Abstract
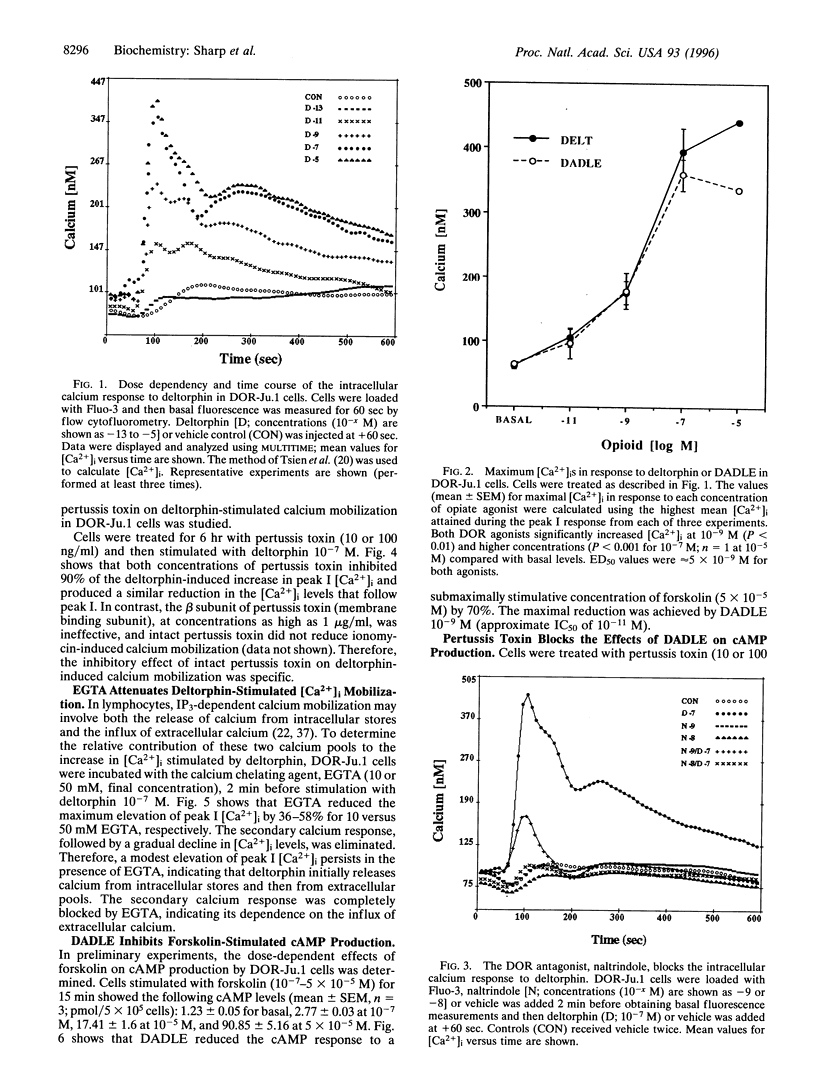
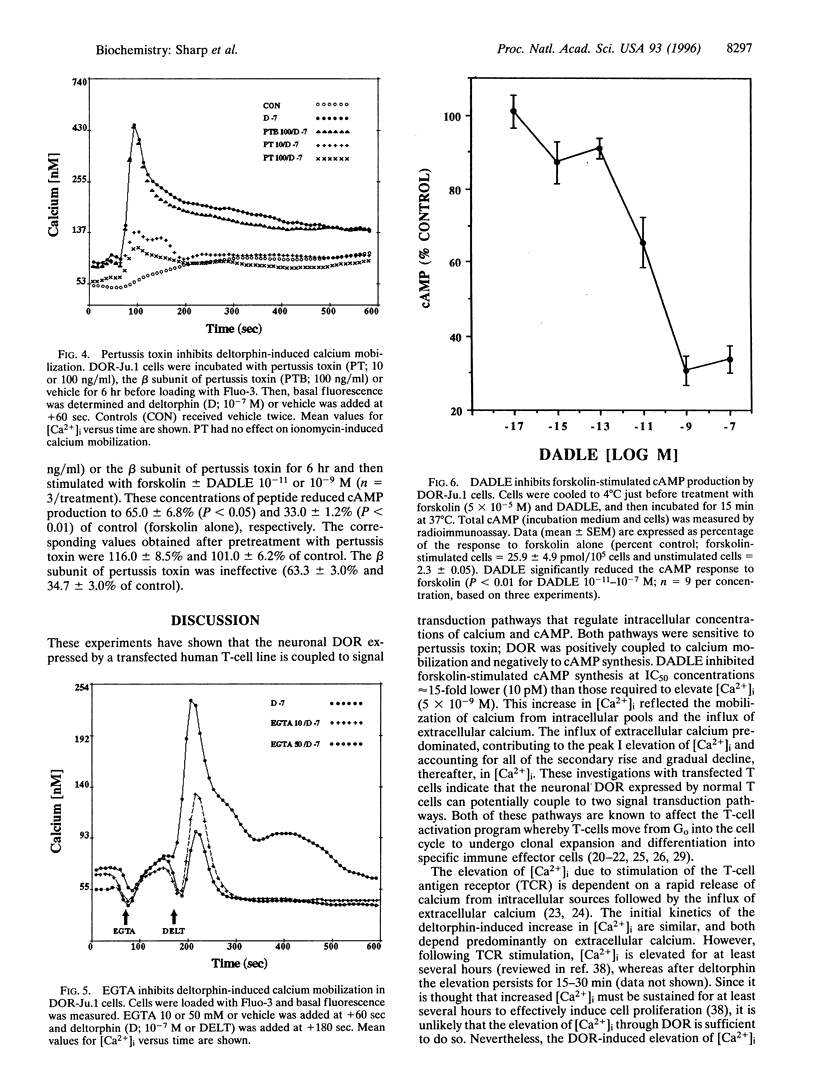
Opiates are known to function as immunomodulators, in part by effects on T cells. However, the signal transduction pathways mediating the effects of opiates on T cells are largely undefined. To determine whether pathways that regulate free intracellular calcium ([Ca2+]i) and/or cAMP are affected by opiates acting through delta-type opioid receptors (DORs), a cDNA encoding the neuronal DOR was expressed in a stably transfected Jurkat T-cell line. The DOR agonists, deltorphin and [D-Ala2, D-Leu5]-enkephalin (DADLE), elevated [Ca2+]i, measured by flow cytofluorometry using the calcium-sensitive dye, Fluo-3. At concentrations from 10(-11)-10(-7) M, both agonists increased [Ca2+]i from 60 nM to peak concentrations of 400 nM in a dose-dependent manner within 30 sec (ED50 of approximately 5 x 10(-9) M). Naltrindole, a selective DOR antagonist, abolished the increase in [Ca2+]i, and pretreatment with pertussis toxin was also effective. To assess the role of extracellular calcium, cells were pretreated with EGTA, which reduced the initial deltorphin-induced elevation of [Ca2+]i by more than 50% and eliminated the second phase of calcium mobilization. Additionally, the effect of DADLE on forskolin-stimulated cAMP production was determined. DADLE reduced cAMP production by 70% (IC50 of approximately equal to 10(-11) M), and pertussis toxin inhibited the action of DADLE. Thus, the DOR expressed by a transfected Jurkat T-cell line is positively coupled to pathways leading to calcium mobilization and negatively coupled to adenylate cyclase. These studies identify two pertussis toxin-sensitive, G protein-mediated signaling pathways through which DOR agonists regulate the levels of intracellular messengers that modulate T-cell activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcover A., Weiss M. J., Daley J. F., Reinherz E. L. The T11 glycoprotein is functionally linked to a calcium channel in precursor and mature T-lineage cells. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2614–2618. doi: 10.1073/pnas.83.8.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine J. A., Secrist J. P., Daniels J. K., Leibson P. J., Abraham R. T. Signal transduction through the T cell antigen receptor. Activation of phospholipase C through a G protein-independent coupling mechanism. J Immunol. 1991 May 1;146(9):2889–2897. [PubMed] [Google Scholar]
- Bacon K. B., Premack B. A., Gardner P., Schall T. J. Activation of dual T cell signaling pathways by the chemokine RANTES. Science. 1995 Sep 22;269(5231):1727–1730. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- Barinaga M. Pot, heroin unlock new areas for neuroscience. Science. 1992 Dec 18;258(5090):1882–1884. doi: 10.1126/science.1335165. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989 Jul 13;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Camps M., Carozzi A., Schnabel P., Scheer A., Parker P. J., Gierschik P. Isozyme-selective stimulation of phospholipase C-beta 2 by G protein beta gamma-subunits. Nature. 1992 Dec 17;360(6405):684–686. doi: 10.1038/360684a0. [DOI] [PubMed] [Google Scholar]
