Abstract
The ecological effects of ocean acidification (OA) from rising atmospheric carbon dioxide (CO2) on benthic marine communities are largely unknown. We investigated in situ the consequences of long-term exposure to high CO2 on coral-reef-associated macroinvertebrate communities around three shallow volcanic CO2 seeps in Papua New Guinea. The densities of many groups and the number of taxa (classes and phyla) of macroinvertebrates were significantly reduced at elevated CO2 (425–1100 µatm) compared with control sites. However, sensitivities of some groups, including decapod crustaceans, ascidians and several echinoderms, contrasted with predictions of their physiological CO2 tolerances derived from laboratory experiments. High CO2 reduced the availability of structurally complex corals that are essential refugia for many reef-associated macroinvertebrates. This loss of habitat complexity was also associated with losses in many macroinvertebrate groups, especially predation-prone mobile taxa, including crustaceans and crinoids. The transition from living to dead coral as substratum and habitat further altered macroinvertebrate communities, with far more taxa losing than gaining in numbers. Our study shows that indirect ecological effects of OA (reduced habitat complexity) will complement its direct physiological effects and together with the loss of coral cover through climate change will severely affect macroinvertebrate communities in coral reefs.
Keywords: carbon dioxide, pH, habitat quality, reef structural complexity, scleractinian coral cover, reef-associated macroinvertebrate fauna
1. Introduction
For marine ecosystems, the consequences of long-term exposure to rising carbon dioxide (CO2) concentrations are poorly understood. The partial pressure of CO2 (pCO2) in seawater is now higher than it has been for at least 800 000 years and has already reduced global mean seawater pH by 0.1 units [1], a process called ocean acidification (OA). If anthropogenic CO2 emissions continue unabated, pCO2 will increase to two to three times pre-industrial values towards the end of this century [2], reducing global mean pH by up to 0.3 units (a 150% increase in hydrogen ions) and halving carbonate ion concentrations [1–3].
Controlled experiments are essential to identify physiological responses, tolerance curves and thresholds to OA. Such laboratory experiments are being carried out on a rapidly increasing number of species in one or several of their life stages [4–6]. However, experiments have a limited capacity to predict in situ ecosystem responses to increasing CO2. Some taxa that perform poorly at high CO2 under laboratory conditions may be able to acclimatize to high CO2 in the longer term. Others that perform well under experimental CO2 exposure may be at risk during life stages that have not been experimentally assessed [5,6] or may be indirectly affected by OA-induced ecological changes in habitat properties (e.g. microbial surface biofilms and coralline algae), food webs, competition, diseases and/or community structures [7–9].
Shallow submarine CO2 seeps are among the few sites in the world within which the ecological consequences of long-term (multi-decadal) exposure to high CO2 can be assessed in situ. A small number of such CO2 seep systems have been investigated to date, including temperate rocky-shore communities in the Mediterranean Sea [8,10,11], a tropical coral reef in Japan [12], a Mexican groundwater spring system where brackish water high in CO2 and alkalinity emerges into coral reefs [13], and three volcanic CO2 seeps within coral reefs in Papua New Guinea (PNG) [7].
The ecological consequences of OA are likely to be most pronounced in ecosystems built by calcifying biota. The existence of coral reefs depends on the skeletal growth of scleractinian corals, coralline algae and other calcifying organisms, which ‘hypercalcify’ at low CO2 owing to the three to fourfold carbonate supersaturation state in tropical seawaters, accreting several kg m−2 yr−1 of calcium carbonate. In healthy coral reefs, structurally complex branching, tabulate and foliose morphologies are abundant (figure 1a), and provide important habitat and refugia for reef-associated organisms [14,15]. However, OA and climate-change-related disturbances (e.g. coral bleaching or severe storms) will increasingly affect the survival and growth of scleractinian corals, with the more sensitive structural corals likely to be most severely affected [16,17]. Coral reefs may therefore shift towards two contrasting structural forms. First, chronic exposure to elevated CO2, upwelling or pollution typically leads to structurally simplified coral communities dominated by massive growth forms (often the tough and persistent boulder corals of the genus Porites spp.; figure 1b), whereas the more sensitive structurally complex branching corals are under-represented [7,13]. Such communities may have high coral cover but low structural and taxonomic diversity. Second, episodic and severe disturbances can lead to widespread coral mortality and ‘reef flattening’ (i.e. the loss of three-dimensional habitat structure; figure 1c), which may persist for years to decades after such disturbances [16]. Again, structurally complex corals are more sensitive to these disturbances than massive growth forms [18], further simplifying habitat structures.
Figure 1.

Indo-Pacific reef communities with contrasting structural complexity. (a) Structurally complex reef (complexity rating value 4–5) with branching, tabulate and foliose coral morphologies. (b) Structurally simplified communities dominated by massive coral morphologies (complexity 2–3), as found in areas of chronic disturbance as shown here from one of the PNG CO2 seeps. (c) Flattened reef lacking three-dimensional structure (complexity 0–1), as found after major disturbance. (Online version in colour.)
Coral reefs are estimated to house 600 000 to more than 9 million species [19], a large proportion of which are macroinvertebrates. For reef-associated fishes, the availability of refugia, both ‘permanent’ (e.g. holes in reef substrata) and ‘transient’ (thickets of living coral branches), is known to increase local species richness [20], and the loss of habitat complexity and coral cover detrimentally affects their communities [15]. For the far more speciose macroinvertebrates, the consequences of losing habitat complexity and live coral remain largely unknown, and the few studies of this topic have resulted in contrasting conclusions. Some documented reduced diversity but unaltered [21] or reduced densities [22] with the erosion of reef framework. Others reported higher cryptofauna diversity and densities on reefs that had lost their three-dimensional habitat structure and living coral cover [23]. Given that the majority of these macroinvertebrates are relatively small-bodied and vulnerable to predation, ecological theory predicts that loss of habitat structure and refugia would have ramifications for the abundance and diversity of their communities [24].
