Abstract
Apis mellifera capensis is unique among honeybees in that unmated workers can produce pseudo-clonal female offspring via thelytokous parthenogenesis. Workers use this ability to compete among themselves and with their queen to be the mother of new queens. Males could therefore enhance their reproductive success by imprinting genes that enhance fertility in their daughter workers. This possibility sets the scene for intragenomic conflict between queens and drones over worker reproductive traits. Here, we show a strong parent-of-origin effect for ovary size (number of ovarioles) in reciprocal crosses between two honeybee subspecies, A. m. capensis and Apis mellifera scutellata. In this cross, workers with an A. m. capensis father had 30% more ovarioles than genotypically matched workers with an A. m. scutellata father. Other traits we measured (worker weight at emergence and the presence/absence of a spermatheca) are influenced more by rearing conditions than by parent-of-origin effects. Our study is the first to show a strong epigenetic (or, less likely, cytoplasmic maternal) effect for a reproductive trait in the honeybee and suggests that a search for parent-of-origin effects in other social insects may be fruitful.
Keywords: Apis mellifera capensis, reciprocal effects, imprinting, parent-of-origin effects, intragenomic conflict, interspecific crosses
1. Introduction
When the reproductive interests of males and females differ, their genes may be in conflict [1]. The probability that a mother's genes will be present in all her offspring is 1, whereas the probability that a male's genes will be present in his mating partner's offspring is 1 only if she is monogamous [2,3]. In species where polyandry is the rule, the reproductive interests of the male will be favoured if genes inherited from the father (‘patrigenes’) can secure additional resources for their offspring from the mother at the expense of half-siblings [1]. For example, a patrigene that is upregulated so that a fetus grows larger at the expense of half-sibling fetuses should be favoured. By contrast, the ‘matrigene’ version of the same gene will favour the reproductive interests of the mother in most circumstances if all fetuses, both current and future, share their mother's resources equally. Insulin-like growth factor II (IGF II), which regulates fetal growth in mammals, was the first concrete example of this phenomenon [4]. When IGF II is transmitted to offspring as a matrigene, it is imprinted to be switched off to reduce the growth of the fetus, whereas when it is transmitted as a patrigene, it is not imprinted and is not switched off, increasing fetal growth [5]. Reciprocally, the receptor of IGF II, IGFIIR is paternally imprinted to be switched off. Haig & Graham [4] argue that the active maternal receptor acts to ‘mop up’ IGFII transcribed from the active paternal allele, providing a clear example of an evolutionary arms race between reciprocally imprinted genes [6]. In another example from mice, Grb1 is only expressed in its patrigene form in the brain, where it engenders male-typical social dominance [7].
Colonies of haplodiploid Hymenopteran (bees, wasps and ants) insects provide a situation that is ripe with potential for intragenomic conflict [8–10]. Queens mate early in life, carrying the sperm of their mating partners in a special organ, the spermatheca, with which to fertilize eggs [11,12]. In some species queens are polyandrous [13], and in a few species queens mate with over 10 different males [14,15]. The diploid female workers of most species do not reproduce [16,17] and lack a spermatheca, but have functional ovaries and are able to produce functional haploid male offspring parthenogenetically [18]. Thus, the patrigenes in workers can be favoured if they increase the probability that workers will escape sterility and lay eggs, a phenomenon that is rare but reported from honeybees [19–22], stingless bees [23,24], wasps [25,26] and ants [27,28]. By contrast, the matrigenes in workers will normally be favoured if they act to ensure that workers are sterile and the queen is the sole reproductive female. This leads to the prediction that a gene that affects worker fecundity might be epigenetically modified so that it is differentially spliced or expressed depending on whether it has been inherited from a male or a female [1,8,9,29,30].
Genomic imprinting describes the modification of DNA in a parent in ways that alter the transcriptome of offspring. Imprinting often involves ‘methylation’, the binding of a methyl group to the 5′ carbon of cytosine in a cytosine–guanine dinucleotide [31]. Methylation can interfere with transcription, leading to gene silencing [31] or change the splicing of exons [32]. The honeybee, Apis mellifera, has a fully functional methylation system [33] and DNA methylation is central to the regulation of caste differentiation in many social insects [33–35]. Thus, theory predicts that social insects are likely to evolve to use their methylation system to differentially imprint some genes, depending on whether they are inherited from a queen or a male [29].
If a gene suppresses worker fertility, then selection will favour epigenetic modifications in males that modify this gene to increase the fertility of their worker offspring. Queens, on the other hand, should be selected to remove such epigenetic marks when they pass on their paternally inherited allele to their female offspring [30]. However, there is also a theoretical expectation that queens may be more constrained in the kinds of epigenetic modifications they can make to genes than males are. Male imprinting can only increase the fecundity of daughters because males have no sons, whereas queen imprinting that decreases the fecundity of daughters may adversely affect sons as well. Thus, imprinting of genes affecting reproduction may be more likely to evolve in males than queens [8].