- Chang M. M., Leeman S. E. Isolation of a sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem. 1970 Sep 25;245(18):4784–4790. [PubMed] [Google Scholar]
- Childers S. R. Opioid receptor-coupled second messenger systems. Life Sci. 1991;48(21):1991–2003. doi: 10.1016/0024-3205(91)90154-4. [DOI] [PubMed] [Google Scholar]
- Chouaib S., Welte K., Mertelsmann R., Dupont B. Prostaglandin E2 acts at two distinct pathways of T lymphocyte activation: inhibition of interleukin 2 production and down-regulation of transferrin receptor expression. J Immunol. 1985 Aug;135(2):1172–1179. [PubMed] [Google Scholar]
- Chuang L. F., Chuang T. K., Killam K. F., Jr, Chuang A. J., Kung H. F., Yu L., Chuang R. Y. Delta opioid receptor gene expression in lymphocytes. Biochem Biophys Res Commun. 1994 Aug 15;202(3):1291–1299. doi: 10.1006/bbrc.1994.2071. [DOI] [PubMed] [Google Scholar]
- Chuang T. K., Killam K. F., Jr, Chuang L. F., Kung H. F., Sheng W. S., Chao C. C., Yu L., Chuang R. Y. Mu opioid receptor gene expression in immune cells. Biochem Biophys Res Commun. 1995 Nov 22;216(3):922–930. doi: 10.1006/bbrc.1995.2709. [DOI] [PubMed] [Google Scholar]
- Clarke B. L., Bost K. L. Differential expression of functional adrenocorticotropic hormone receptors by subpopulations of lymphocytes. J Immunol. 1989 Jul 15;143(2):464–469. [PubMed] [Google Scholar]
- Cook S. J., McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993 Nov 12;262(5136):1069–1072. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- Cotecchia S., Kobilka B. K., Daniel K. W., Nolan R. D., Lapetina E. Y., Caron M. G., Lefkowitz R. J., Regan J. W. Multiple second messenger pathways of alpha-adrenergic receptor subtypes expressed in eukaryotic cells. J Biol Chem. 1990 Jan 5;265(1):63–69. [PubMed] [Google Scholar]
- Fargin A., Raymond J. R., Regan J. W., Cotecchia S., Lefkowitz R. J., Caron M. G. Effector coupling mechanisms of the cloned 5-HT1A receptor. J Biol Chem. 1989 Sep 5;264(25):14848–14852. [PubMed] [Google Scholar]
- Felten D. L., Felten S. Y., Bellinger D. L., Carlson S. L., Ackerman K. D., Madden K. S., Olschowki J. A., Livnat S. Noradrenergic sympathetic neural interactions with the immune system: structure and function. Immunol Rev. 1987 Dec;100:225–260. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- Fowler C. J., Fraser G. L. Mu-, delta-, kappa-opioid receptors and their subtypes. A critical review with emphasis on radioligand binding experiments. Neurochem Int. 1994 May;24(5):401–426. doi: 10.1016/0197-0186(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Gardner P. Calcium and T lymphocyte activation. Cell. 1989 Oct 6;59(1):15–20. doi: 10.1016/0092-8674(89)90865-9. [DOI] [PubMed] [Google Scholar]
