Abstract
Signals of dominance and fighting ability (i.e. status signals) are found in a wide range of taxa and are used to settle disputes between competitive rivals. Most previous research has considered status-signal phenotype as an attribute of the individual; however, it is more likely that signal expression is an emergent property that also incorporates aspects of the social environment. Furthermore, because an individual's signal phenotype is likely to influence its social interactions, the relationships between status signals, social environment and individual quality are probably much more complex than previously appreciated. Here, we explore the dynamic relationship between social interactions and signal expression in a previously undescribed status signal, the frontal shield of the pukeko (Porphyrio porphyrio melanotus: Aves). We demonstrate that frontal shield size is a strong predictor of dominance status within social groups, even after controlling for potentially confounding variables. Then, we evaluate the relationship between social interactions and signal expression by testing whether manipulating apparent shield size influences (i) dominance interactions and (ii) future signal expression. By showing that decreasing apparent shield size causes both an increase in the amount of aggression received and a decrease in an individual's true shield size, we provide the first evidence of dynamic feedback between signal expression and social interactions. Our study provides important insight into the role of receiver-dependent (i.e. social) costs in maintaining signal honesty and demonstrates a unique approach to studying status signalling applicable to future studies on dynamic morphological signals.
Keywords: badges of status, handicap hypothesis, honest signalling, dominance, resource-holding potential
1. Introduction
Signals of dominance and fighting ability are used to settle disputes over mates and other resources, and are found in a wide range of taxa [1]. In some species, signals used in a competitive context are inherently related to some aspect of biological quality (e.g. mammalian acoustical formant frequency is intrinsically tied to body size [2,3]), and thus the production of reliable signals is enforced. However, many animals use convention-based signals of quality (i.e. ‘arbitrary signals’) that are not obviously physically tied to any aspect of quality. Understanding the factors that maintain a correlation between these signal traits and the unobservable qualities that they reveal has been a major focus of behavioural biology for the last 40 years [4–9].
A prevailing view in animal communication research is that conventional signals are honest because their production is differentially costly to individuals of different quality (the handicap principle [4,5]). Under this model, signal phenotypes are determined by the intrinsic quality of the individual, and signal reliability is maintained by receiver-independent costs. Receivers are selected to attend to these signal traits because they provide reliable information that can be incorporated into deciding whether to engage in a physical contest with a rival. However, this model is problematic for two reasons: first, there is considerable evidence that conventional signal expression is influenced by social interactions [10–15]. For example, previous studies have demonstrated that changes in dominance rank per se can cause corresponding changes in status-signal phenotypes [12–14], and also that population density can influence signal honesty [15]. Second, the aspects of quality that status signals are purported to correlate with (e.g. health status, androgen levels) are highly dynamic and themselves respond to various social factors [16,17]. Thus, signal expression in many species is probably an emergent property that incorporates both aspects of an individual's intrinsic quality and also the individual's social environment. As a result, the simplistic model in which status signals influence social interactions in a unidirectional manner is probably unrealistic, and the relationships between signal expression, social interactions and individual quality are likely to be much more complex than is widely appreciated.
Perhaps the best method of investigating the relationship between signals and social interactions is through cosmetic signal manipulation. While many studies have examined the effect of signal manipulation on receiver behaviour [7], we know much less about the feedback effects of signal manipulation on the signaller themselves (but see [18,19]). Because cosmetic signal manipulation does not have a direct effect on the receiver-independent costs of signal production, any observed differences in the cost of bearing signals is likely to be owing to changes in social interactions (i.e. the receiver-dependent costs). If signal expression is influenced by receiver-dependent costs, then manipulation of signal phenotype should cause changes in the social interactions experienced by the signaller. Furthermore, because the social environment can have a strong influence on animal phenotypes [10–15], it is also possible that changes in apparent signal phenotype may provide feedback on the same processes that control true signal expression. While such a feedback mechanism of signal expression is yet to be demonstrated, recent evidence suggests that the relationship between signal expression and individual quality is more dynamic than previously appreciated [20]. In a study on North American barn swallows (Hirundo rustica erythrogaster), Safran et al. [19] found that experimentally enhancing male chest plumage reverses a seasonal decline in androgens, likely owing to changes in social interactions experienced by the manipulated individuals. Because androgens have been widely implicated in the regulation of status signals, it seems likely that signal expression could be influenced by the very social interactions that are the outcome of the signal itself.