South Africa is home to two honeybee subspecies: A. m. capensis and A. m. scutellata [36–38]. Apis mellifera capensis (hereafter ‘Capensis’) is unique among honeybee subspecies in that queenless workers produce diploid female offspring via thelytokous parthenogenesis [39,40]. In all other honeybee subspecies, including A. m. scutellata (hereafter ‘Scutellata’), thelytoky is rare or absent, and workers produce haploid male offspring via arrhenotokous parthenogenesis (usually only in queenless colonies) [11]. The consequences of thelytoky are profound because workers have evolved to compete with their queen and each other over the production of queens because queen progeny are so much more reproductively valuable than worker progeny [18,41,42]. Queens are not genetically disadvantaged if their workers reproduce because they are equally related to their daughters and thelytokously produced granddaughters [43]. Strikingly, over 50% of queen larvae are daughters of workers rather than daughters of the queen [44–46]. Frequent, successful reproduction by workers no doubt explains the unusually high expression of reproductive traits in Capensis workers, including a much higher ovariole number than any other honeybee subspecies, high body weight and presence of a spermathaeca at high frequency [47–50].
The ability of Capensis workers to produce female (and particularly queen) offspring via thelytoky increases the selective advantage of males imprinting genes that enhance the reproductive potential of their worker offspring. Whereas in all other honeybee (sub-) species, males can only produce a few grandsons via their worker-daughters, Capensis drones can potentially father (in genetic terms) queens if their worker-daughters produce the colony's next queen. The stronger selection pressure on Capensis males to influence the reproductive potential of their worker-daughters may explain previously observed reciprocal effects for some worker-expressed traits in crosses between Scutellata and Capensis. When Scutellata queens are crossed with Capensis males, the worker offspring are heavier and much more likely to have a spermatheca than when Capensis queens are crossed with Scutellata males [51]. However, it is unclear how much of this difference is owing to the rearing environment or to direct parental effects on the offspring. Rearing environment greatly affects the development of reproductive traits in Capensis workers. Capensis workers that develop in a Scutellata colony are heavier, have more ovarioles, fewer basitarsal hairs on their pollen basket and are more likely to have a large spermatheca than if they are reared in a colony of their own subspecies [52,53].
To determine whether there are direct parental effects distinct from the effects of rearing environment, we generated replicate reciprocal crosses between a colony of A. m. capensis and a colony of A. m. scutellata. We then determined the effect of the direction of the cross on the expression of emergence weight, number of ovarioles and the presence/absence of spermatheca while controlling for rearing environment. If traits related to worker reproduction are expressed to a greater degree when a worker is derived from a Capensis male than from a Capensis queen, then this would indicate that Capensis males epigenetically modify the genome of their spermatozoa to enhance the reproductive capacities of their worker offspring.
2. Material and methods
We used instrumental insemination [54] to produce reciprocal crosses between a Capensis colony and a Scutellata colony. The Scutellata parent was obtained from Douglas (29.05° S, 23.77° E), and transported to Stellenbosch (33.92° S, 18.86° E). The Capensis colony originated from Stellenbosch. We grafted queen cells from the two parent colonies and reared queens of the two parents in another Scutellata cell building colony using methods described in [54]. In each case, we inseminated the daughter queens from each colony with a single male from the other colony [54]. We successfully obtained progeny from three Scutellata queens mated to a Capensis male and four Capensis queens mated to a Scutellata male.
To standardize rearing conditions, we selected an unrelated Scutellata and an unrelated Capensis host colony to rear eggs from the seven offspring queens. We removed a brood comb from the seven offspring colonies and cut an approximately 10 × 5 cm section containing only eggs from each comb. We cut similar-sized sections from a brood comb in the host colonies and pushed the sections containing eggs of the offspring queens into the host comb. The sections were positioned haphazardly in the host comb so that there were no systematic position effects. We returned the patchwork combs to the host colonies.
We removed the patchwork combs from the host colonies 16 days after the introduction of the eggs. We cut out the previously inserted sections, now containing sealed pupae, and placed each section into an individual wooden cage. We placed the cages in an incubator at 34.5°C. Over the next 3 days, we harvested newly emerged workers three times per day until we had up to 100 workers per offspring colony. Because Capensis and Scutellata workers take more than 20 days to develop from egg to adult [55], we were confident that no emerged workers were daughters of the host queen. In any case, hybrid workers are readily distinguishable from either of the parental types based on cuticle colour. We did not see any evidence of host-colony-queen-derived workers.
We transferred the newly emerged workers to preweighed labelled 0.5 ml microcentrifuge tubes, weighed them to the nearest milligram and froze them. Later, we dissected the workers, counted the number of ovarioles in the left ovary and determined the presence or absence of a spermatheca [49]. If we failed to locate the left ovary, we substituted it with the right ovary.
We repeated the experiment with a new Scutellata and a new Capensis host colony.
(a). Statistics
We analysed our continuous data (weight and number of ovarioles) using GLM with fixed effects. We examined the effects of cross direction (CD), trial (T), host colony subspecies (H) and colony of origin (i.e. the seven source colonies) nested within cross direction as main effects, the interaction of CD × H, and where appropriate, the interaction of T × CD. We tested the homogeneity of error variances between groups using Levene's tests but no significant violations were found. All means are least-square means derived from the GLM models.