In this study, we investigate the consequences of long-term in situ exposure to elevated seawater CO2 on benthic macroinvertebrate communities. We compare densities and taxonomic richness of mobile and sessile macroinvertebrate communities along gradients of habitat complexity at the CO2 seeps of PNG and adjacent control sites, on patches occupied by live coral and on substrata devoid of living corals. Our results demonstrate some of the mechanisms of how both direct physiological responses and indirect effects of OA may co-determine future macroinvertebrate communities in a high-CO2 world.
2. Material and methods
(a). Study sites and seawater chemistry
Studies were conducted at three shallow submarine volcanic CO2 seeps and three adjacent control sites with similar geomorphology, seawater temperature and salinity, in Milne Bay Province, PNG [7]. The three seep locations (Dobu, Esa'Ala and Upa Upasina) are located along an active tectonic fault line, and almost pure CO2 gas has been streaming through the reef substrata for an unknown period of time (confirmed for approx. 70 years, but possibly much longer [7]), resulting in locally reduced seawater pH. Areas of intense seeping with a median pHtotal < 7.7 (approx. 1100 µatm pCO2) were not included in the surveys, because no reef development is found beyond this apparent threshold. Mean hard coral cover was similar at the seeps compared with the adjacent control sites (33% ± 2.4 s.e. versus 31% ± 3.4 s.e.). However, at high CO2, the cover of massive Porites corals is twice that of the control sites, the cover of structurally complex corals with branching, foliose and tabulate growth forms is reduced threefold, and coral diversity is reduced by 39% [7]. Seawater temperature and salinity are similar between seep and control sites [7].
A total of 968 discrete seawater samples of pH were taken at the six sites from approximately 0.5 m above the benthos (see electronic supplementary material). The data include all samples collected between 2010 and 2012, representing a range of tidal, irradiance and wave conditions to characterize the ranges encountered by the organisms. The samples were immediately analysed for pH, temperature and salinity, and a subset of 450 samples were preserved for later determination of total alkalinity and dissolved inorganic carbon. Other relevant seawater carbonate parameters (aragonite saturation state, dissolved inorganic carbon, pCO2) were calculated from pH, total alkalinity, dissolved inorganic carbon, salinity and temperature using the R program Seacarb v. 2.4.8 [25].
(b). Survey methods
Surveys were based on intensive searches along belt transects at the high-CO2 and control sites, excluding specimens less than 1 cm in size and fauna within deep crevices that would have required destructive sampling (cryptofauna and infauna). Invertebrates were classified into 46 operational taxonomic units (OTUs), including all major calcifying and non-calcifying groups of mobile macroinvertebrates more than 1 cm in size, and many sessile groups, and comprised a total of 4910 organisms (see electronic supplementary material, table S1). Additionally, 29 OTUs within the echinoderm classes Echinoidea, Holothuroidea and Asteroidea were recorded to the highest possible taxonomic level. PNG is located within the most speciose marine biogeographic region on the Earth (the ‘Coral Triangle’), yielding a large proportion of extant marine species (e.g. estimates for decapod crustaceans: approx. 15 000 species; gastropods: approx. 30 000 species). As the majority of taxa are rare, undescribed or in need of taxonomic revision, OTUs were essential for non-destructive sampling, and groupings were defined to permit consistent in situ identification. Taxa were later grouped into phyla and classes (7 and 12 of which were represented in the surveys, respectively), into mobile versus sessile groups, and calcifying versus non-calcifying groups (see electronic supplementary material, table S1).
Thirty-five fine-scale surveys were conducted during two ship-based expeditions to these remote sites (20 surveys in April 2011 and 15 surveys in April 2012). The surveys were conducted at night (more than 1.5 h after the onset of darkness) because many mobile invertebrates remain cryptic during the day. Transect tapes were laid parallel to the slope at 3 m depth. The surveys were conducted within a belt of 20 × 1 m, with a 10 m gap between replicate surveys. Additionally, the larger echinoderm OTUs were recorded along belts 2 m wide along the 50 m length of the two replicate 20 m transects plus gap (total area of 100 m2) during both expeditions. During the fine-scale surveys in 2012, the 20 m transects were subdivided into 0.5 × 0.5 m2 quadrats (80 quadrats of 0.25 m2 per transect), and data were recorded separately for each quadrat. These data also included visual estimates of fine-scale complexity of the reef substrata (six levels [26]; figure 1; electronic supplementary material, tables S1 and S2) and dominant (more than 50% coverage) substratum types: ‘HC’ (living hard corals and Millepora), ‘DS’ (substrata devoid of macrofauna and macroflora, but dominated by coralline/turf algae growing on dead coral or coral rubble) or ‘other substrata’. Only 69 of the 1200 quadrats were dominated by ‘other’ substrata, and these were excluded from the second set of analyses.
(c). Statistical analyses
The analyses were based on two sets of data: (a) the combined 2011 and 2012 datasets (n = 35 surveys) were used to assess CO2 effects only; (b) the 2012 data (n = 1131 quadrats from 15 surveys) were used to assess the joint effects of CO2, complexity and substratum.
