Abstract
The average surface pH of the ocean is dropping at a rapid rate due to the dissolution of anthropogenic CO2, raising concerns for marine life. Additionally, some coastal areas periodically experience upwelling of CO2-enriched water with reduced pH. Previous research has demonstrated ocean acidification (OA)-induced changes in behavioural and sensory systems including olfaction, which is due to altered function of neural gamma-aminobutyric acid type A (GABAA) receptors. Here, we used a camera-based tracking software system to examine whether OA-dependent changes in GABAA receptors affect anxiety in juvenile Californian rockfish (Sebastes diploproa). Anxiety was estimated using behavioural tests that measure light/dark preference (scototaxis) and proximity to an object. After one week in OA conditions projected for the next century in the California shore (1125 ± 100 µatm, pH 7.75), anxiety was significantly increased relative to controls (483 ± 40 µatm CO2, pH 8.1). The GABAA-receptor agonist muscimol, but not the antagonist gabazine, caused a significant increase in anxiety consistent with altered Cl− flux in OA-exposed fish. OA-exposed fish remained more anxious even after 7 days back in control seawater; however, they resumed their normal behaviour by day 12. These results show that OA could severely alter rockfish behaviour; however, this effect is reversible.
Keywords: gabazine, muscimol, Sebastes, upwelling
1. Introduction
A large proportion of anthropogenic CO2 produced by burning of fossil fuels is readily absorbed by the ocean, lowering oceanic pH in a process called ocean acidification (OA) [1]. Worldwide, the average ocean pH has already decreased by more than 0.1 pH units, which represents a 30% increase in the concentration of hydrogen ions (H+). These unprecedented changes in ocean CO2 partial pressure (pCO2) and pH levels are predicted to severely impact marine organisms [2]. To date, calcifying phytoplankton and invertebrates such as corals and mollusks have received the most attention due to the potential impact of acidification on carbonate shell integrity and formation [3,4]. By contrast, fish were initially believed to be safe from the effects of OA, as early studies demonstrated a lack of mortality under extremely high CO2 levels (greater than 10 000 µatm) [5]. However, an increasing body of evidence suggests that OA-relevant CO2/pH levels induce various sub-lethal effects in fish, including otolith over-growth [6,7], a shift in behavioural lateralization [8,9], alterations in olfaction that affect detection of cues from substrates, parents [10], prey [11] and predators [8,12,13], and impaired learning [9,14]. Because the gamma-aminobutyric acid type A (GABAA) receptor antagonist, gabazine, restores proper discrimination of predator odour, learning of predatory cues [14] and behavioural lateralization in OA-exposed fish [8], at least some of the deleterious effects of OA in fish seem to be related to altered neurotransmitter function. If this model is correct, an alteration of GABA function is bound to have several other profound effects on behaviour owing to the ubiquitous distribution of GABA receptors in the brain.
Activation of the ligand-gated GABAA receptor is the major inhibitory mechanism in the central nervous system of vertebrate animals, and is involved in reducing anxiety in humans [15], rodents [16] and fish [16,17]. GABAA receptors are the main target for pharmaceuticals that decrease anxiety (anxiolytics) such as benzodiazepines, which have been found in surface waters at concentrations that can alter food intake, social behaviour and activity levels in fish [18]. The opening of ionotropic GABAA receptors typically results in a shunting/hyperpolarizing effect on neural excitability via an influx of Cl− ions from the extracellular to intracellular space. However, the concentration of intracellular Cl− ions in some immature neurons is higher compared with mature neurons, causing the flow of Cl− ions to reverse and move outward through GABAA receptors, thus resulting in depolarization [19]. Similarly, the compensatory response of fish to hypercapnic acidosis involves accumulating HCO3− in plasma to buffer pH [20,21], which leads to a decrease in plasma Cl− concentration (to maintain charge balance). The resulting altered Cl− concentration gradient between cells and plasma is thought to turn some GABAA receptors from inhibitory to excitatory, which, if correct, will have profound effects on anxiety.
To date, OA research on behaviour has predominantly been investigated in Australian fish that live close to or on coral reefs, a notable exception being a recent study on three-spined stickleback, a temperate fish [9]. Non-reef fish that live near kelp forest ecosystems are candidates to be especially vulnerable because it has recently been demonstrated that upwelling events can significantly affect seawater chemistry [22]. Short-term fluctuations in ocean dynamics can create localized areas of low pH (approx. 7.8) and higher than normal pCO2 that can reach as high as 820 µatm at shallow depths (7 m) and as high as 1016 µatm at greater depths (17 m) for at least 5–7 days [22]. Therefore, the California coast already regularly experiences CO2-acidified water and will be exposed to more extreme OA conditions earlier than in other locations [23,24].
The aim of this study was to investigate whether CO2 OA alters anxiety in a Californian fish, and whether (consistent with studies on reef fish) OA affects GABAA-receptor function. In addition to the GABAA-receptor antagonist gabazine, we used the GABAA-receptor agonist muscimol to verify the mechanism proposed by Nilsson et al. [8]. Finally, we explored whether any potential effects of OA on rockfish behaviour were reversible after placing rockfish back into normal seawater.