For the discrete spermathecal data, we used contingency tables to determine whether the direction of the cross affected the probability that a worker would have a spermatheca.
3. Results
In the second trial, the Capensis rearing colony failed to rear brood. Therefore, our data are analysed either without a replicate Capensis colony or solely within the two Scutellata colonies.
(a). Emergence weights
The source queen, host colony and trial all had a significant effect on emergence weight, but not the direction of the cross (table 1). There was a significant host by cross direction interaction (table 1).
Table 1.
Effects of reciprocal crosses between A. m. capensis and A. m. scutellata and the rearing colony on the emergence weight and number of ovarioles of F1 workers.
| source of variation | emergence weight |
no. ovarioles |
||||||
|---|---|---|---|---|---|---|---|---|
| d.f. | mean square | F | p | d.f. | mean square | F | p | |
| trial (T) | 1 | 730.41 | 1446.12 | <0.001 | 1 | 225.23 | 15.15 | <0.001 |
| cross direction (CD) | 1 | 0.039 | 0.076 | 0.78 | 1 | 554.29 | 37.27 | <0.001 |
| host (H) | 1 | 62.83 | 124.39 | <0.001 | 1 | 262.47 | 17.65 | <0.001 |
| source colony (cross direction)a | 4 | 7.79 | 15.42 | <0.001 | 4 | 184.67 | 12.42 | <0.001 |
| T × CD | 1 | 0.56 | 1.11 | 0.29 | 1 | 1.48 | 0.10 | 0.75 |
| CD × H | 1 | 12.91 | 25.56 | <0.001 | 1 | 474.31 | 31.90 | <0.001 |
| error | 1380 | 0.50 | 1352 | 14.87 | ||||
aSource colony nested in cross direction.
When we analysed our data derived from the two Scutellata host colonies only, thus excluding the individuals reared by the Capensis host colony, the effect of cross direction was highly significant (figure 1, F1,1074 = 34.48, p < 0.001) and consistent across the two trials (CD × T interaction F1,1074 = 8.94, p = 0.17). Daughters of the Scutellata queens mated to Capensis males were heavier than the daughters of Capensis queens mated to Scutellata males (figure 1).
Figure 1.
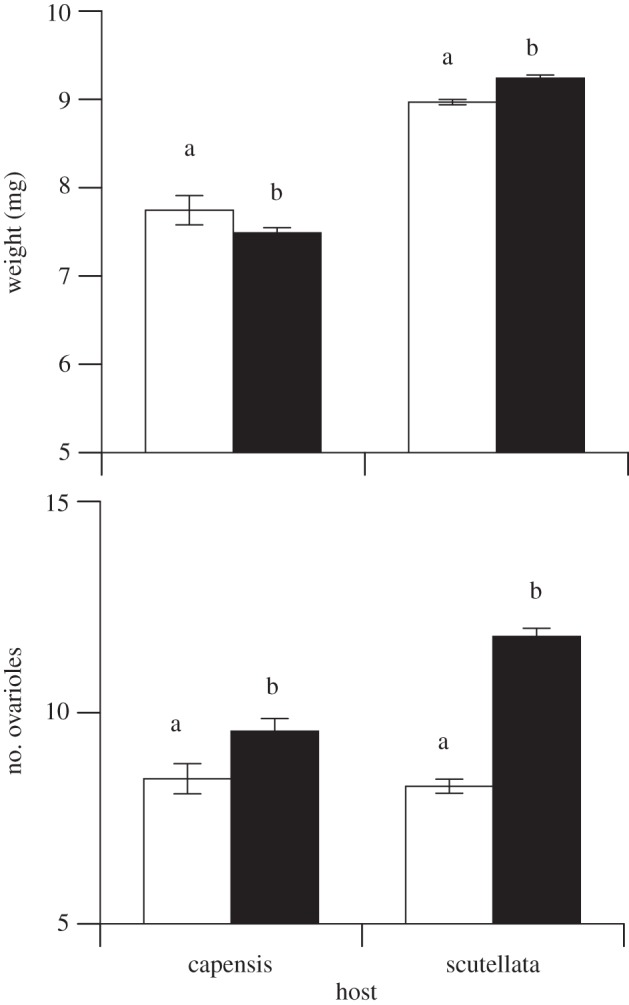
Reciprocal effects on emergence weight and ovariole number in crosses between A. m. scutellata queens inseminated with a single A. m. capensis male (sxc, black bars) and A. m. capensis queens inseminated with an A. m. scutellata male (cxs, white bars). Eggs from two to four queens per cross were reared in different A. m. scutellata (Scutellata) and A. m. capensis (Capensis) host colonies. Error bars are standard errors of the means. Within a column, bars with a different letter are significantly different (5% least significant difference).
When we analysed the individuals reared in the Capensis host colony alone, there was a highly significant effect of cross direction (F1,303 = 7.8, p = 0.006). However, in this case workers with a Capensis mother were slightly heavier than workers with a Scutellata mother (figure 1).