In this study, we explore putative feedback effects between social interactions and signal expression in a hitherto undescribed badge of status, the frontal shield of the pukeko (Porphyrio porphyrio melanotus). This cooperatively breeding bird lives in permanent, mixed-sex social groups [21,22]. Within each group, individuals have frequent agonistic interactions over access to resources [23,24]. Both male and female pukeko have conspicuous frontal shield ornaments that extend from the bill upwards to cover the front of the crown. These ornaments, which are found in several species in the family Rallidae, are testosterone-dependent and have the ability to change size over short time periods [25,26]. Pukeko prominently display their frontal shields during aggressive interactions [23], and population differences in shield dimorphism are thought to be owing to variation in the intensity of intrasexual competition [27].
To explore the relationship between dominance and frontal shields, we first investigated the relationship between frontal shield size and dominance status, while controlling for other traits that could be important in determining social rank. As pukeko prominently display frontal shields in aggressive interactions, we predicted that frontal shield size would be correlated with dominance status. We then explored whether there is a dynamic relationship between signal phenotype and social interactions by testing two key predictions of the model outlined above: (i) that changes in signal expression influence social interactions and (ii) that changes in signal expression cause feedback effects that alter future signal expression. In order to test these predictions, we reduced the apparent size of the frontal shield in two separate experiments, and assessed whether the manipulation caused changes in dominance interactions and true shield size, respectively.
2. Material and methods
(a). Behavioural observations
This study was conducted at the Tawharanui Open Sanctuary, New Zealand (36°22′ S, 174°49′ E). In 2010 and 2012, pukeko were banded as part of a larger study on social behaviour. Upon capture, a suite of morphological measurements were taken including measures of body size (mass) and frontal shield size. Birds were also sexed by measurement according to Craig et al. [28]. Shield area (as determined using digital photography) is highly correlated with field-measured maximum shield width (R2 = 0.87, n = 50), thus we use maximum shield width as our measure of shield size throughout, as it is straightforward and highly repeatable (standard error of measurement = 0.13 mm, mean adult shield size = 24.8 mm). Previous research has demonstrated that frontal shield colour does not correlate with dominance status, and thus we do not consider shield colour in this study (C. J. Dey 2012, unpublished data).
In 2012, we performed detailed behavioural observations on 11 social groups in which all group members were banded. Observations were conducted during January and February 2012, which is outside of the peak breeding season for pukeko at this site (breeding typically occurs between August and November at this site). Each group was observed for 30 min per day between 06.30 and 10.00, for 10 days. Approximately 50 g of dried maize was placed in a small pile on the territory of the focal group immediately prior to each observation period to increase the frequency of interaction between group members [24]. Pukeko were accustomed to human presence at this site and quickly resumed normal behaviours following this disturbance. Observers were concealed in a camouflaged hide and recorded all dominance interactions during the observation period.
(b). Frontal shield manipulations I: effects on dominance behaviour
From April 2013 to June 2013, we performed a shield reduction experiment to test the prediction that changes in signal phenotype would influence dominance interactions involving the focal individual. We randomly selected one adult male out of the banded males in each of 22 social groups (on average 68% of the birds were banded in these groups). Next, we performed a series of five baseline behavioural observations (duration: 30 min each) on each of the focal males (one per day for 5 days). These observations were similar to the group behavioural observations described above, except that the observer followed a single individual (i.e. the focal individual) and recorded the observation period with a video camera (Sony HDR-PJ260, Tokyo, Japan). Following these baseline observations, the focal individual was trapped (n = 6 individuals could not be trapped and were therefore excluded), and alternately assigned to a shield reduction (n = 8) or a control treatment (n = 8). The shield reduction treatment was conducted by applying a small amount of black paint (Spraypack Quick Dry, Dulux, Lower Hutt, New Zealand) to the perimeter of the shield using a small brush, such that the paint made a 6 mm border surrounding the shield. As the plumage surrounding the shield is also black, this treatment caused the shield of the manipulated individuals to appear smaller. Individuals assigned to the control group had red paint applied in a similar fashion. This treatment did not change the apparent shield size in the control individuals. A pilot study demonstrated that such treatments last for 4–6 days and that the paint used closely matches the reflectance of the plumage (black) and shield (red), respectively (see the electronic supplementary material). The paint was allowed to dry for 5 min before birds were released back onto their territory. To determine whether manipulation of apparent signal phenotype influenced aggressive interactions, we then conducted a further five behavioural observations (duration: 30 min each) on each focal individual, beginning 2 days after the manipulation. Videos collected from this experiment were reviewed by two individuals who were blind to the treatments and the study objectives. They observed the videos in a randomized order and recorded all dominance interactions that occurred between the focal individual and other group members.