2. Material and methods
Juvenile rockfish (Sebastes diploproa; n = 30; 4.5–6 cm; 1.5–3.2 g) were caught from kelp drifting off the shores of San Diego–La Jolla and maintained in the Scripps Institution of Oceanography flowing seawater aquarium for at least 20 days prior to experimentation. Rockfish were randomly assigned to either the control (two tanks) or OA group (two tanks) and housed in identical 20 l tanks with constantly flowing seawater (0.3 l min−1). Within each group, fish from the two control tanks did not have different dark preference (Ctl 1: 449 ± 143 s, n = 7; Ctl 2: 461 ± 140 s, n = 8; p = 0.9581, unpaired t-test), and nor did the two OA tanks (OA1: 699 ± 88 s, n = 8; OA2: 808 ± 52 s, n = 7; p = 0.3969 Mann–Whitney U-test), so the observations were pooled together. All fish were fed tropical fish flake food (Aquatic Eco-Systems, Apopka, FL) ad libitum, once daily after behavioural testing. Manipulation of seawater CO2 was performed in a 200 l reservoir tank located upstream of the tank holding the experimental fish. Experimental seawater CO2 was manipulated using a pH-stat system (IKS, Karlsbad, Germany) or Reef Fanatic pH controller (Indianapolis, IN) that continuously measured pH and injected CO2. Aquaria were vigorously aerated to maintain appropriate air saturation and to promote mixing, which reduced the response time of the pH-stat system. The average pH value in the reservoir tank throughout the experiment was 7.78 ± 0.03 (measured with a National Standards Bureau-scale pH electrode at 1 min intervals for the duration of the study, calibrated with appropriate buffers as needed). The pH in the reservoir and experimental tanks were additionally monitored with a second electrode at least twice per day. Control seawater (pH 8.10 ± 0.03) was treated in an identical fashion, except for the CO2 injection. Temperature was continuously monitored at 1 min intervals throughout the duration of the experiment and was 22.1 ± 0.4°C. Total alkalinity (AT) was measured at days 1, 3 and 7 for control tanks (2244.4 ± 0.7 µmol kg−1), and at days 1, 3, 7, 11, 15 and 19 for high-CO2 tanks (2245.1 ± 0.5 µmol kg−1). These values lay within the average AT in the La Jolla Shores seawater system (where the seawater for the experimental aquarium is pumped from), which is 2223 µmol kg−1 with a range from 2200 to 2250 µmol kg−1 [25]. Salinity was 33.695 ± 0.005%. As it is impossible to measure AT continuously, we chose to use the average AT for the seawater system to calculate pCO2 using the Matlab version of CO2SYS [26,27], with dissociation constants from Mehrbach et al. [28] as refitted by Dickson & Millero [29]. Using the upper and lower limits of AT, an average salinity of 33.7, and measured pHNBS and temperature (°C) resulted in an average range for each calculated value of pCO2 of 26 μatm. All pCO2 values are reported at average AT. Using these values, CO2 in control and experimental seawater was 483 ± 40 µatm and 1125 ± 100 µatm (mean ± s.e.m.), respectively.
(a). Behavioural testing
All testing took place between 9.00 and 18.00. The light/dark arena was 9.5 cm wide by 55 cm long and 9.5 cm deep, and had a white plastic floor; water level was 6.5 cm. The walls of the arena were lined with white or black non-reflective waterproof paper affixed to the sides of the arena with Velcro. Prior to each trial, the arena was rotated 180° to control for potential subtle differences in lighting and refilled with fresh control or high-CO2 seawater. Experimental scototaxic (light/dark preference) testing was performed as previously described [30]. Fish were released into the centre of the arena facing parallel to the long axis to prevent biasing to the light or dark zone. Trials began 1–5 s after fish were released into the arena and lasted 15 min, consistent with other light/dark testing in fish [30–32]. The shelter test arena was 31 cm in diameter and refilled with 10 cm of water prior to each trial with control or high-CO2 seawater. The object in the centre was a 5 cm tall Lego figurine constructed of a variety of colours to avoid any innate colour preference of the fish. Time spent near the object was quantified with a circle of 12 cm diameter placed on top of the object in Ethovision XT (v. 7.0, Noldus, Leesburg, VA). This diameter of circle was chosen because it is approximately twice the body length of juvenile rockfish. Trials began approximately 1–5 s after fish were placed into the arena. Rockfish movement was tracked with Ethovision XT motion tracking software. Time spent in zones (light versus dark, centre versus outer), average velocity and immobility (5% threshold based on Pham et al. [33]) were recorded (n = 7 for the 7 and 12 day recovery groups; one fish died of undetermined causes prior to light/dark testing). Data were analysed using GraphPad Prism v. 4.0B (La Jolla, CA).
(b). Drug administration
Gabazine (4 mg l−1, 10.9 μM) and muscimol (1 mg l−1, 8.8 μM) were purchased from Sigma (St Louis, MO) and dissolved in 500 ml of seawater (high-CO2 seawater for the CO2 group). Heat, sonication and gentle stirring were used to dissolve the compounds. Fish were placed in the drug solution for 30 min, and then placed in the experimental arena filled with control or high-CO2 water. Gabazine and muscimol were applied to Ctl 1 and OA 1 groups on days 9 and 10, respectively. Gabazine is a specific GABAA-receptor antagonist [34] that has become a popular tool to study the effects of OA on fish behaviour [8,14]. Muscimol is a specific agonist of GABAA receptors [35], including fish GABAA receptors [36–38]. To the best of our knowledge, this is the first study to use muscimol to characterize the effects of OA on fish behaviour.
(c). Statistical analyses
Normality was tested with either the D'Agostino and Pearson omnibus test or the Kolmogorov–Smirnov test depending on the sample size. Time in the dark zone was analysed with one-sample t-tests or Wilcoxon signed-rank tests to assess statistically significant differences, as commonly used in these types of study [11,30,32,39]. Two-tailed paired and unpaired t-tests and one-way ANOVAs were used for parametric data, and Mann–Whitney U-tests and Kruskal–Wallis tests with Dunn's multiple comparison post hoc tests for data that were not normally distributed. An α-level of p < 0.05 and 95% confidence intervals were used for assessing statistical significance in all tests. Data were analysed using GraphPad Prism v. 4.0B. Data are presented as mean ± s.e.m.