(b). Number of ovarioles
The direction of the cross had a highly significant effect on the number of ovarioles, especially when the bees were reared in Scutellata colonies (table 1). In the Scutellata host colonies, workers with a Capensis father had a significantly greater number of ovarioles than genotypically similar workers with a Scutellata father (figure 1). The seven individual source colonies also contributed significantly to variation to ovariole number (table 1).
When we excluded the bees reared in the Capensis host colony, the effect of cross direction remained highly significant (F1,1050 = 190.94, p < 0.001). Workers with a Capensis father and Scutellata mother had 30% more ovarioles than workers with a Scutellata father and Capensis mother (figure 1). Although the effect of the two trials was also highly significant (F1,1050 = 19.37, p < 0.001), the effect of the direction of the cross was consistent across the two trials (trial × cross interaction, F1,1050 = 0.065, p = 0.80).
The strong reciprocal effect was also evident in the workers reared in the Capensis colony (F1,299 = 7.64, p = 0.016), and again workers with a Capensis father had more ovarioles than workers with a Scutellata father (figure 1).
(c). Presence of a spermatheca
Only one of the 308 workers reared by the Capensis host colony developed a spermatheca. This worker was a daughter of a Capensis queen inseminated with a Scutellata drone.
By contrast, in the Scutellata host colonies, 12.0% of workers developed a spermatheca in trial 1 and 56.9% in trial 2 (figure 2). In both trial 1 (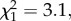 p = 0.08) and trial 2 (
p = 0.08) and trial 2 (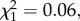 p = 0.80), there was no significant effect of the direction of the cross on the proportion of workers that had a spermatheca, though this effect was significant when the two trials were combined (
p = 0.80), there was no significant effect of the direction of the cross on the proportion of workers that had a spermatheca, though this effect was significant when the two trials were combined (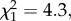 p = 0.04).
p = 0.04).
Figure 2.
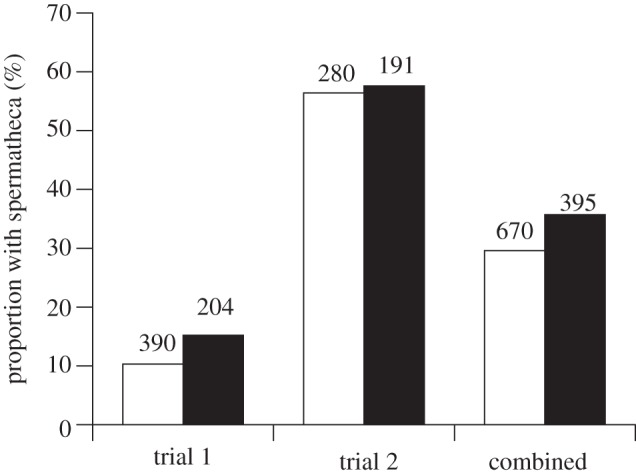
The effect of paternity on the presence of a spermatheca in reciprocal crosses between A. m. scutellata (cxs, white bars) and A. m. capensis (sxc, black bars) reared in two unrelated A. m. scutellata colonies. Numbers above the bars are the sample size.
4. Discussion
This study strongly suggests that genes involved in determination of ovariole number are affected by the parent-of-origin. Workers with a Capensis father consistently had a greater number of ovarioles than workers with a Scutellata father, irrespective of the subspecies of the host colony that reared them. Strong and consistent reciprocal effects suggest that Capensis parents make epigenetic modifications to their gametes, thereby altering the phenotype of their offspring. These modifications are sex (queen or drone) dependent, so that the direction of the cross alters the phenotype of their worker offspring.
In our crosses, the queen genotype and the drone genotype are confounded: Scutellata queens were mated to Capensis drones and Capensis queens to Scutellata drones. Thus, it is not possible to determine whether the parent-of-origin effects originated in the queen or drone or both. That is we cannot definitively say whether patrigenes increase ovariole number or whether matrigenes or maternally derived cytoplasmic factors decrease it. However, we have previously generated Capensis × Scutellata hybrid queens and backcrossed these to either Scutellata or Capensis males [49]. In that experiment, we showed that workers derived from a backcross to a Capensis father had a greater number of ovarioles than workers derived from a backcross to a Scutellata father. However, because these crosses involved variable numbers of multiple fathers and because the workers that we scored were reared in their own colony, it was not possible to determine whether the differences were owing to additive genetic effects, rearing environment effects or epigenetic effects. In combination, our two studies strongly suggest that Capensis males make epigenetic modifications to their spermatozoa that increase the number of ovarioles in their worker offspring. Nonetheless, we cannot exclude the possibility that it is Capensis queens that make epigenetic modifications to eggs, or transmit a cytoplasmic factor that reduces the number of ovarioles in their worker offspring. In either case, our finding of strong parent-of-origin effects on ovary size is robust.