(c). Frontal shield manipulations II: effects on shield size
During March and April 2012, we manipulated frontal shield size to test the prediction that changes in apparent shield size would cause changes in true shield size. In 25 social groups, we captured a single male pukeko per group. The first male to enter the trap in each social group was used. Morphometric measurements were taken as described above. Captured individuals were then randomly assigned to a shield reduction treatment (n = 13) or a control treatment (n = 12) by coin-flip, which was identical to the procedure described above. Approximately one week after the treatment (mean = 6.1 days, range = 6–9 days), we recaptured as many manipulated and control individuals as possible (n = 9 control, n = 7 treatment). Upon capture, individuals were subjected to the same set of morphological measurements as were performed prior to the treatment. In most birds, the treatment had worn off by the time of recapture and the measurements were performed blind to the treatment. However, in four birds the treatment was visible during this recapture event, and thus analyses were performed both with and without these birds.
(d). Analysis
All analysis was conducted using R v. 3.0.1 [29]. First, we examined the relationship between social status (David's dominance score [30]) and frontal shield size. Typically, studies of status signalling test for a correlation between signal phenotype and some measure (or proxy measure) of dominance to suggest that the focal signal is informative. We followed these methods, but importantly we also tested to see whether social dominance is predicted by shield size after controlling for confounds. We used two linear mixed-effect models to evaluate the relationship between social dominance and shield size, using normalized David's score as our response variable (see the electronic supplementary material). In our first model, we used shield width as the sole fixed effect to estimate the potential information content of frontal shield status signals. In our second model, we included shield width, mass, sex and two-way interactions between shield width and sex, and mass and sex. These interactions were included because the relationship between badge size and dominance, and mass and dominance, could vary between the sexes owing to differential selective pressure on competitive traits [31,32]. If shield width is a significant predictor of dominance even after controlling for these covariates, it would suggest a strong relationship between signal phenotype and dominance that is independent of body size and sex.
We used a general linear model to test for treatment effects on true shield size. In this analysis, the shield width at recapture was modelled as a function of treatment (reduction/control) and the individual's pretreatment shield width. Additionally, we used three Poisson family generalized linear mixed models (GLMMs) to analyse how our shield reduction treatment affected the dominance interactions directed at our focal individuals. In each of these models, we included two fixed effects: time (before/after treatment) and treatment (reduction/control), as well as the interaction between these effects. Thus, a significant interaction term would support our prediction that manipulating frontal shield size should cause changes in dominance interactions. The response variables considered in these models were: (i) the number of aggressive challenges received (‘upright aggressive’ displays [23]), (ii) the number of attacks received (‘kicks’, the primary form of physical aggression [23]) and (iii) the number of ‘wings up’ displays received (an aggressive display used towards non-group members [23]). Further details on model fitting are described in the electronic supplementary material. Data are available from the Dryad Digital Repository [33]. All figures were created using the ggplot2 package in R [34].
3. Results
(a). Behavioural observations
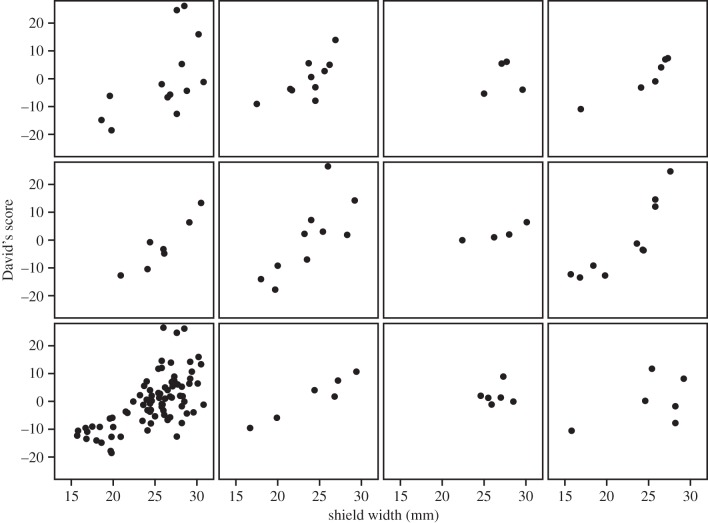
Dominance rank was highly correlated with frontal shield size (model 1, table 1; figure 1; partial R2 = 0.51), and this finding was conserved even after controlling for body size and sex (model 2, table 1).
Table 1.
Linear mixed-effect model of factors predicting social dominance in pukeko. The model allows for a random intercept for each social group and includes data from 82 individuals in 11 groups. Significant p-values are shown in italic.
| fixed effect | estimate | 95% CI estimate | p-value |
|---|---|---|---|
| model 1 | |||
| shield width | 0.19 | 0.15, 0.24 | <0.0001 |
| model 2 | |||
| shield width | 0.09 | 0.03, 0.16 | 0.008 |
| mass | 0.005 | 0.002, 0.007 | 0.0002 |
| sex | 0.001 | −0.27, 0.27 | 0.99 |
| shield width × sex | 0.05 | −0.01, 0.11 | 0.10 |
| mass × sex | 0.001 | −0.002, 0.003 | 0.62 |
Figure 1.