3. Results
In our first set of experiments, we tested for scototaxis (light/dark preference), which is a validated behavioural test for anxiety in fish [31]. Control rockfish (n = 15) housed in seawater with present-day CO2 levels for 7 days (483 ± 40 μatm) showed no significant preference for either the light (413 ± 100 s) or dark zone (455 ± 96 s; paired t-test, p = 0.8314; figure 1a). However, rockfish that were exposed to high seawater CO2 (1125 ± 100 μatm) (n = 15) for 7 days demonstrated a significant preference for the dark zone, indicative of increased anxiety (light: 150 ± 53 s, dark: 750 ± 53 s; Mann–Whitney test, p < 0.0001; figure 1a). There were no significant differences in distance moved (control: 1066 ± 122 cm, n = 15; CO2: 1420 ± 304 cm, n = 15; Mann–Whitney test, p = 0.6783; electronic supplementary material, table S1), nor immobility (control: 517 ± 32 s, n = 15; CO2: 485 ± 53 s, n = 15; unpaired t-test, p = 0.6924; electronic supplementary material, table S1) between the control and OA groups, suggesting that there were no changes in basic locomotion caused by OA. Next, we administered gabazine to both control and OA fish prior to a second scototaxic test in the same fish (control: n = 7, CO2 n = 8). Because decreased GABAA-receptor activity is correlated with an increase in anxiety in many species, we predicted that gabazine would have a pronounced effect on the control group and not the OA group. Indeed, figure 1 demonstrates that the administration of gabazine resulted in a significant dark preference in the control group (light: 156 ± 124 s, dark: 744 ± 144 s; Mann–Whitney test, p = 0.002) but did not change the dark preference of the OA group (figure 1b).
Figure 1.
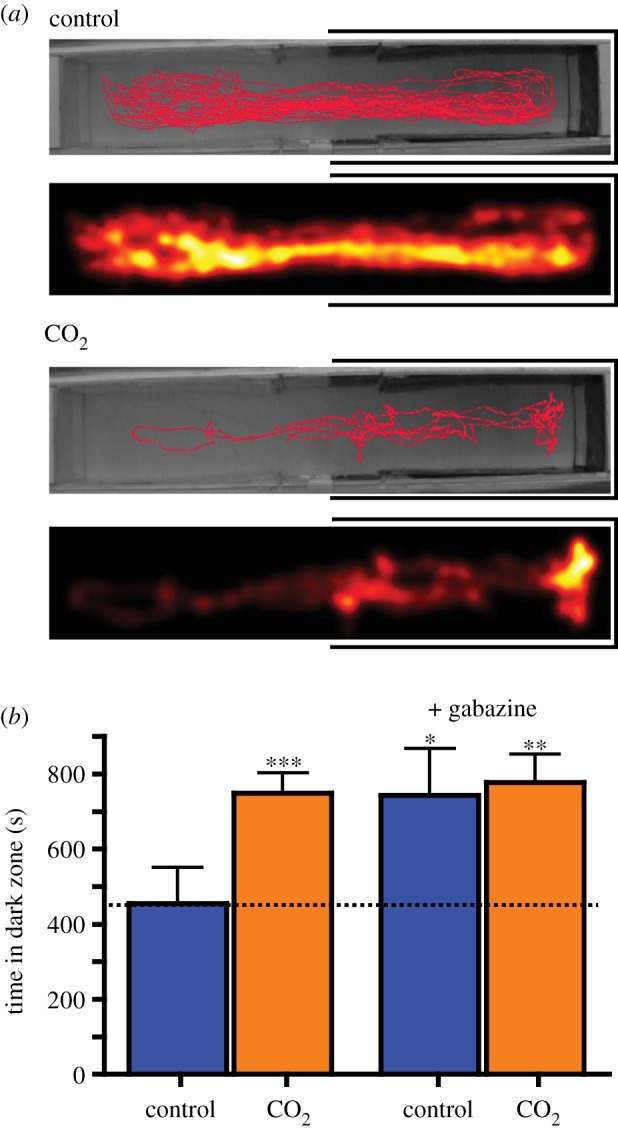
Both high CO2 and gabazine induce dark preference. (a) Control and OA-exposed fish were individually placed in the light/dark preference test arena and their location was recorded for 900 s. The red trace illustrates the movement of one representative fish from each treatment over the trial. The heatmap (below) is the coloured representation of rockfish movement from the same fish throughout the trial, and proportional to the time the fish spent in each pixel. (b) Control fish (n = 15) had no preference for the dark zone (455 ± 96 s; p = 0.9587), whereas OA-exposed fish (n = 15) spent significantly more time in the dark zone (750 ± 53 s; p = 0.0004), indicating increased anxiety. Gabazine caused a dark preference in the control group (n = 7; dark zone 744 ± 124 s; p = 0.0234), and caused no behavioural change in the time spent in the dark zone by the OA-exposed group (n = 8; dark zone 778 ± 76, p = 0.0078). Values are mean ± s.e.m., analysed with a one-sample t-test or Wilcoxon signed-rank test, difference from 450. ***p < 0.001, **p < 0.01, *p < 0.05.
To examine whether the OA-induced increase in anxiety is reversible, 10-day OA-exposed rockfish were placed into normal seawater and tested for light/dark preference. After 7 days of recovery, we calculated dark preference and still found a robust preference for dark (light: 112 ± 29 s, dark: 788 ± 29 s; n = 7, p < 0.0001; electronic supplementary material, figure S1). However, after 12 days in normal seawater there was a significant shift in behaviour with the previously OA-exposed rockfish no longer displaying dark preference (light: 360 ± 78 s, dark: 540 ± 78 s; n = 7, p = 0.2932; electronic supplementary material, figure S1), suggesting the increase in anxiety is reversible.