That drones and/or queens might make epigenetic modifications that increase ovariole number in their worker offspring when the father is of the Capensis subspecies is consistent with predictions of the kinship theory of genomic imprinting as it applies to social insects [1,8,9,29,30,56]. A queen honeybee mates with 10–20 males, storing the semen in her spermatheca, and using the spermatozoa throughout her life [11]. Thus, especially in a population where workers can be thelytokous, a drone can increase his relative fitness over other competing males if he can increase the reproductive success of the workers he sires. This provides the selective advantage that makes the evolution of a germline imprinting system to increase the number of ovarioles particularly likely for Capensis males. We also note Queller's prediction [8] that in polyandrous social insects paternal imprinting is more likely to evolve than maternal imprinting. This prediction is consistent with the hypothesis that it is Capensis males that imprint genes that increase ovariole number.
The genetic architecture underlying emergence weights and the presence of a spermatheca is more complex and we find little evidence for epigenetic inheritance of these traits. Overall, there was little or no significant parent-of-origin effect for weight or spermathecal presence. These traits are more affected by the genotype of the rearing colony and the trial than the direction of the cross. This phenomenon has been observed previously. Allsopp et al. [53] showed that when Capensis larvae are reared by Scutellata workers, the resulting offspring are much heavier than when they are reared by Capensis workers. This result was interpreted as evidence that Capensis worker larvae solicit food more strongly than Scutellata worker larvae, and are thus able to grow larger [49,53]. Importantly, these larger workers had no more ovarioles than the smaller Capensis workers raised in a colony of their own subspecies. This suggests that variation in worker weight and spermathecal development is mostly influenced by rearing environment and additive genetic effects. By contrast, ovariole number is mostly influenced by epigenetic and additive effects.
If a male is imprinting a gene so that it enhances the reproductive success of his worker offspring, and this enhancement is to the detriment of genes inherited maternally, modifiers are predicted to evolve that reduce the effect of the paternal imprinting [8,57]. Thus, within a subspecies, an equilibrium between paternal and maternal epigenetic modifications is expected to evolve in which neither sex is greatly disadvantaged [29,58]. Such coevolved associations between paternal and maternal modifications to genes are disrupted in crosses between subspecies. In the workers studied here, all genes derived from males were of either the Capensis or the Scutellata subspecies, and therefore potentially epigenetically incompatible with the matrigenes from the other subspecies. Thus, interspecific crosses can reveal epigenetically derived parent-of-origin effects, which may not be apparent within a subspecies. That is, interspecific crosses provide a window that is more likely to reveal epigenetic effects than crosses with more closely related individuals.
Our data suggest that Capensis host workers favoured larvae that had been laid by a Capensis queen: workers with a Capensis mother were slightly heavier than workers with a Scutellata mother despite their identical genotype. This may indicate that some of the factors that cause Capensis larvae to be fed more than Scutellata larvae [52,53] are either maternally imprinted or cytoplasmically inherited. This effect might have been revealed more decisively had our second Capensis host colony reared its brood and warrants further investigation.
Our study provides the first phenotypic evidence of parent-of-origin effects that influence worker reproduction in a social insect. The reproductive success of a male Capensis is enormously increased if one of his daughter workers is successful in becoming the thelytokous mother of a new queen. Thus, the benefits of male competition are substantially greater in thelytokous Capensis than in arrhenotokous Scutellata, and plausibly drove the evolution of imprinting of genes that influence ovary size in Capensis. Nonetheless, although the selective advantage of parental (and particularly paternal) imprinting is likely to be greater in a thelytokous social insect species than that in an arrhenotokous species, males of an arrhenotokous species are still advantaged if their daughter workers produce male offspring or are more likely to become a new queen than the daughters of other males. Importantly, there is strong evidence of a genetic (and possibly epigenetic) component to the probability that a female larva will be reared as a queen in both honeybees [59,60] and leafcutter ants [61]. This variance in reproductive success among female subfamilies provides a plausible route to the evolution of paternal imprinting in social insects [8,30,56]. While parent-of-origin effects may be more subtle in arrhenotokous species than in thelytokous subspecies like Capensis, our demonstration of reciprocal effects in Capensis argues that we should not ignore the possibility of parental imprinting in the more typical arrhenotokous species. The use of reciprocal crosses between widely diverged subspecies may be helpful in disrupting coevolved gene complexes that may mask parent-of-origin effects within a subspecies.
Acknowledgements
We thank Theunis Engelbrecht for Scutellata colonies and Christian Fransman for field assistance.
Data accessibility
Data supporting this paper are available as the electronic supplementary material.
Funding statement
This work was funded by Australian Research Council grants to B.P.O. and M.B. and by the University of Sydney sabbatical leave programme.