- Gettys T. W., Okonogi K., Tarry W. C., Johnston J., Horton C., Taylor I. L. Examination of relative rates of cAMP synthesis and degradation in crude membranes of adipocytes treated with hormones. Second Messengers Phosphoproteins. 1990;13(1):37–49. [PubMed] [Google Scholar]
- Gilman S. C., Schwartz J. M., Milner R. J., Bloom F. E., Feldman J. D. beta-Endorphin enhances lymphocyte proliferative responses. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4226–4230. doi: 10.1073/pnas.79.13.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan L., Townsend R., Eisenstein T. K., Adler M. W., Rogers T. J. Both T cells and macrophages are targets of kappa-opioid-induced immunosuppression. Brain Behav Immun. 1994 Sep;8(3):229–240. doi: 10.1006/brbi.1994.1021. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Hazum E., Chang K. J., Cuatrecasas P. Specific nonopiate receptors for beta-endorphin. Science. 1979 Sep 7;205(4410):1033–1035. doi: 10.1126/science.224457. [DOI] [PubMed] [Google Scholar]
- Heagy W., Laurance M., Cohen E., Finberg R. Neurohormones regulate T cell function. J Exp Med. 1990 May 1;171(5):1625–1633. doi: 10.1084/jem.171.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heagy W., Shipp M. A., Finberg R. W. Opioid receptor agonists and Ca2+ modulation in human B cell lines. J Immunol. 1992 Dec 15;149(12):4074–4081. [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W., Lee N. M., Loh H. H., Thayer S. A. Dual excitatory and inhibitory effects of opioids on intracellular calcium in neuroblastoma x glioma hybrid NG108-15 cells. Mol Pharmacol. 1992 Dec;42(6):1083–1089. [PubMed] [Google Scholar]
- Johnson K. W., Davis B. H., Smith K. A. cAMP antagonizes interleukin 2-promoted T-cell cycle progression at a discrete point in early G1. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6072–6076. doi: 10.1073/pnas.85.16.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A., Wu D., Simon M. I. Subunits beta gamma of heterotrimeric G protein activate beta 2 isoform of phospholipase C. Nature. 1992 Dec 17;360(6405):686–689. doi: 10.1038/360686a0. [DOI] [PubMed] [Google Scholar]
- Kuno M., Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987 Mar 19;326(6110):301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., June C. H., Grosmaire L. S., Rabinovitch P. S. Crosslinking of surface antigens causes mobilization of intracellular ionized calcium in T lymphocytes. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1384–1388. doi: 10.1073/pnas.84.5.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A., Jacobson B., Miller R. A. Cyclic AMP concentrations modulate both calcium flux and hydrolysis of phosphatidylinositol phosphates in mouse T lymphocytes. J Immunol. 1988 Feb 1;140(3):936–940. [PubMed] [Google Scholar]