To further support the theory that OA is shifting anion flux through GABAA receptors, we tested whether activation of GABAA receptors induced a differential effect on the behaviour of control and OA-exposed fish. However, testing this hypothesis required a method that can detect both an increase and decrease in anxiety responses. The light/dark assay is not suitable because the OA-exposed fish were already at or close to a ceiling effect, as demonstrated with the application of gabazine, and lack of observable increase in anxiety (figure 1). Therefore, we designed a test better suited to analyse anxiety across a spectrum: the shelter test consists of a circular arena with a novel object placed in the centre. Time (s) spent in close proximity to the object is used as an index of anxiety. This test was based on previously observed behaviour in juvenile rockfish, which spend significant time near or underneath drifting kelp for protection from predators [40]. Although OA-exposed fish demonstrated a tendency for increased anxiety there was no statistically significant difference between control (180 ± 80 s, n = 7) and OA-exposed rockfish (302 ± 82 s, n = 8; unpaired t-test, p = 0.3115; figure 2) in this shelter test, suggesting that this test is a less sensitive measure of anxiety than the light/dark assay. Also similar to the light/dark assay, OA exposure did not result in any changes in locomotion (distance travelled or immobility; electronic supplementary material, table S2). To test whether the activation of GABAA receptors has opposing effects on control and OA-exposed fish, we applied muscimol, which is a potent GABAA-receptor agonist. Muscimol acts independent of GABA release and reduces anxiety in animal models [16,41]. If OA exposure shifts the action of GABAA receptors from shunting (inhibitory) to depolarizing (excitatory), the administration of the normally anxiolytic (anxiety-reducing) muscimol should be anxiogenic (anxiety-inducing) in OA-exposed fish (figure 3). As predicted, muscimol produced an opposing and significant shift in the location preference of the control (164 ± 98 s, n = 7) and OA-exposed rockfish (503 ± 60 s, n = 7; p < 0.05; figure 2). Importantly, there were no significant differences in basic locomotion (see electronic supplementary material, table S2). This indicates that the effect of muscimol is probably due to neurological effects that increase anxiety in OA-exposed fish, whereas muscimol causes control fish to explore the arena away from the shelter object, which is indicative of reduced anxiety.
Figure 2.
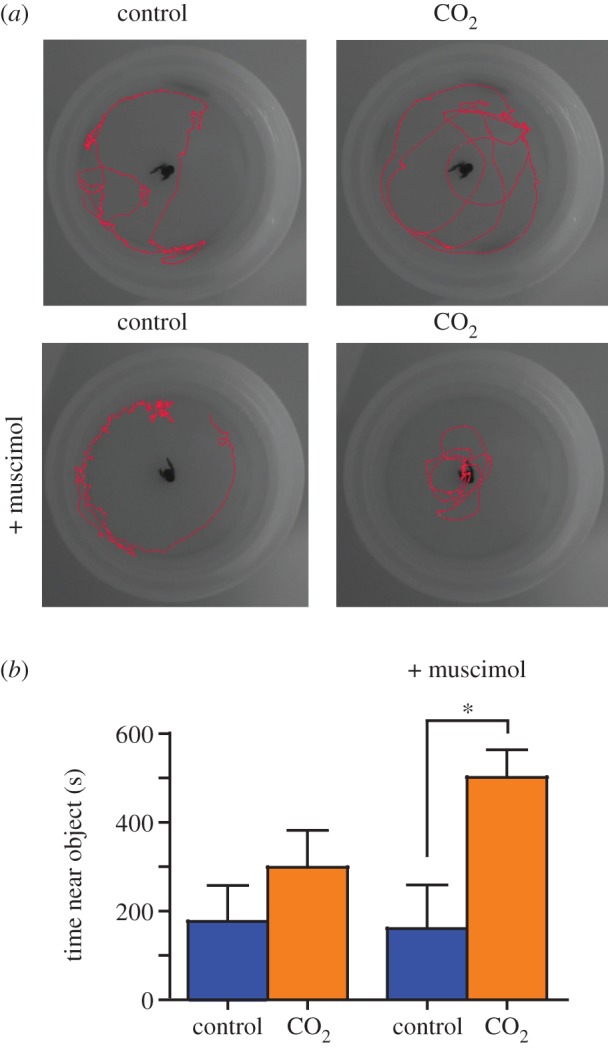
Muscimol produces opposing effects on behaviour. (a) Control and OA-exposed fish were placed in the shelter test arena and their location was recorded for 600 s. Two days later, muscimol (1 mg l−1) was administered for 30 min prior to a second shelter test. The red lines illustrate the movement of one representative fish from each treatment over the trial. (b) There was no difference in time spent near the object for control (180 ± 80 s, n = 7) versus the OA-exposed fish (302 ± 82 s, n = 8; p > 0.05). Muscimol induced a significant difference in time spent near the object (control: 164 ± 98 s, n = 7; OA: 503 ± 60, n = 7; p < 0.05; Kruskal–Wallis test (p = 0.0382) with Dunn's Multiple Comparision post hoc test). Values are mean ± s.e.m. *p < 0.05.
Figure 3.
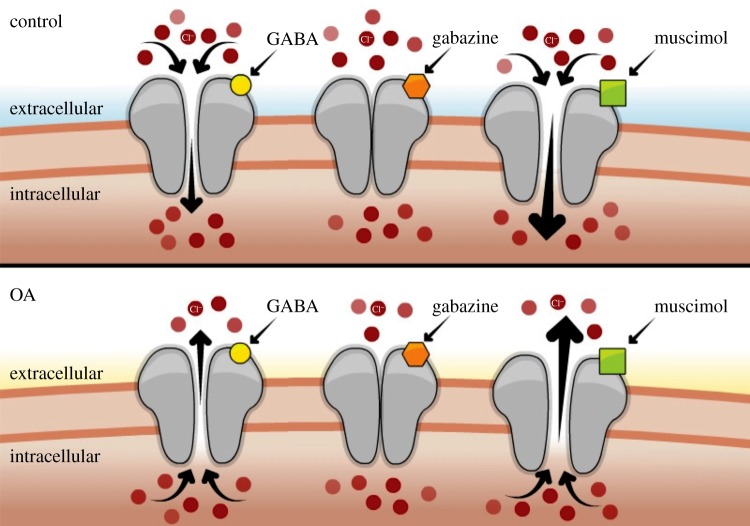
Model of OA-induced changes in GABAA-receptor ionic gradients. Under normal conditions, the concentration of Cl− ions is slightly higher in the cerebrospinal fluid (extracellular) relative to the cytoplasm (intracellular), and the equilibrium potential for Cl− (ECl) is near the resting membrane potential. When GABAA receptors open, Cl− flows into the neuron and counters depolarization, keeping the membrane potential more negative, and reducing neuronal activity. OA induces hypercapnic acidosis in plasma, which fish counteract by excreting excess H+ and accumulating HCO3−. This leads to a decrease in plasma [Cl−] (to maintain charge balance), thus leading to an alteration in ECl. In this condition, opening of GABAA receptors results in net Cl− movement out of the neuron, causing membrane depolarization and increasing excitation of neural pathways. Gabazine is an allosteric antagonist of GABAA receptors that prevents the channel from opening, whereas muscimol is a selective GABAA agonist that binds to the same site as GABA and increases channel opening (independent of presynaptic GABA release). Muscimol has opposite effects in control and OA-exposed fish when the membrane potential is close to resting because ECl is altered.