For (a), generalized linear mixed models (GLMMs) were used to assess the effects of CO2 [27]. The models included fixed effects of CO2, and random effects of location (Dobu, Esa'Ala and Upa Upasina) and transects within location. The significances of CO2 effects were estimated using quasi-F tests [28]. Residual plots were used to assess the mean–variance relationships. For the number of OTUs, classes and phyla per quadrat, the models comprised a log link function and variance proportional to the mean (quasi-Poisson) in order to account for over-dispersion. For densities, the variance increased more rapidly with mean values, and there were also large proportions of absences, thus each response was modelled using a negative binomial GLMM [29].
For (b), the analyses included the joint effects of CO2, complexity and substratum type on the biotic responses. The densities of the main groupings and the number of taxonomic classes (all per 0.25 m2) were analysed using GLMMs as for (a), but the predictors were CO2 and substratum type (categories), and complexity (numeric). The latter was based on quadratic trends across its six levels owing to the nonlinearity of responses. There was no support for higher-order trends across the six levels of complexity. Although CO2 and substratum were well balanced, complexity was not well balanced with either CO2 or substratum (see electronic supplementary material, table S2), and hence the interactions between the profiles of complexity and each of CO2 and substratum were not precisely estimable. First-order interaction effects between CO2, substratum and complexity were small and non-significant when adjusted for multiple comparisons across the responses. Hence, we only included main effects of CO2, substratum and complexity. These results are presented as partial effects plots and analyses of deviance. All statistical analyses used the statistical software R [30].
3. Results
(a). Seawater chemistry
At the three high-CO2 sites, median seawater pH values were 7.95, 7.81 and 7.72 (total scale), pCO2 concentrations ranged from 441 to 998 µatm, and aragonite saturation state (Ωarag) ranged from 3.7 to 2.1 (table 1; electronic supplementary material, figures S1 and S2). By contrast, at the three adjacent control sites, the median pH ranged from 8.02 to 7.98 units, pCO2 from 346 to 413 µatm, and Ωarag from 4.2 to 3.7.
Table 1.
Seawater carbonate chemistry at the three CO2 seep and control locations. Medians, and 5th and 95th percentiles, of measured pH, total alkalinity (TA) and salinity, and calculated concentrations of dissolved inorganic carbon (DIC), partial pressure of carbon dioxide (pCO2) and aragonite saturation state (Ωarag) are shown (see also [7]; electronic supplementary material, figures S1 and S2).
| location | site | pH | TA (µmoles kg−1) | DIC (µmoles kg−1) | pCO2 (µatm) | Ωarag | salinity |
|---|---|---|---|---|---|---|---|
| Dobu | control | 8.01 (7.91,8.10) | 2235 (2221,2293) | 1942 (1924,1924) | 368 (279,558) | 4.16 (3.07,4.35) | 34.8 (33.8,36.0) |
| Dobu | high CO2 | 7.72 (7.08,7.99) | 2295 (2260,2326) | 2147 (1962,1962) | 998 (423,3541) | 2.10 (0.65,3.75) | 34.8 (34.0,36.0) |
| Esa'Ala | control | 8.02 (7.94,8.04) | 2262 (2247,2290) | 1967 (1885,1885) | 413 (258,499) | 3.74 (3.34,4.86) | 34.8 (34.5,35.0) |
| Esa'Ala | high CO2 | 7.95 (7.83,8.04) | 2291 (2224,2304) | 1995 (1947,1947) | 441 (257,762) | 3.66 (3.15,5.07) | 34.8 (34.5,35.0) |
| Upa-U | control | 7.98 (7.91,8.09) | 2261 (2225,2326) | 1982 (1947,1947) | 346 (302,653) | 4.08 (2.82,4.57) | 34.8 (34.5,36.0) |
| Upa-U | high CO2 | 7.81 (7.28,8.01) | 2308 (2240,2378) | 2049 (1964,1964) | 624 (410,1564) | 2.89 (1.39,3.59) | 34.8 (33.8,35.9) |
(b). CO2 effects
The total density of macroinvertebrates and the number of taxa per transect were significantly reduced at the high-CO2 compared with the control sites (figure 2; electronic supplementary material, table S3). Total density was reduced to 48% of control values (from 10.9 to 5.2 individuals m−2), whereas the number of OTUs, classes and phyla were reduced to 77%, 79% and 85%, respectively. Losses at high CO2 were significant in both the groupings of calcifying and non-calcifying invertebrates (down to 51% and 38%, respectively). When taxa were grouped by their mobility, a general trend emerged despite the variability between taxa. The total density of the combined mobile groups was strongly reduced at high CO2 (down to 43% of control values), whereas that of the combined sessile groups was not significantly different. Of the mobile taxa, the most severely affected groups were the decapod crustaceans (down to 22%) and feather stars (Crinoidea, down to 25%; figure 2; electronic supplementary material, table S3). By contrast, the sea urchins (Echinoidea) increased in density, especially Diadema savignyi and Echinothrix spp., which are often found around massive Porites and other boulder-shaped substrata with low structural complexity. Total sea star (Asteroidea) density was similar at the seeps and controls; however, when Linckia multifora was excluded (a species with three times higher densities at the seeps compared with the controls), the density of the remaining asteroids was approximately 50% of control values. Of the sessile invertebrates, the most severely reduced taxa were the sea squirts (Ascidiacea, 22% of controls) and terebellid polychaetes (44%). Mean densities of date mussels (Lithophaga spp.) and vermetid gastropods, which live embedded within living massive Porites and other substrata, increased approximately 50-fold and eightfold at high CO2, respectively, but differences were not statistically significant owing to high variability. In other taxonomic groups, densities were similar at the high-CO2 and control sites (see electronic supplementary material, table S3).