References
- 1.Haig D. 2000. The kinship theory of genomic imprinting. Annu. Rev. Ecol. Syst. 31, 9–32 (doi:10.1146/annurev.ecolsys.31.1.9) [Google Scholar]
- 2.Haig D. 1999. Multiple paternity and genomic imprinting. Genetics 151, 1229–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker GA. 1984. Sperm competition and the evolution of animal mating strategies. In Sperm competition and the evolution of animal mating systems (ed. Smith RL.), pp. 1–60 Orlando, FL: Academic Press [Google Scholar]
- 4.Haig D, Graham C. 1991. Genomic imprinting and the strange case of the insulin-like growth factor II receptor. Cell 64, 1045–1046 (doi:10.1016/0092-8674(91)90256-X) [DOI] [PubMed] [Google Scholar]
- 5.Rappolee DA, Sturm KS, Behrendtsen O, Schultz GA, Pedersen RA, Werb Z. 1992. Insulin-like growth factor-II acts through an endogenous growth pathway regulated by imprinting in early mouse embryos. Genes Dev. 8, 939–952 (doi:10.1101/gad.6.6.939) [DOI] [PubMed] [Google Scholar]
- 6.Williams C, et al. 2012. An exon splice enhancer primes IGF2:IGF2R binding site structure and function evolution. Science 338, 1209–1213 (doi:10.1126/science.1228633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garfield AS, et al. 2011. Distinct physiological and behavioural functions for parental alleles of imprinted Grb10. Nature 469, 534–538 (doi:10.1038/nature09651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Queller DC. 2003. Theory of genomic imprinting conflict in social insects. BMC Evol. Biol. 3, 15 (doi:10.1186/1471-2148-3-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kronauer DJC. 2008. Genomic imprinting and kinship in the social Hymenoptera: what are the predictions? J. Theor. Biol. 254, 737–740 (doi:10.1016/j.jtbi.2008.06.019) [DOI] [PubMed] [Google Scholar]
- 10.Haig D. 1992. Intragenomic conflict and the evolution of eusociality. J. Theor. Biol. 156, 401–403 (doi:10.1016/S0022-5193(05)80683-6) [DOI] [PubMed] [Google Scholar]
- 11.Winston ML. 1987. The biology of the honey bee, p. 281 Cambridge, UK: Harvard University Press [Google Scholar]
- 12.Hölldobler B, Wilson EO. 1990. The ants, p. 732 Berlin, Germany: Springer [Google Scholar]
- 13.Hughes WOH, Oldroyd BP, Beekman M, Ratnieks FLW. 2008. Ancestral monogamy shows kin selection is the key to the evolution of eusociality. Science 320, 1213–1216 (doi:10.1126/science.1156108) [DOI] [PubMed] [Google Scholar]
- 14.Palmer KA, Oldroyd BP. 2000. Evolution of multiple mating in the genus Apis. Apidologie 31, 235–248 (doi:10.1051/apido:2000119) [Google Scholar]
- 15.Kronauer DJC, Schoning C, Pedersen JA, Boomsma JJ, Gadau J. 2004. Extreme queen mating frequency and colony fission in African army ants. Mol. Ecol. 13, 2381–2388 (doi:10.1111/j.1365-294X.2004.02262.x) [DOI] [PubMed] [Google Scholar]
- 16.Walin L, Sundstrom L, Seppa P, Rosengren R. 1998. Worker reproduction in ants: a genetic analysis. Heredity 81, 604–612 (doi:10.1046/j.1365-2540.1998.00434.x) [Google Scholar]
- 17.Barron AB, Oldroyd BP, Ratnieks FLW. 2001. Worker reproduction in honey-bees (Apis) and the anarchic syndrome: a review. Behav. Ecol. Sociobiol. 50, 199–208 (doi:10.1007/s002650100362) [Google Scholar]
- 18.Beekman M, Oldroyd BP. 2008. When workers disunite: intraspecific parasitism in eusocial bees. Annu. Rev. Entomol. 53, 19–37 (doi:10.1146/annurev.ento.53.103106.093515) [DOI] [PubMed] [Google Scholar]
- 19.Oldroyd BP, Smolenski AJ, Cornuet J-M, Crozier RH. 1994. Anarchy in the beehive. Nature 371, 749 (doi:10.1038/371749a0) [Google Scholar]
- 20.Châline N, Ratnieks FLW, Bourke T. 2002. Anarchy in the UK: detailed genetic analysis of worker reproduction in a naturally-occurring British anarchistic honeybee, Apis mellifera, colony using DNA microsatellites. Mol. Ecol. 11, 1795–1803 (doi:10.1046/j.1365-294X.2000.01569.x) [DOI] [PubMed] [Google Scholar]
- 21.Montague CE, Oldroyd BP. 1998. The evolution of worker sterility in honey bees: an investigation into a behavioral mutant causing a failure of worker policing. Evolution 52, 1408–1415 (doi:10.2307/2411310) [DOI] [PubMed] [Google Scholar]
- 22.Holmes MJ, Oldroyd BP, Duncan M, Allsopp MH, Beekman M. 2013. Cheaters sometimes prosper: targeted worker reproduction in honeybee (Apis mellifera) colonies during swarming. Mol. Ecol. 22, 4298–4306 (doi:10.1111/mec.12387) [DOI] [PubMed] [Google Scholar]