4. Discussion
In this study, we increased CO2 to levels projected for the start of the twenty-second century and measured the impact on anxiety behaviour in juvenile rockfish. As fish exposed to high CO2 (1125 ± 100 μatm) demonstrated increased anxiety compared with controls (483 ± 40 μatm), we applied GABAA-receptor modulators to test the model that OA alters Cl− ion flux through these receptors (figure 3; see also [8]). Consistent with OA-induced GABAA-receptor-mediated alteration of olfactory ability and lateralization [8], antagonizing GABAA receptors increased anxiety in control rockfish but had no effect on OA-exposed rockfish. Activation of GABAA receptors decreased anxiety in controls, but increased anxiety in OA-exposed rockfish.
Until now, anxiety testing in fish has predominantly been performed on freshwater goldfish and zebrafish. Common tests of anxiety in fish include the novel tank test, open field test, light/dark test, predator avoidance task and novel object approach tests [17,31,42]. To date, these tests have not been used with marine fish; however, a recent study has demonstrated a decrease in boldness in sticklebacks (Gasterosteus aculeatus) in a novel object approach test after 20 days of OA exposure [10]. Here, we have shown that control rockfish have no innate preference in the light/dark test under our experimental conditions. This is different from the strong preference for the dark area observed in zebrafish (Danio rerio), tetra (Paracheirodon axelrodi), swordtail (Xiphophorus helleri) and guppy (Poecilia reticulata) [31], but similar to mosquitofish (Gambusia holbrooki) behaviour. Our observed lack of dark preference in control rockfish may be related to the fluctuation in light levels in kelp forests, which is the natural habitat of juvenile rockfish.
The light/dark test has been validated as an appropriate test for anxiety, because pharmacological compounds that reduce anxiety (anxiolytics) have profound effects on location preference. For example, anxiolytics cause zebrafish to switch their preference from the dark to the light zone [43]. In our study, we used the selective GABAA antagonist gabazine as an anxiety-generating (anxiogenic) compound, which caused control rockfish to prefer the dark zone, thus establishing that, for rockfish, dark preference in the light/dark test is indicative of increased anxiety. As OA-exposed rockfish display a strong preference for the dark zone, we conclude that high CO2 levels result in increased anxiety. Gabazine had no further effect, indicating that the already anxious CO2 group had reached a ceiling level of anxiety. When tested for recovery in normal seawater the OA-exposed group returned to control levels after 12 days. Any potential habituation to the light/dark test was not investigated in this study; however, previous work in zebrafish suggests that habituation does not occur in the light/dark test even after 5 consecutive days of testing [44]. As we administered the light/dark test on a minimal schedule and fish were rarely handled, and the change in dark preference was robust between recovery days 7 and 12, habituation to the test was unlikely to occur. It is more probable that there was a change in GABAA-receptor functioning that explains the absence of dark preference with recovery.
Until now, the role of GABAA in OA-induced altered behaviour has been exclusively inferred using the GABAA antagonist gabazine [8,14]. However, experimentation with pharmacological compounds can never rule out undesired effects on unspecific targets. As genetic manipulation (e.g. gene knockdown or silencing) is not yet feasible in marine fish, we opted to confirm the involvement of GABAA receptors by applying a second, structurally different drug. If OA results in a shift in the equilibrium potential for Cl− (ECl) and reversal of Cl− flux across GABAA receptors, the GABAA-receptor agonist muscimol should produce opposing effects in OA-exposed compared with control fish (figure 3). The effect of muscimol was tested using the ‘shelter test’, which is designed to measure the amount of time that the fish spends near an object located in the centre of the arena. Both control and experimental fish had the same baseline behaviour, which allowed us to test the hypothesis that activation of GABAA receptors leads to opposing behaviours in OA-exposed and control fish. Control fish moved away from the object upon muscimol administration; as muscimol is an anxiolytic, this behaviour is indicative of reduced anxiety in rockfish. On the other hand, OA-exposed fish moved closer to the object, consistent with the anxiogenic effects of OA shown in figure 1. These results clearly indicate that the activation of GABAA receptors results in normal anxiolytic action in control fish, but anxiogenesis in OA-exposed fish. All together, these results are consistent with Cl− influx through GABAA receptors in control fish, but Cl− efflux in OA-exposed fish (figure 3).
In previous studies [8,14], gabazine restored the OA-induced effects on impaired olfactory discrimination, unlike our results, in which gabazine has an effect only on control fish. Increased activity of olfactory neural circuitry because of a shifted ECl would result in a depolarizing action on GABAA receptors leading to impaired olfactory signalling downstream to brain areas that regulate the escape response. The underlying mechanisms are not known in fish but the impaired response may be because of decreased tonic inhibition that mediates olfactory discrimination in the olfactory bulb, as described in mice [45]. Gabazine could then act to suppress the overactivity, resulting in a normal escape response.