Figure 2.
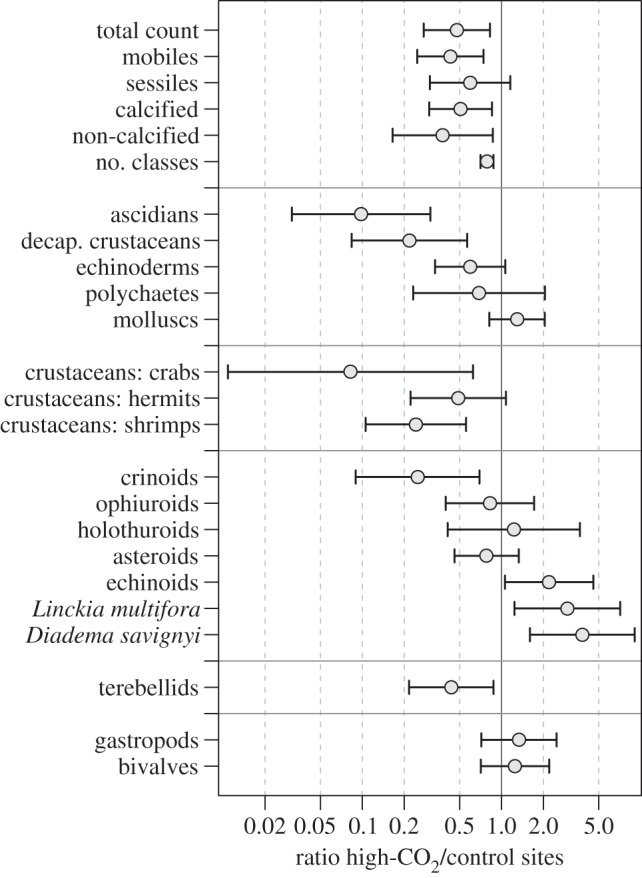
Ratios of the densities or number of taxonomic units of macroinvertebrates at high-CO2 relative to control sites (n = 35 surveys). Circles mark the estimated ratios, error bars show lower and upper 95% CIs (see electronic supplementary material, tables S5 and S6). Differences are significant (p < 0.05) if the error bars do not include the value 1.0.
(c). Combined effects of CO2, structural complexity and substratum type
The 2012 survey data confirmed that fine-scale structural complexity of the reef habitat was significantly reduced in the transects at the high-CO2 compared with control sites (F1,9 = 24.2, p < 0.001; electronic supplementary material, figure S3 and table S4), with similar losses of complexity at all three locations (F2,9 = 3.4, p = 0.078), and non-significant interactions between locations and CO2 (F2,9 = 0.61, p = 0.570). The proportions of quadrats with live HC and those with substrata devoid of macrofauna (DS) were similar at high-CO2 compared with control sites (53% HC at high CO2 versus 49% HC at controls; n = 1131, χ2 = 1.93, p = 0.16; electronic supplementary material, table S2).
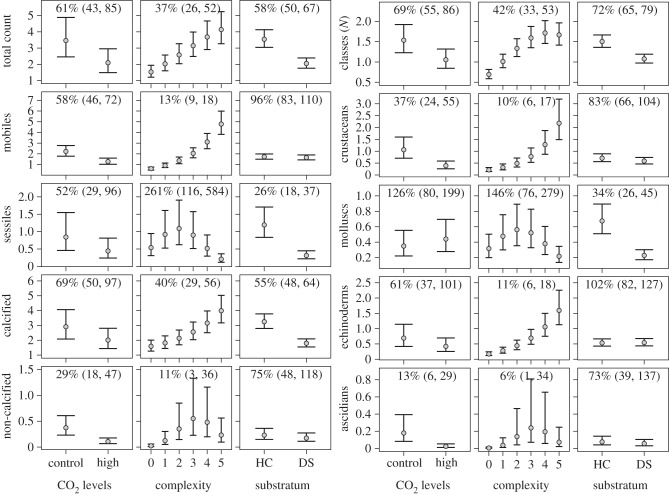
Differences in CO2, complexity and substratum type each accounted for large proportions of variation in the macroinvertebrate communities (figure 3a,b; electronic supplementary material, tables S4 and S5). Partial effects analyses showed that the total density of all macroinvertebrates combined not only declined at high CO2 to 61% of control values, but additionally declined to 37% at low compared with high complexity, and to 58% on DS compared with HC. Similarly, the number of classes per quadrat declined to 69% at high CO2, to 42% from high to low substratum complexity, and to 72% in quadrats dominated by DS compared with those dominated by HC. The densities of all mobile macroinvertebrates combined (of crinoids, decapod crustaceans, ascidians and terebellid polychaetes) were well explained by both direct CO2 effects and the loss in complexity, while substratum type was less important for these groups. Overall, there were significant losses at high CO2 in 12 of 22 responses (densities and number of taxa for which effects were estimable; electronic supplementary material, table S5), and increases in one response (Asteroidea). Additionally, 14 of the 22 responses significantly declined with declining complexity, suggesting strong indirect effects of OA on macroinvertebrate communities (figure 3a,b). For example, crustacean and echinoderm densities were reduced to 10 and 11% in low-compared with high-complexity quadrats. Four other responses (total density of sessile organisms, bivalves, polychaetes and sabellids) had higher densities at low levels of complexity, and several responses had highest values at intermediate complexity. Finally, 14 of the responses also showed significant differences between substratum types, with all but two (Asteroidea and Echinoidea) having reduced densities (18–68%) and number of taxa (72–74%) on DS compared with HC.