- Linner K. M., Beyer H. S., Sharp B. M. Induction of the messenger ribonucleic acid for proenkephalin A in cultured murine CD4-positive thymocytes. Endocrinology. 1991 Feb;128(2):717–724. doi: 10.1210/endo-128-2-717. [DOI] [PubMed] [Google Scholar]
- Linner K. M., Quist H. E., Sharp B. M. Met-enkephalin-containing peptides encoded by proenkephalin A mRNA expressed in activated murine thymocytes inhibit thymocyte proliferation. J Immunol. 1995 May 15;154(10):5049–5060. [PubMed] [Google Scholar]
- McKean D. J., Huntoon C., Bell M. Ligand-induced desensitization of interleukin 1 receptor-initiated intracellular signaling events in T helper lymphocytes. J Exp Med. 1994 Oct 1;180(4):1321–1328. doi: 10.1084/jem.180.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. Mechanisms of multifunctional signalling by G protein-linked receptors. Trends Pharmacol Sci. 1993 Jun;14(6):239–244. doi: 10.1016/0165-6147(93)90019-g. [DOI] [PubMed] [Google Scholar]
- Park D. J., Min H. K., Rhee S. G. Inhibition of CD3-linked phospholipase C by phorbol ester and by cAMP is associated with decreased phosphotyrosine and increased phosphoserine contents of PLC-gamma 1. J Biol Chem. 1992 Jan 25;267(3):1496–1501. [PubMed] [Google Scholar]
- Park D. J., Rho H. W., Rhee S. G. CD3 stimulation causes phosphorylation of phospholipase C-gamma 1 on serine and tyrosine residues in a human T-cell line. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5453–5456. doi: 10.1073/pnas.88.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit A., Gallo-Payet N., Bellabarba D., Lehoux J. G., Bélisle S. The modulation of placental lactogen release by opioids: a role for extracellular calcium. Mol Cell Endocrinol. 1993 Jan;90(2):165–170. doi: 10.1016/0303-7207(93)90148-d. [DOI] [PubMed] [Google Scholar]
- Portoghese P. S., Sultana M., Takemori A. E. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur J Pharmacol. 1988 Jan 27;146(1):185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- Pronin A. N., Gautam N. Interaction between G-protein beta and gamma subunit types is selective. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6220–6224. doi: 10.1073/pnas.89.13.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. G., Choi K. D. Multiple forms of phospholipase C isozymes and their activation mechanisms. Adv Second Messenger Phosphoprotein Res. 1992;26:35–61. [PubMed] [Google Scholar]
- Saravia F., Ase A., Aloyz R., Kleid M. C., Ines M., Vida R., Nahmod V. E., Vindrola O. Differential posttranslational processing of proenkephalin in rat bone marrow and spleen mononuclear cells: evidence for synenkephalin cleavage. Endocrinology. 1993 Apr;132(4):1431–1437. doi: 10.1210/endo.132.4.8462445. [DOI] [PubMed] [Google Scholar]