GABAA receptors are constantly active in the mammalian amygdala, maintaining a tonic state of neuronal inhibition that keeps anxiety at a low behavioural level [46]. Conversely, prolonged antagonism of GABAA receptors in this brain area removes this tonic inhibition and results in a chronic increased anxiety state [46]. Because the same behavioural response takes place in control rockfish after gabazine administration in our study, we hypothesize that the fear associated with the light area is regulated by brain areas that are analogous to the mammalian amygdala. Therefore, reversal of function of GABAA receptors via OA or pharmacological antagonism (gabazine) will induce an anxiogenic state (figure 1). This explains why gabazine does not have any further anxiogenic effect on OA-exposed rockfish.
A clear picture is emerging whereby alterations in GABAA receptor function have profound impacts on fish behaviour. OA has been reported to affect behaviours triggered by olfactory cues such as predator–prey interactions [8,10,11] and homing ability [10]. In addition, contamination of freshwater with wastewater effluents containing the anxiolytic drug oxazepam increases boldness of exposed fish [18]. The apparent shift in GABAA receptor function due to OA found here in rockfish results in increased anxiety, which has an opposite behavioural effect compared with pollution caused by oxazepam, yet both are a danger to wild fish populations. The proposed mechanism (figure 3) is evident after 7 days of OA exposure; however, the onset and long-lasting consequences are unknown. When OA-exposed rockfish were placed back into seawater containing the present-day CO2 level, they still demonstrated increased anxiety 7 days later, but not 12 days later. Thus, the impact of OA on rockfish anxiety seems to be reversible. This is similar to previous studies on reef fish, which showed restoring of predator avoidance 2 days after placing damselfish larvae in normal seawater [13]. Differences in the time OA-induced changes return to normal could be due to sensitivity or net Cl− flux through various subtypes of GABAA receptors involved, with more sustained effects on anxiety relative to olfactory circuitry. Species-specific and life stage differences, as well as magnitude of OA, are other potential explanations.
Short-term fluctuations in ocean dynamics, such as upwellings or currents that are characteristic of the southern California near-shore environment, result in low pH and high CO2 levels that are similar to our experimental OA condition. For example, while the pH of seawater is normally approximately 8.05 units and pCO2 is approximately 400 μatm, they can reach approximately 7.70 pH units and 1016 μatm for several days [22] (approx. 5 days in that particular study, but as long as 11 days in other surveys that estimated upwelling from dissolved O2 [47]). These values are very similar to our control (pH: 8.10; pCO2: 483 μatm) and experimental (pH: 7.75; pCO2: 1125 μatm) conditions and duration of the exposure (7 days). Therefore, it is possible that rockfish off the coast of southern California commonly experience increased anxiety during upwelling events.
GABAA receptors are present in the nervous system of vertebrates and invertebrates, and therefore the effect of OA on anxiety could be widespread. Interestingly, during development GABA receptors are expressed prior to the main excitatory neurotransmitter, glutamate. Giant depolarizing potentials are generated by GABAA receptors because of the high intracellular Cl− concentration present at birth, but as glutamatergic innervation increases, the intracellular Cl− concentration decreases and GABA then becomes inhibitory [41]. The excitatory action of GABA during development has been identified in every animal studied, including rats, mice, rabbits, frogs, kittens and ferrets [41]. Evolutionarily, it can be assumed that this development-induced shift in excitatory to inhibitory action of GABAA receptors is also present in fish, although the exact timing during development is unknown. Prior to this shift, the OA-induced shift in ECl is unlikely to induce any major effects on behaviour. However, as development proceeds and GABAA receptors become inhibitory, there may be profound consequences of OA on brain physiology. As GABAA receptors are ubiquitous and act physiologically to dampen excitatory circuitry throughout the brain, there are a multitude of potential neurological effects beyond what has been studied here. For example, in addition to the increased anxiety reported here, epileptic seizures may occur, as reported in the brain of adult mammals containing low GABA levels [41]. All of these effects would greatly influence population dynamics and other ecological interactions (e.g. predator–prey, dispersal). However, it is also possible that longer exposures to OA result in compensatory responses such as GABAA-receptor internalization or decreased GABA release in juvenile and adult fish, which would ameliorate the anxiolytic effect of OA on fish behaviour.
In summary, prolonged exposure to OA has detrimental effects on the neurophysiology of marine fish, resulting in increased anxiety behaviour. Future studies need to investigate the extent of OA-induced effects both physiologically and behaviourally, how these effects influence fish fitness in the wild, and whether fish are capable of adapting to OA.
Acknowledgements
We greatly appreciated the assistance of Christina Frieder (SIO) (seawater chemistry calculations), Rodney Schmaltz and Nicole Anderson (software), and Jan Kowalczeski (figure 3 design). Many thanks to Phil Zerofsky for helping with aquarium matters, and to Stuart Sandin (SIO) for supplying the fish used in this study. The authors confirm that there is no conflict of interest. T.J.H. and M.T. conceived and designed the study; T.J.H. and A.H. performed the study; T.J.H., A.H. and M.T. analysed the data; T.J.H. and M.T. wrote the paper.
All experiments were approved by the SIO-UCSD animal care committee under protocol no. S10320 in compliance with the IACUC guidelines for the care and use of experimental animals.
Funding statement
This study was funded by a MacEwan Research Office and Arts and Science grant to T.J.H., MacEwan Student Enrichment Fund to A.H., and USA National Science Foundation grant no. EF-1220641, UCSD Academic Senate award and SIO Funds and Alfred P. Sloan Research Fellowship (grant no. BR2013-103) to M.T.