Figure 3.
Partial effects plots showing the relationships of macroinvertebrates to CO2, complexity and substratum type (HC: quadrats dominated by living hard corals; DS: substrata devoid of macrofauna). Circles indicate estimated marginal mean densities or number of taxonomic classes per 0.25 m−2 (total counts = all organisms combined; mobile/sessile and calcifying/non-calcifying = densities of all mobile/sessile and calcifying/non-calcifying organisms combined). Error bars are lower and upper 95% CIs. Estimated percentage change (high CO2 to control, complexity rating = 0 to complexity rating = 5, and DS to HC) and their 95% CIs are shown in the panels.
4. Discussion
(a). Physiological and ecological effects of ocean acidification
This study demonstrates that OA exerts both direct and indirect effects on tropical marine macroinvertebrate communities in their natural environment. To date, few tropical macroinvertebrates in few life stages have been investigated for their physiological sensitivity to OA, and sensitivities can vary substantially between species. Nevertheless, reviews and meta-analyses of the existing physiological studies of temperate and tropical taxa have supported general predictions about the OA sensitivities of phyla, classes or orders [5,6,31–33]. A comparison of OA sensitivities derived from these reviews with the in situ responses observed at the PNG and Mediterranean seeps shows some commonalities, yet also several notable contrasts (table 2), as summarized below.
Table 2.
Predicted and observed responses of macroinvertebrates to OA. Predictions are based on the generalized main conclusions of meta-analyses and reviews of physiological laboratory experiments [5,6,33]. Observed in situ responses are from the CO2 seeps in PNG (this study), and the Mediterranean at a pH of more than 7.7 [8,10,11]. Thick arrows represent widespread responses from many taxa; thin arrows represent data from fewer taxa; direction of arrows represents increase (⇑, ↑), little difference (⇔, ↔) or decline/greater reduction (⇓, ↓).
| predicted response (laboratory) | observed in situ response (CO2 seeps) |
||
|---|---|---|---|
| PNG | Mediterranean | ||
| decapod crustaceans | ⇔ ↓ | ⇓ | ⇓ |
| molluscs | ↔ ⇓ | ⇔ | ⇔ |
| echinoderms | ↔ ⇓ | ↑ ⇔ ↓ | ↑ ⇓ |
| polychaetes | ↔ ↓ | ↔ ↓ | ↔ ⇓ |
| ascidians | ↑ | ⇓ | |
| mobile groups (cf. sessile groups) | ⇑ | ⇓ | |
| calcifying groups (cf. non-calcifying groups) | ⇓ | ⇔ | |
For decapod crustaceans, our field data have documented severe losses at high CO2. By contrast, most physiological laboratory studies predict this aerobic mobile group to be highly CO2-tolerant, with negative effects typically only at very high CO2 concentrations [4–6], although new studies suggest greater sensitivities in some species [34]. For molluscs, we found no significant in situ changes in total densities and the densities of most specific OTUs, whereas experiments suggest either weak or detrimental OA effects on benthic molluscs [4–6,31,33,35]. For echinoderms, our field data also showed only minor changes at high CO2 in most classes, while laboratory experiments have typically predicted echinoderms (and, as for molluscs, especially their larvae) to be relatively OA-sensitive, attributable to their highly soluble high-magnesium calcite skeletal structures [5,6,31,33,36] (but see also [32]). Crinoids were the only echinoderm class showing a steep decline. We are not aware of any laboratory study on this class but note that they were decimated during the high-CO2 period marking the Permian to Triassic transition. The observation that molluscs and echinoderms appear to be often most OA-sensitive as larvae [5,33] may partly explain the weak responses at the seeps, where most organisms get exposed to high CO2 only after settlement. All mollusc and echinoderm OTUs that increased at the seeps (Lithophaga spp., Vermetidae, D. savignyi, Echinothrix spp. and L. multifora) are often associated with massive Porites, suggesting their densities to be more constrained by habitat availability than by post-settlement exposure to pH at levels above 7.7. Polychaetes are a diverse class comprising calcifying and non-calcifying groups, sessile and mobile groups, and many trophic levels. Not surprisingly, results are mixed for both field and experimental data on their OA tolerance. At the seeps, the Terebellidae showed steep declines at high CO2, while the total density of polychaetes (and specifically those of the Sabellidae, despite their common association with Porites) remained similar. In the laboratory, responses range from reduced calcification and metabolic depression to high CO2 tolerance [37,38], but too few taxa have been investigated for such a diverse group to draw any general conclusions. Finally, ascidians are soft-bodied sessile filter-feeding tunicates, with their tunics made of cellulose-like polysaccharides and proteins. While the field data showed severely reduced ascidian densities at high CO2, the few existing laboratory experiments all showed positive survival and developmental responses [33].
There were also substantial differences between field and laboratory results based on the groupings by mobility and calcification (table 2). At the seeps, the decline of the total density of all mobile organisms combined was far greater than that of sessile organisms, whereas calcifying organisms had similar losses in density compared with non-calcifying organisms. By contrast, physiological data predict the most active aerobic mobile groups to be the physiologically most OA tolerant groups owing to effective acid–base regulation of their body fluids, and conclude calcification to be one of the most sensitive responses to OA [5,6]. These discrepancies are not surprising because the groupings by mobility and calcification are composites of diverse taxonomic groups. Nonetheless, the data show that on their own, the physiological mechanisms developed to support mobility and calcification do not render organisms in the field more or less OA-resilient than their sessile and non-calcifying counterparts.