- Schall T. J., Bacon K., Toy K. J., Goeddel D. V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990 Oct 18;347(6294):669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- Shahabi N. A., Linner K. M., Sharp B. M. Murine splenocytes express a naloxone-insensitive binding site for beta-endorphin. Endocrinology. 1990 Mar;126(3):1442–1448. doi: 10.1210/endo-126-3-1442. [DOI] [PubMed] [Google Scholar]
- Shahabi N. A., Sharp B. M. Antiproliferative effects of delta opioids on highly purified CD4+ and CD8+ murine T cells. J Pharmacol Exp Ther. 1995 Jun;273(3):1105–1113. [PubMed] [Google Scholar]
- Stanisz A. M., Scicchitano R., Dazin P., Bienenstock J., Payan D. G. Distribution of substance P receptors on murine spleen and Peyer's patch T and B cells. J Immunol. 1987 Aug 1;139(3):749–754. [PubMed] [Google Scholar]
- Stiene-Martin A., Mattson M. P., Hauser K. F. Opiates selectively increase intracellular calcium in developing type-1 astrocytes: role of calcium in morphine-induced morphologic differentiation. Brain Res Dev Brain Res. 1993 Dec 17;76(2):189–196. doi: 10.1016/0165-3806(93)90207-q. [DOI] [PubMed] [Google Scholar]
- Taub D. D., Eisenstein T. K., Geller E. B., Adler M. W., Rogers T. J. Immunomodulatory activity of mu- and kappa-selective opioid agonists. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):360–364. doi: 10.1073/pnas.88.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig R., Iñiguez-Lluhi J. A., Gilman A. G. Inhibition of adenylyl cyclase by Gi alpha. Science. 1993 Jul 9;261(5118):218–221. doi: 10.1126/science.8327893. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- Tsunoo A., Yoshii M., Narahashi T. Block of calcium channels by enkephalin and somatostatin in neuroblastoma-glioma hybrid NG108-15 cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9832–9836. doi: 10.1073/pnas.83.24.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar L., Muca C., Magni M., Albert P., Bunzow J., Meldolesi J., Civelli O. Differential coupling of dopaminergic D2 receptors expressed in different cell types. Stimulation of phosphatidylinositol 4,5-bisphosphate hydrolysis in LtK- fibroblasts, hyperpolarization, and cytosolic-free Ca2+ concentration decrease in GH4C1 cells. J Biol Chem. 1990 Jun 25;265(18):10320–10326. [PubMed] [Google Scholar]
- Weiss A., Imboden J., Shoback D., Stobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybran J., Appelboom T., Famaey J. P., Govaerts A. Suggestive evidence for receptors for morphine and methionine-enkephalin on normal human blood T lymphocytes. J Immunol. 1979 Sep;123(3):1068–1070. [PubMed] [Google Scholar]
- Yu V. C., Sadée W. Phosphatidylinositol turnover in neuroblastoma cells: regulation by bradykinin, acetylcholine, but not mu- and delta-opioid receptors. Neurosci Lett. 1986 Nov 11;71(2):219–223. doi: 10.1016/0304-3940(86)90562-8. [DOI] [PubMed] [Google Scholar]