References
- 1.Caldeira K, Wickett ME. 2003. Oceanography: anthropogenic carbon and ocean pH. Nature 425, 365 (doi:10.1038/425365a) [DOI] [PubMed] [Google Scholar]
- 2.Munday PL, McCormick MI, Nilsson GE. 2012. Impact of global warming and rising CO2 levels on coral reef fishes: what hope for the future? J. Exp. Biol. 215, 3865–3873 (doi:10.1242/jeb.074765) [DOI] [PubMed] [Google Scholar]
- 3.Riebesell U, Zondervan I, Rost B, Tortell PD, Zeebe RE, Morel FM. 2000. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 407, 364–367 (doi:10.1038/35030078) [DOI] [PubMed] [Google Scholar]
- 4.Raven J, Caldeira K, Elderfield H, Hoegh-Guldberg O, Liss P, Riebesell U, Shepherd J, Turley C, Watson A. 2005. Ocean acidification due to increasing atmospheric carbon dioxide, p. 60 London, UK: The Royal Society [Google Scholar]
- 5.Ishimatsu A, Hayashi M, Kikkawa T. 2008. Fishes in high-CO2, acidified oceans. Mar. Ecol. Prog. Ser. 373, 295–302 (doi:10.3354/meps07823) [Google Scholar]
- 6.Checkley DM, Dickson AG, Takahashi M, Radich JA, Eisenkolb N, Asch R. 2009. Elevated CO2 enhances otolith growth in young fish. Science 324, 1683 (doi:10.1126/Science.1169806) [DOI] [PubMed] [Google Scholar]
- 7.Bignami S, Sponaugle S, Cowen RK. 2013. Response to ocean acidification in larvae of a large tropical marine fish, Rachycentron canadum. Glob. Change Biol. 19, 996–1006 (doi:10.1111/gcb.12133) [DOI] [PubMed] [Google Scholar]
- 8.Nilsson GE, Dixson DL, Domenici P, McCormick MI, Sørensen C, Watson S-A, Munday PL. 2012. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat. Clim. Change 2, 201–204 (doi:10.1038/nclimate1352) [Google Scholar]
- 9.Jutfelt F, Bresolin de Souza K, Vuylsteke A, Sturve J. 2013. Behavioural disturbances in a temperate fish exposed to sustained high-CO2 levels. PLoS ONE 8, e65825 (doi:10.1371/journal.pone.0065825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, Doving KB. 2009. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl Acad. Sci. USA 106, 1848–1852 (doi:10.1073/pnas.0809996106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cripps IL, Munday PL, McCormick MI. 2011. Ocean acidification affects prey detection by a predatory reef fish. PLoS ONE 6, e22736 (doi:10.1371/journal.pone.0022736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixson DL, Munday PL, Jones GP. 2010. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 13, 68–75 (doi:10.1111/j.1461-0248.2009.01400.x) [DOI] [PubMed] [Google Scholar]
- 13.Munday PL, Dixson DL, McCormick MI, Meekan M, Ferrari MC, Chivers DP. 2010. Replenishment of fish populations is threatened by ocean acidification. Proc. Natl Acad. Sci. USA 107, 12 930–12 934 (doi:10.1073/pnas.1004519107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chivers DP, McCormick MI, Nilsson GE, Munday PL, Watson S-A, Meekan MG, Mitchell MD, Corkill KC, Ferrari MCO. In press. Impaired learning of predators and lower prey survival under elevated CO2: a consequence of neurotransmitter interference. Glob. Change Biol. (doi:10.1111/gcb.12291) [DOI] [PubMed] [Google Scholar]
- 15.Shin LM, Liberzon I. 2010. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35, 169–191 (doi:10.1038/npp.2009.83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodgers RJ, Dalvi A. 1997. Anxiety, defence and the elevated plus-maze. Neurosci. Biobehav. Rev. 21, 801–810 (doi:10.1016/S0149-7634(96)00058-9) [DOI] [PubMed] [Google Scholar]
- 17.Stewart A, et al. 2011. Pharmacological modulation of anxiety-like phenotypes in adult zebrafish behavioral models. Prog. Neuropsychopharmacol. Bol. Psychiatry 35, 1421–1431 (doi:10.1016/j.pnpbp.2010.11.035) [DOI] [PubMed] [Google Scholar]
- 18.Brodin T, Fick J, Jonsson M, Klaminder J. 2013. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science 339, 814–815 (doi:10.1126/science.1226850) [DOI] [PubMed] [Google Scholar]
- 19.Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. 1989. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. 416, 303–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heisler N. 1984. Acid-base regulation in fishes. In Fish physiology (eds Hoar WS, Randall DJ.), pp. 315–401 New York, NY: Academic Press [Google Scholar]
- 21.Heisler N. 1988. Acid-base regulation. In Physiology of elasmobranch fishes (ed. Shuttleworth TJ.), pp. 215–252 Berlin, Germany: Springer [Google Scholar]
- 22.Frieder CA, Nam SH, Martz TR, Levin LA. 2012. High temporal and spatial variability of dissolved oxygen and pH in a nearshore California kelp forest. Biogeosciences 9, 3917–3930 (doi:10.5194/bg-9-3917-2012) [Google Scholar]
- 23.Hauri C, et al. 2013. Spatiotemporal variability and long-term trends of ocean acidification in the California current system. Biogeosciences 10, 193–216 (doi:10.5194/bg-10-193-2013) [Google Scholar]
- 24.Gruber N, Hauri C, Lachkar Z, Loher D, Frölicher TL, Plattner G-K. 2012. Rapid progression of ocean acidification in the California current system. Science 337, 220–223 (doi:10.1126/science.1216773) [DOI] [PubMed] [Google Scholar]
- 25.Bockmon EE, Frieder CA, Navarro MO, White-Kershek LA, Dickson AG. 2013. Controlled experimental aquarium system for multi-stressor investigation: carbonate chemistry, oxygen saturation, and temperature. Biogeosci. Discuss. 10, 3431–3453 (doi:10.5194/bgd-10-3431-2013) [Google Scholar]
- 26.Lewis E, Wallace DWR.1998. CO2SYS program developed for the CO2 system calculations. Report ORNL/CDIAC-105. Oak Ridge, TN: Oak Ridge National Laboratory, Carbon Dioxide Information Analysis Center.