While there are some distinct contrasts between the outcomes of physiological and field studies, a comparison with macroinvertebrate data from the Mediterranean CO2 seeps shows many commonalities (table 2). Mediterranean sites with less than 7.7 pH were not considered for this comparison, and the rocky-shore communities of the temperate Mediterranean seeps do not face the issue of loss in structural complexity nor the loss of living coral as substrata. At the Mediterranean seeps, decapod densities were reduced to approximately 40% of control values [8], and there were no major changes in gastropod and bivalve densities [8,11]. Twenty of the 28 more common Mediterranean polychaete OTUs declined in abundances towards higher CO2 [8], with the most insensitive species showing genetic adaptation and/or physiological acclimatization [39]. The main contrast between the two regions was a high variability in echinoderm responses in PNG, while most Mediterranean echinoderms appear to be severely affected [11], although a few more CO2-tolerant echinoderms are now also being reported [40].
Despite our strong results, certain limitations in the use of CO2 seeps as OA proxies should be noted. First, the pCO2 range at the seeps is more variable than in open ocean systems, with unknown physiological consequences. However, pCO2 fluctuations only slightly lower than those at the seeps are also being reported from some coastal systems, lagoons or embayments, in zones of upwelling and within metabolically active photosynthetic communities [41]. Second, seeps comprise open populations (i.e. although all sessile and many mobile individuals have had a post-settlement lifetime of exposure to high CO2, exposure commences only during larval settlement, and only few taxa with poorly dispersive larvae may have multi-generational exposure). Many of the abundant small crabs, shrimps and crinoids are relatively stationary, with high affinities to specific coral heads or patches of substrata, but larger mobile taxa may have immigrated to the seeps later in their life. Third, seawater temperatures at the seeps represent present-day rather than future conditions, with thermal stress expected to worsen the OA effects [6]. Fourth, the reported associations are not all the effects of CO2 and habitat complexity, as additional factors will contribute to the ecological outcomes, such as CO2-induced reduced settlement from the loss of crustose coralline algae or altered microbial biofilms. These limitations have to be balanced against the limitations of laboratory perturbation experiments, most of which do not provide for long-term acclimatization nor capture the ecological changes that await organisms in their natural environment as CO2 levels continue to rise. Furthermore, responses at higher taxonomic levels (classes or phyla) do not necessarily indicate responses of specific taxa within those classes. For example, the echinoderm data that contained species-level information demonstrated that the apparent lack of response in the class Asteroidea was probably masked by the threefold increased densities of a single species (L. multifora). More species-level studies and studies on density compensation are needed to complement existing higher-level data. Nevertheless, the mismatches between in situ and laboratory data suggest that indirect ecological effects of OA may become important mechanisms of change, magnifying any direct physiological effects. They also show that our current understanding of which marine taxa will be adversely affected by OA is still far from comprehensive.
(b). Loss of structural complexity and live coral as habitat
Our study documented reduced fine-scale structural complexity in coral reefs at high CO2, confirming the results of an earlier study [7]. The loss of habitat complexity was associated with severe losses in the densities, in particular, of some mobile groups (e.g. decapod crustaceans and crinoids), with their nocturnal behaviour suggesting high predation pressure by visually hunting fish. By contrast, sessile groups protected from predators by shells or toxins (bivalvia, ascidians), and mobile groups protected by shells (gastropods, hermit crabs), large sizes or toxins (some of the echinoderms, nudibranchs), were far less dependent on structural complexity. Our study also showed richest macroinvertebrate communities in quadrats occupied by living coral, suggesting that live corals represent essential habitat rather than competitors for space or food for many groups. The observed reduction in macroinvertebrates associated with the loss of structural complexity and live coral is of concern, because cover and structural complexity of coral reefs have been declining at an alarming rate around the world [16,42,43]. A substantial proportion of the decline is attributed to rising temperatures, OA, more frequent coral bleaching and intense storm events, and greater rainfall variability from increasing CO2 emissions [16,42,43].
In conclusion, our study shows that reef-inhabiting macroinvertebrates will be affected by several interrelated pathways in a high-CO2 world (figure 4). First, the loss of live coral cover owing to climate change and other anthropogenic disturbances [42,43] will cause severe losses not only to coral cover but also to reef-inhabiting macroinvertebrates. Second, as shown here, the loss of refugia and habitat is an important additional mechanism of how OA will affect macroinvertebrate communities. Third, as demonstrated in numerous experimental studies, OA will directly affect the physiology, calcification, reproduction, behaviour, neuronal functions and survival of many groups. As sensitivities to these disturbances vary between groups, we anticipate shifts in food webs, altered competitive advantages and functional replacements in reef communities. Thus, it is essential to combine empirical evidence from both controlled CO2 perturbation experiments and the field, to improve our understanding of the combined physiological and ecological mechanisms and thresholds. Despite incomplete knowledge, however, our data strongly suggest that unless urgent action is taken to prevent further substantial CO2 rises, the biodiversity of many benthic communities will continue to decline.
Figure 4.
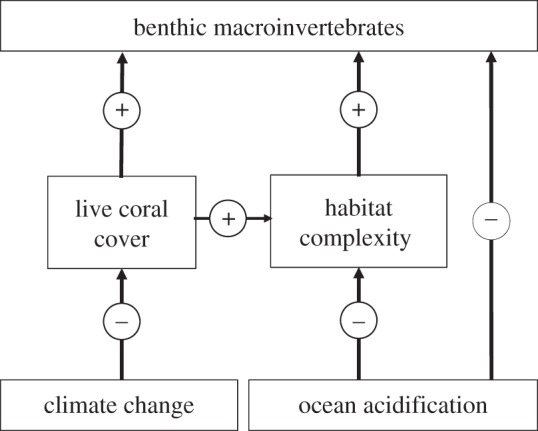
Conceptual diagram of the loss of coral cover owing to climate-change-related disturbances and the indirect and direct effects of OA on reef-associated macroinvertebrates.