- 23.Drumond PM, Oldroyd BP, Osborne KE. 2000. Worker reproduction in the Australian stingless bee Austroplebeia australis (Hymenoptera: Apidae, Meliponini). Ins. Soc. 47, 333–336 (doi:10.1007/PL00001725) [Google Scholar]
- 24.Imperatriz-Fonseca VL, Kleinert ADP. 1998. Worker reproduction in the stingless bee species Friesella schrottkyi (Hymenoptera: Apidae: Meliponinae). Entomol. Gen. 23, 169–175 [Google Scholar]
- 25.Foster KR, Ratnieks FLW, Gyllenstrand N, Thoren PA. 2001. Colony kin structure and male production in Dolichovespula wasps. Mol. Ecol. 10, 1003–1010 (doi:10.1046/j.1365-294X.2001.01228.x) [DOI] [PubMed] [Google Scholar]
- 26.Foster KR, Ratnieks FLW. 2001. Paternity, reproduction and conflict in vespine wasps: a model system for testing kin selection predictions. Behav. Ecol. Sociobiol. 50, 1–8 (doi:10.1007/s002650100336) [Google Scholar]
- 27.Heinze J, Stratz M, Pedersen JS, Haberl M. 2000. Microsatellite analysis suggests occasional worker reproduction in the monogynous ant Crematogaster smithi. Ins. Soc. 47, 299–301 (doi:10.1007/PL00001719) [Google Scholar]
- 28.Choe JC. 1988. Worker reproduction and socal evolution in ants. In Advances in myrmecology (ed. Trager JC.), pp. 163–187 New York, NY: E.J. Brill [Google Scholar]
- 29.Burt A, Trivers R. 2006. Genes in conflict: the biology of selfish genetic elements, p. 623 Cambridge, UK: Harvard University Press [Google Scholar]
- 30.Drewell RA, Lo N, Oxley PR, Oldroyd BP. 2012. Kin conflict in insect societies: a new epigenetic perspective. Trends Ecol. Evol. 27, 367–373 (doi:10.1016/j.tree.2012.02.005) [DOI] [PubMed] [Google Scholar]
- 31.Laird PW. 2010. Principles and challenges of genome-wide DNA methylation analysis. Nat. Rev. Genet. 11, 191–203 (doi:10.1038/nrg2732) [DOI] [PubMed] [Google Scholar]
- 32.Lyko F, Foret S, Kucharski R, Wolf S, Falckenhayn C, Maleszka R. 2010. The honey bee epigegenomes: differential methylation of brain DNA in queens and workers. PLoS Biol. 8, e1000506 (doi:10.1371/journal.pbio.1000506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Jorda M, Jones PL, Maleszka R, Ling X, Robertson HM, Mizzen CA, Peinado MA, Robinson GE. 2006. Functional CpG methylation system in a social insect. Science 314, 645–647 (doi:10.1126/science.1135213) [DOI] [PubMed] [Google Scholar]
- 34.Kucharski R, Maleszka J, Foret S, Maleszka R. 2008. Nutritional control of reproductive status in honeybees via DNA methylation. Science 319, 1827–1830 (doi:10.1126/science.1153069) [DOI] [PubMed] [Google Scholar]
- 35.Weiner SA, Galbraith DA, Adams DC, Valenzuela N, Noll FD, Grozinger CM, Toth AL. 2013. A survey of DNA methlyation across social insect species, life stages and castes reveals abundant caste-associated methylation in a primitively eusocial wasp. Naturwissenschaften 100, 795–799 (doi:10.1007/s00114-013-1064-z) [DOI] [PubMed] [Google Scholar]
- 36.Beekman M, Allsopp M, Wossler TC, Oldroyd BP. 2008. Factors affecting the dynamics of the honey bee (Apis mellifera) hybrid zone of South Africa. Heredity 100, 13–18 (doi:10.1038/sj.hdy.6801058) [DOI] [PubMed] [Google Scholar]
- 37.Ruttner F. 1988. Biogeography and taxonomy of honeybees, p. 284 Berlin, Germany: Springer [Google Scholar]
- 38.Hepburn HR, Radloff SE, Fuchs S. 1998. Population structure and the interface between Apis mellifera capensis and Apis mellifera scutellata. Apidologie 29, 333–346 (doi:10.1051/apido:19980404) [Google Scholar]
- 39.Anderson RH. 1963. The laying worker in the Cape honeybee Apis mellifera capensis. J. Apic. Res. 2, 85–92 [Google Scholar]
- 40.Onions GW. 1912. South African ‘fertile worker bees’. Ag. J. Union S. Afr. 1, 720–728 [Google Scholar]
- 41.Beekman M, Good G, Allsopp MH, Radloff S, Pirk CWW, Ratnieks FLW. 2002. A non-policing honey bee colony (Apis mellifera capensis). Naturwissenschaften 89, 479–482 (doi:10.1007/s00114-002-0365) [DOI] [PubMed] [Google Scholar]
- 42.Moritz RFA, Kryger P, Allsopp MH. 1996. Competition for royalty in bees. Nature 384, 31 (doi:10.1038/384031a0) [Google Scholar]
- 43.Greeff JM. 1996. Effects of thelytokous worker reproduction on kin-selection and conflict in the Cape honeybee, Apis mellifera capensis. Phil. Trans. R. Soc. Lond. B 351, 617–625 (doi:10.1098/rstb.1996.0060) [Google Scholar]