- 27.van Heuven S, Pierrot D, Rae JWB, Lewis E, Wallace DRW. 2011. MATLAB program developed for CO2 system calculations. Report ORNL/CDIAC-105b. Oak Ridge, TN: Oak Ridge National Laboratory, Carbon Dioxide Information Analysis Center. [Google Scholar]
- 28.Mehrbach C, Culberson C, Hawley J, Pytkowicz R. 1973. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907 (doi:10.4319/lo.1973.18.6.0897) [Google Scholar]
- 29.Dickson AG, Millero F. 1987. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res. 34, 1733–1743 (doi:10.1016/0198-0149(87)90021-5) [Google Scholar]
- 30.Holcombe A, Howorko A, Powell RA, Schalomon M, Hamilton TJ. 2013. Reversed scototaxis during withdrawal after daily-moderate, but not weekly-binge, administration of ethanol in zebrafish. PLoS ONE 5, e63319 (doi:10.1371/journal.pone.0063319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maximino C, Marques de Brito T, Dias CA, Gouveia A, Jr, Morato S. 2010. Scototaxis as anxiety-like behavior in fish. Nat. Protoc. 5, 209–216 (doi:10.1038/nprot.2009.225) [DOI] [PubMed] [Google Scholar]
- 32.Maximino C, et al. 2007. A comparative analysis of the preference for dark environments in five teleosts. Int. J. Comp. Psychol. 20, 351 [Google Scholar]
- 33.Pham J, Cabrera SM, Sanchis-Segura C, Wood MA. 2009. Automated scoring of fear-related behavior using EthoVision software. J. Neurosci. Methods 178, 323–326 (doi:10.1016/j.jneumeth.2008.12.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heaulme M, Chambon JP, Leyris R, Molimard JC, Wermuth CG, Biziere K. 1986. Biochemical characterization of the interaction of three pyridazinyl-GABA derivatives with the GABAA receptor site. Brain Res. 384, 224–231 (doi:10.1016/0006-8993(86)91158-3) [DOI] [PubMed] [Google Scholar]
- 35.Andrews PR, Johnston GA. 1979. GABA agonists and antagonists. Biochem. Pharmacol. 28, 2697–2702 (doi:10.1016/0006-2952(79)90549-5) [DOI] [PubMed] [Google Scholar]
- 36.Lee S-Y, Jung C-S. 2009. Intraocular Injection of muscimol induces illusory motion reversal in goldfish. Korean J. Physiol. Pharmacol. 13, 469–473 (doi:10.4196/kjpp.2009.13.6.469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yazulla S, Brecha N. 1980. Binding and uptake of the GABA analogue, 3H-muscimol, in the retinas of goldfish and chicken. Invest. Ophtalmol. Vis. Sci. 19, 1415–1426 [PubMed] [Google Scholar]
- 38.Clements S, Schreck CB. 2001. The GABAa agonist muscimol enhances locomotor activity, but does not alter the behavioural effects of CRH in juvenile spring chinook salmon (Oncorhynchus tshawytscha). Fish Physiol. Biochem. 24, 41–48 (doi:10.1023/A:1011102420877) [Google Scholar]
- 39.Mathur P, Guo S. 2011. Differences of acute versus chronic ethanol exposure on anxiety-like behavioral responses in zebrafish. Behav. Brain Res. 219, 234–239 (doi:10.1016/j.bbr.2011.01.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson PA. 2001. Behavioral ecology of young-of-the-year kelp rockfish, Sebastes atrovirens Jordan and Gilbert (Pisces: Scorpaenidae). J. Exp. Mar. Biol. Ecol. 256, 33–50 (doi:10.1016/S0022-0981(00)00305-1) [DOI] [PubMed] [Google Scholar]
- 41.Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. 2007. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 87, 1215–1284 (doi:10.1152/physrev.00017.2006) [DOI] [PubMed] [Google Scholar]
- 42.Maximino C, de Brito TM, da Silva Batista AW, Herculano AM, Morato S, Gouveia A., Jr 2010. Measuring anxiety in zebrafish: a critical review. Behav. Brain Res. 214, 157–171 (doi:10.1016/j.bbr.2010.05.031) [DOI] [PubMed] [Google Scholar]
- 43.Maximino C, Puty B, Benzecry R, Araujo J, Lima MG, de Jesus Oliveira Batista E, Renata de Matos Oliveira K, Crespo-Lopez ME, Herculano AM. 2013. Role of serotonin in zebrafish (Danio rerio) anxiety: relationship with serotonin levels and effect of buspirone, WAY 100635, SB 224289, fluoxetine and para-chlorophenylalanine (pCPA) in two behavioral models. Neuropharmacology 71, 83–97 (doi:10.1016/j.neuropharm.2013.03.006) [DOI] [PubMed] [Google Scholar]
- 44.Maximino C, de Brito TM, Colmanetti R, Pontes AA, de Castro HM, de Lacerda RI, Morato S, Gouveia A., Jr 2010. Parametric analyses of anxiety in zebrafish scototaxis. Behav. Brain Res. 210, 1–7 (doi:10.1016/j.bbr.2010.01.031) [DOI] [PubMed] [Google Scholar]
- 45.Labarrera C, London M, Angelo K. 2013. Tonic inhibition sets the state of excitability in olfactory bulb granule cells. J. Physiol. 591, 1841–1850 (doi:10.1113/jphysiol.2012.241851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shekhar A, Sajdyk TJ, Gehlert DR, Rainnie DG. 2003. The amygdala, panic disorder, and cardiovascular responses. Ann. N. Y. Acad. Sci. 985, 308–325 (doi:10.1111/j.1749-6632.2003.tb07090.x) [DOI] [PubMed] [Google Scholar]
- 47.Send U, Nam S. 2012. Relaxation from upwelling: the effect on dissolved oxygen on the continental shelf. J. Geophys. Res.-Oceans 117, C04024 (doi:10.1029/2011jc007517) [Google Scholar]