Acknowledgements
We thank the owners of the Upa Upasina, Dobu and Esa'Ala reefs for allowing us to conduct the surveys on their reefs, and to the councillors of the Dobu RLLG, the Milne Bay Province Research Committee, and the Department of Environment and Conservation of Papua New Guinea for permits and logistical support. Special thanks to Rob van der Loos and the crew of the MV Chertan, the National Research Institute of Papua New Guinea, and QantasLink for their ongoing logistical support.
Data accessibility
The data are available through the MEST Data Archive of the Australian Institute of Marine Science.
Funding statement
The research was financially supported by the Australian Institute of Marine Science.
References
- 1.Solomon S, Quin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL. 2007. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Feely RA, Doney SC, Cooley SR. 2009. Ocean acidification: present conditions and future changes in a high-CO2 world. Oceanography 22, 36–47 (doi:10.5670/oceanog.2009.95) [Google Scholar]
- 3.Hoegh-Guldberg O, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (doi:10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 4.Pörtner HO. 2008. Ecosystem effects of ocean acidification in times of ocean warming: a physiologist's view. Mar. Ecol. Prog. Ser. 373, 203–217 (doi:10.3354/meps07768) [Google Scholar]
- 5.Kroeker KJ, Kordas RL, Crim RN, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso JP. 2013. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Change Biol. 19, 1884–1896 (doi:10.1111/gcb.12179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wittmann AC, Pörtner H-O. 2013. Sensitivities of extant animal taxa to ocean acidification. Nat. Clim. Change 3, 995–1001 (doi:10.1038/nclimate1982) [Google Scholar]
- 7.Fabricius K, et al. 2011. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Change 1, 165–169 (doi:10.1038/nclimate1122) [Google Scholar]
- 8.Kroeker KJ, Micheli F, Gambi MC, Martz TR. 2011. Divergent ecosystem responses within a benthic marine community to ocean acidification. Proc. Natl Acad. Sci. USA 108, 14 515–14 520 (doi:10.1073/pnas.1107789108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connell SD, Kroeker KJ, Fabricius KE, Kline DI, Russell BD. 2013. The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Phil. Trans. R. Soc. B 368, 20120442 (doi:10.1098/rstb.2012.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cigliano M, Gambi MC, Rodolfo-Metalpa R, Patti FP, Hall-Spencer JM. 2010. Effects of ocean acidification on invertebrate settlement at volcanic CO2 vents. Mar. Biol. 157, 2489–2502 (doi:10.1007/s00227-010-1513-6) [Google Scholar]
- 11.Hall-Spencer J, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner S, Rowley S, Tedesco D, Buia M. 2008. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454, 96–99 (doi:10.1038/nature07051) [DOI] [PubMed] [Google Scholar]
- 12.Inoue S, Kayanne H, Yamamoto S, Kurihara H. 2013. Spatial community shift from hard to soft corals in acidified water. Nat. Clim. Change 3, 683–687 (doi:10.1038/NCLIMATE1855) [Google Scholar]
- 13.Crook ED, Potts D, Rebolledo-Vieyra M, Hernandez L, Paytan A. 2012. Calcifying coral abundance near low-pH springs: implications for future ocean acidification. Coral Reefs 31, 239–245 (doi:10.1007/s00338-011-0839-y) [Google Scholar]
- 14.Hoegh-Guldberg O, Bruno JF. 2010. The impact of climate change on the world's marine ecosystems. Science 328, 1523–1528 (doi:10.1126/science.1189930) [DOI] [PubMed] [Google Scholar]
- 15.Jones GP, McCormick MI, Srinivasan M, Eagle JV. 2004. Coral decline threatens fish biodiversity in marine reserves. Proc. Natl Acad. Sci. USA 101, 8251–8253 (doi:10.1073/pnas.0401277101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez-Filip L, Dulvy NK, Gill JA, Cote IM, Watkinson AR. 2009. Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc. R. Soc. B 276, 3019–3025 (doi:10.1098/rspb.2009.0339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan NCS, Connolly SR. 2013. Sensitivity of coral calcification to ocean acidification: a meta-analysis. Glob. Change Biol. 19, 282–290 (doi:10.1111/gcb.12011) [DOI] [PubMed] [Google Scholar]
- 18.Wakeford M, Done TJ, Johnson CR. 2008. Decadal trends in a coral community and evidence of changed disturbance regime. Coral Reefs 27, 1–13 (doi:10.1007/s00338-007-0284-0) [Google Scholar]
- 19.Reaka-Kudla ML. 1997. The global biodiversity of coral reefs: a comparison with rainforests. In Biodiversity II: understanding and protecting our natural resources (eds Reaka-Kudla ML, Wilson DE, Wilson EO.), pp. 83–108 Washington, DC: Joseph Henry/National Academy Press [Google Scholar]
- 20.Caley MJ, St John J. 1996. Refuge availability structures assemblages of tropical reef fishes. J. Anim. Ecol. 65, 414–428 (doi:10.2307/5777) [Google Scholar]
