Becker D. M., Kavaler J., Davis M. M. Genomic organization and sequence of T-cell receptor beta-chain constant- and joining-region genes. Nature. 1984 Aug 2;310(5976):387–391. doi: 10.1038/310387a0. [DOI] [PubMed] [Google Scholar]
- Gasson J. C., Bourgeois S. A new determinant of glucocorticoid sensitivity in lymphoid cell lines. J Cell Biol. 1983 Feb;96(2):409–415. doi: 10.1083/jcb.96.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold D. P., Puck J. M., Pettey C. L., Cho M., Coligan J., Woody J. N., Terhorst C. Isolation of cDNA clones encoding the 20K non-glycosylated polypeptide chain of the human T-cell receptor/T3 complex. Nature. 1986 May 22;321(6068):431–434. doi: 10.1038/321431a0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Kappler J., Kubo R., Haskins K., White J., Marrack P. The mouse T cell receptor: comparison of MHC-restricted receptors on two T cell hybridomas. Cell. 1983 Oct;34(3):727–737. doi: 10.1016/0092-8674(83)90529-9. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Lesley J., Hyman R., Schulte R., Trotter J. Expression of transferrin receptor on murine hematopoietic progenitors. Cell Immunol. 1984 Jan;83(1):14–25. doi: 10.1016/0008-8749(84)90220-x. [DOI] [PubMed] [Google Scholar]
- MacLeod C. L., Hays E. F., Hyman R., Bourgeois S. A new murine model system for the in vitro development of thymoma cell heterogeneity. Cancer Res. 1984 May;44(5):1784–1790. [PubMed] [Google Scholar]
- MacLeod C. L., Weinroth S. E., Streifinger C., Glaser S. M., Hays E. F. SL12 murine T-lymphoma: a new model for tumor cell heterogeneity. J Natl Cancer Inst. 1985 Apr;74(4):875–882. [PubMed] [Google Scholar]
- McIntyre B. W., Allison J. P. The mouse T cell receptor: structural heterogeneity of molecules of normal T cells defined by xenoantiserum. Cell. 1983 Oct;34(3):739–746. doi: 10.1016/0092-8674(83)90530-5. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Acuto O., Hercend T., Schlossman S. F., Reinherz E. L. The human T-cell receptor. Annu Rev Immunol. 1984;2:23–50. doi: 10.1146/annurev.iy.02.040184.000323. [DOI] [PubMed] [Google Scholar]
- Oettgen H. C., Pettey C. L., Maloy W. L., Terhorst C. A T3-like protein complex associated with the antigen receptor on murine T cells. Nature. 1986 Mar 20;320(6059):272–275. doi: 10.1038/320272a0. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Mak T. W., Van den Elsen P., Yanagi Y., Yoshikai Y., Calman A. F., Terhorst C., Stobo J. D., Weiss A. Reconstitution of an active surface T3/T-cell antigen receptor by DNA transfer. Nature. 1985 Aug 15;316(6029):606–609. doi: 10.1038/316606a0. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Lugo J. P. Differentiation and cell division in the mammalian thymus. Dev Biol. 1985 Nov;112(1):1–17. doi: 10.1016/0012-1606(85)90114-9. [DOI] [PubMed] [Google Scholar]
- Royer H. D., Ramarli D., Acuto O., Campen T. J., Reinherz E. L. Genes encoding the T-cell receptor alpha and beta subunits are transcribed in an ordered manner during intrathymic ontogeny. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5510–5514. doi: 10.1073/pnas.82.16.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelson L. E., Germain R. N., Schwartz R. H. Monoclonal antibodies against the antigen receptor on a cloned T-cell hybrid. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6972–6976. doi: 10.1073/pnas.80.22.6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelson L. E., Harford J. B., Klausner R. D. Identification of the components of the murine T cell antigen receptor complex. Cell. 1985 Nov;43(1):223–231. doi: 10.1016/0092-8674(85)90027-3. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Lindsten T., Fowlkes B. J., van den Elsen P., Terhorst C., Davis M. M., Germain R. N., Schwartz R. H. Expression of genes of the T-cell antigen receptor complex in precursor thymocytes. 1985 Jun 27-Jul 3Nature. 315(6022):765–768. doi: 10.1038/315765a0. [DOI] [PubMed] [Google Scholar]
- Siu G., Kronenberg M., Strauss E., Haars R., Mak T. W., Hood L. The structure, rearrangement and expression of D beta gene segments of the murine T-cell antigen receptor. 1984 Sep 27-Oct 3Nature. 311(5984):344–350. doi: 10.1038/311344a0. [DOI] [PubMed] [Google Scholar]
- Snodgrass H. R., Kisielow P., Kiefer M., Steinmetz M., von Boehmer H. Ontogeny of the T-cell antigen receptor within the thymus. Nature. 1985 Feb 14;313(6003):592–595. doi: 10.1038/313592a0. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Lesley J., Trotter J., Hyman R. Thymocyte subpopulation enriched for progenitors with an unrearranged T-cell receptor beta-chain gene. Nature. 1985 Jun 20;315(6021):666–669. doi: 10.1038/315666a0. [DOI] [PubMed] [Google Scholar]
- Weinroth S. E., MacLeod C. L., Minning L., Hays E. F. Genetic complexity of glucocorticoid-induced lysis of murine T-lymphoma cells. Cancer Res. 1985 Oct;45(10):4804–4809. [PubMed] [Google Scholar]
- Yagüe J., White J., Coleclough C., Kappler J., Palmer E., Marrack P. The T cell receptor: the alpha and beta chains define idiotype, and antigen and MHC specificity. Cell. 1985 Aug;42(1):81–87. doi: 10.1016/s0092-8674(85)80103-3. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Anatoniou D., Clark S. P., Yanagi Y., Sangster R., Van den Elsen P., Terhorst C., Mak T. W. Sequence and expression of transcripts of the human T-cell receptor beta-chain genes. Nature. 1984 Dec 6;312(5994):521–524. doi: 10.1038/312521a0. [DOI] [PubMed] [Google Scholar]
- van den Elsen P., Bruns G., Gerhard D. S., Pravtcheva D., Jones C., Housman D., Ruddle F. A., Orkin S., Terhorst C. Assignment of the gene coding for the T3-delta subunit of the T3-T-cell receptor complex to the long arm of human chromosome 11 and to mouse chromosome 9. Proc Natl Acad Sci U S A. 1985 May;82(9):2920–2924. doi: 10.1073/pnas.82.9.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elsen P., Shepley B. A., Cho M., Terhorst C. Isolation and characterization of a cDNA clone encoding the murine homologue of the human 20K T3/T-cell receptor glycoprotein. Nature. 1985 Apr 11;314(6011):542–544. doi: 10.1038/314542a0. [DOI] [PubMed] [Google Scholar]