- 44.Jordan LA, Allsopp MH, Oldroyd BP, Wossler TC, Beekman M. 2008. Cheating honeybee workers produce royal offspring. Proc. R. Soc. B 275, 345–351 (doi:10.1098/rspb.2007.1422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holmes MJ, Oldroyd BP, Allsopp MH, Lim J, Wossler TC, Beekman M. 2010. Maternity of emergency queens in the Cape honey bee, Apis mellifera capensis. Mol. Ecol. 19, 2792–2799 (doi:10.1111/j.1365-294X.2010.04683.x) [DOI] [PubMed] [Google Scholar]
- 46.Moritz RFA, Lattorff HMG, Crous KL, Hepburn RH. 2011. Social parasitism of queens and workers in the Cape honeybee (Apis mellifera capensis). Behav. Ecol. Sociobiol. 65, 735–740 (doi:10.1007/s00265-010-1077-y) [Google Scholar]
- 47.Ruttner F, Hesse B. 1981. Specific differences in the development of ovaries and egg-laying of queenless workers of several races of the honeybee Apis mellifera L. Apidologie 12, 159–183 (doi:10.1051/apido:19810206) [Google Scholar]
- 48.Phiancharoen M, Pirk CWW, Radloff SE, Hepburn R. 2009. Clinal nature of the frequencies of ovarioles and spermathecae in cape worker honeybees, Apis mellifera capensis. Apidologie 41, 129–134 (doi:10.1051/apido/2009054) [Google Scholar]
- 49.Jordan LA, Allsopp M, Beekman M, Wossler TC, Oldroyd BP. 2008. Inheritance of traits associated with reproductive potential in Apis mellifera capensis and A. m. scutellata workers. J. Hered. 99, 376–381 (doi:10.1093/jhered/esn023) [DOI] [PubMed] [Google Scholar]
- 50.Goudie F, Allsopp MH, Beekman M, Lim J, Oldroyd BP. 2012. Heritability of worker ovariole number in the Cape honey bee Apis mellifera capensis. Ins. Soc. 59, 351–359 (doi:10.1007/s00040-012-0227-9) [Google Scholar]
- 51.Beekman M, Allsopp MH, Holmes MJ, Lim J, Noach-Pienaar L-A, Wossler TC, Oldroyd BP. 2012. Racial mixing in South African honeybees: the effects of genotype mixing on reproductive traits of workers. Behav. Ecol. Sociobiol. 66, 897–904 (doi:10.1007/s00265-012-1338-z) [Google Scholar]
- 52.Calis JNM, Boot WJ, Allsopp MH, Beekman M. 2002. Getting more than a fair share: nutrition of worker larvae related to social parasitism in the Cape honey bee Apis mellifera capensis. Apidologie 33, 193–202 (doi:10.1051/apido:2002009) [Google Scholar]
- 53.Allsopp MH, Calis JNM, Boot WJ. 2003. Differential feeding of worker larvae affects caste characters in the Cape honeybee, Apis mellifera capensis. Behav. Ecol. Sociobiol. 54, 555–561 (doi:10.1007/s00265-003-0666-4) [Google Scholar]
- 54.Harbo JR. 1986. Propagation and instrumental insemination. In Bee genetics and breeding (ed. Rinderer TE.), pp. 361–389 Orlando, FL: Academic Press [Google Scholar]
- 55.Tribe GD, Allsopp MH. 2001. Life history of the honeybee colony. In Beekeeping in South Africa (ed. Johannsmeier MF.), pp. 17–26 Pretoria, South Africa: Agricultural Research Council [Google Scholar]
- 56.Dobata S, Tsuji K. 2012. Intragenomic conflict over queen determination favours genomic imprinting is eusocial Hymenoptera. Proc. R. Soc. B 279, 2553–2560 (doi:10.1098/rspb.2011.2673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilkins JF, Haig D. 2003. What good is genomic imprinting: the function of parent-specific gene expression. Nat. Rev. Genet. 4, 1–10 (doi:10.1038/nrg1062) [DOI] [PubMed] [Google Scholar]
- 58.Burt A, Trivers R. 1998. Genetic conflicts in genomic imprinting. Proc. R. Soc. Lond. B 265, 2393–2397 (doi:10.1098/rspb.1998.0589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tilley CA, Oldroyd BP. 1997. Unequal representation of subfamilies among queen and worker brood of queenless honey bee (Apis mellifera) colonies. Anim. Behav. 54, 1483–1490 (doi:10.1006/anbe.1997.0546) [DOI] [PubMed] [Google Scholar]
- 60.Châline N, Arnold G, Papin C, Ratnieks FLW. 2003. Patriline differences in emergency queen rearing in the honey bee Apis mellifera. Ins. Soc. 50, 234–236 (doi:10.1007/s00040-003-0664-6) [Google Scholar]
- 61.Hughes WOH, Boomsma JJ. 2008. Genetic royal cheats in leaf-cutting ant societies. Proc. Natl Acad. Sci. USA 105, 5150–5153 (doi:10.1073pnas.0710262105) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting this paper are available as the electronic supplementary material.