- 21.Idjadi JA, Edmunds PJ. 2006. Scleractinian corals as facilitators for other invertebrates on a Caribbean reef. Mar. Ecol. Prog. Ser. 319, 117–127 (doi:10.3354/meps319117) [Google Scholar]
- 22.Bailey-Brock J, Brock R, Kam A, Fukunaga A, Akiyama H. 2007. Anthropogenic disturbance on shallow cryptofaunal communities in a marine life conservation district on Oahu, Hawaii. Int. Rev. Hydrobiol. 92, 291–300 (doi:10.1002/iroh.200610958) [Google Scholar]
- 23.Enochs IC, Toth LT, Brandtneris VW, Afflerbach JC, Manzello DP. 2011. Environmental determinants of motile cryptofauna on an eastern Pacific coral reef. Mar. Ecol. Prog. Ser. 438, U105–U127 (doi:10.3354/meps09259). [Google Scholar]
- 24.MacArthur RH, MacArthur JW. 1961. On bird species diversity. Ecology 42, 594–598 (doi:10.2307/1932254) [Google Scholar]
- 25.Lavigne H, Gattuso JP. 2013. Package ‘seacarb’: seawater carbonate chemistry with R, v. 2.4.8 (ed. R Development Core Team) See http://cran.r-project.org/web/packages/seacarb/index.html
- 26.Wilson SK, Graham NAJ, Polunin NVC. 2007. Appraisal of visual assessments of habitat complexity and benthic composition on coral reefs. Mar. Biol. 151, 1069–1076 (doi:10.1007/s00227-006-0538-3) [Google Scholar]
- 27.McCullagh P, Nelder JA. 1989. Generalized linear models, p. 511 Boca Raton, FL: Chapman and Hall [Google Scholar]
- 28.Eisen EJ. 1966. The quasi-F test for an unnested fixed factor in an unbalanced hierarchal design with a mixed model. Biometrics 22, 937–942 (doi:10.2307/2528083) [Google Scholar]
- 29.Venables WN, BD R. 2002. Modern applied statistics with S, 4th edn New York, NY: Springer [Google Scholar]
- 30.R Development Core Team 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 31.Doney SC, Fabry VJ, Feely RA, Kleypas JA. 2009. Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192 (doi:10.1146/annurev.marine.010908.163834) [DOI] [PubMed] [Google Scholar]
- 32.Dupont S, Ortega-Martinez O, Thorndyke M. 2010. Impact of near-future ocean acidification on echinoderms. Ecotoxicology 19, 449–462 (doi:10.1007/s10646-010-0463-6) [DOI] [PubMed] [Google Scholar]
- 33.Dupont S, Thorndyke MC. 2009. Impact of CO2-driven ocean acidification on invertebrates early life-history: what we know, what we need to know and what we can do. Biogeosci. Discuss. 6, 3109–3131 (doi:10.5194/bgd-6-3109-2009) [Google Scholar]
- 34.Hernroth B, Skold HN, Wiklander K, Jutfelt F, Baden S. 2012. Simulated climate change causes immune suppression and protein damage in the crustacean Nephrops norvegicus. Fish Shellfish Immunol. 33, 1095–1101 (doi:10.1016/j.fsi.2012.08.011) [DOI] [PubMed] [Google Scholar]
- 35.Shirayama Y, Thornton H. 2005. Effect of increased atmospheric CO2 on shallow water marine benthos. J. Geophys. Res. Oceans 110, C09S08 (doi:10.1029/2004JC002618) [Google Scholar]
- 36.Dupont S, Dorey N, Thorndyke M. 2010. What meta-analysis can tell us about vulnerability of marine biodiversity to ocean acidification? Estuarine Coastal Shelf Sci. 89, 182–185 (doi:10.1016/j.ecss.2010.06.013) [Google Scholar]
- 37.Chan VBS, Li CY, Lane AC, Wang YC, Lu XW, Shih KM, Zhang T, Thiyagarajan V. 2012. CO2-driven ocean acidification alters and weakens integrity of the calcareous tubes produced by the serpulid tubeworm, Hydroides elegans. PLoS ONE 7 (doi:10.1371/journal.pone.0042718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Widdicombe S, Needham HR. 2007. Impact of CO2-induced seawater acidification on the burrowing activity of Nereis virens and sediment nutrient flux. Mar. Ecol. Prog. Ser. 341, 111–122 (doi:10.3354/meps341111) [Google Scholar]
- 39.Calosi P, et al. 2013. Adaptation and acclimatization to ocean acidification in marine ectotherms: an in situ transplant experiment with polychaetes at a shallow CO2 vent system. Phil. Trans. R. Soc. B 368, 20120444 (doi:10.1098/rstb.2012.0444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calosi P, Rastrick SPS, Graziano M, Thomas SC, Baggini C, Carter HA, Hall-Spencer JM, Milazzo M, Spicer J. 2013. Distribution of sea urchins living near shallow water CO2 vents is dependent upon species acid–base and ion-regulatory abilities. Mar. Pollut. Bull. 73, 470–484 (doi:10.1016/j.marpolbul.2012.11.040) [DOI] [PubMed] [Google Scholar]
- 41.Duarte CM, Hendriks IE, Moore TS, Olsen YS, Steckbauer A, Ramajo L, Carstensen J, Trotter JA, McCulloch M. 2013. Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuaries Coasts 36, 221–236 (doi:10.1007/s12237-013-9594-3) [Google Scholar]
- 42.Bruno J, Selig E. 2007. Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS ONE 2, e711 (doi:10.1371/journal.pone.0000711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De'ath G, Fabricius KE, Sweatman H, Puotinen M. 2012. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl Acad. Sci. USA 109, 17 995–17 999 (doi:10.1073/pnas.1208909109) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available through the MEST Data Archive of the Australian Institute of Marine Science.