The relationship between shield width and David's score (an index of social dominance) is shown for 82 individuals in 11 pukeko social groups (one per panel). Higher David's scores indicate more dominant individuals. The bottom left panel shows all individuals across all social groups combined. See text for details of statistical analysis.
(b). Frontal shield manipulations I: effects on dominance behaviour
Overall, focal individuals received less aggression in the post-treatment behavioural observations than during the pretreatment behavioural observations (significant effect of time; models 1 and 2, table 2; figure 2). However, the shield reduction group received relatively more aggressive displays and physical attacks in the post-treatment period than did the control group (significant interaction between treatment and time; models 1 and 2, table 2; figure 2). This change in dominance interactions was probably not due to a disruption of individual recognition mechanisms, as there was no significant interaction between time and treatment on the number of wings up displays (table 2). Furthermore, no manipulated individuals were evicted from their group in this study.
Table 2.
Poisson family GLMMs showing the effect of shield size reductions on dominance behaviours directed towards the focal individual. Models include data from 10 behavioural observations for each of 16 focal individuals. Individual ID is included in each model as a random intercept. Significant p-values are shown in italic.
| response variable | fixed effect | estimate | 95% CI estimate | p-value |
|---|---|---|---|---|
| challenges | treatment | 0.20 | −1.19, 1.61 | 0.77 |
| time | −0.79 | −1.56, −0.02 | 0.04 | |
| treatment × time | 1.77 | 0.24, 3.30 | 0.02 | |
| attacks | treatment | −0.93 | −3.00, 1.15 | 0.38 |
| time | −1.60 | −2.87, −0.34 | 0.01 | |
| treatment × time | 2.23 | 0.66, 3.80 | 0.005 | |
| wings up displays | treatment | −1.62 | −3.78, 0.54 | 0.14 |
| time | −0.34 | −0.85, 0.17 | 0.19 | |
| treatment × time | −0.12 | −1.15, 0.90 | 0.81 |
Figure 2.
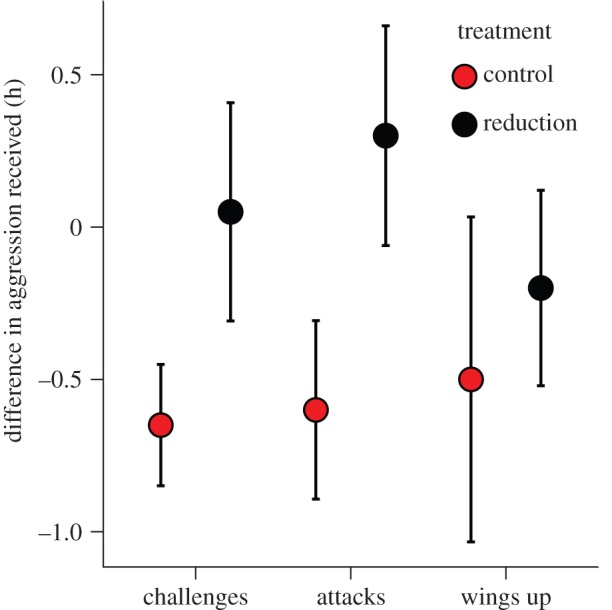
Aggressive behaviours directed at focal individuals with natural (control) or decreased (reduction) apparent shield size. Data are the mean differences in the rate each type of behaviour was received (±s.e.) between the pretreatment and post-treatment observations. See text for details of statistical analysis. (Online version in colour.)
(c). Frontal shield manipulations II: effects on shield size
Prior to treatment, birds assigned to the reduction and control treatments did not differ in shield size (mean shield width ± s.e., reduction: 27.9 mm ± 0.53, control: 27.6 mm ± 0.43, p = 0.40). However, after treatment, birds in the shield reduction group had smaller apparent shield size than those in the control group (reduction: 13.6 ± 0.44 mm, control: as above, p < 0.0001). True shield size at the time of recapture was significantly predicted by pre-treatment shield size (GLMM: estimate = 0.90, 95% CI = (0.77, 1.04), p < 0.0001) and was also influenced by treatment, with birds that received the shield reduction treatment having significantly smaller shields than those with the control treatment (estimate = −0.78, 95% CI = (−1.23, −0.32), p = 0.003; figure 3). Qualitatively similar results were found when only birds who were measured blind to the treatment were included (n = 4 individuals removed).
Figure 3.
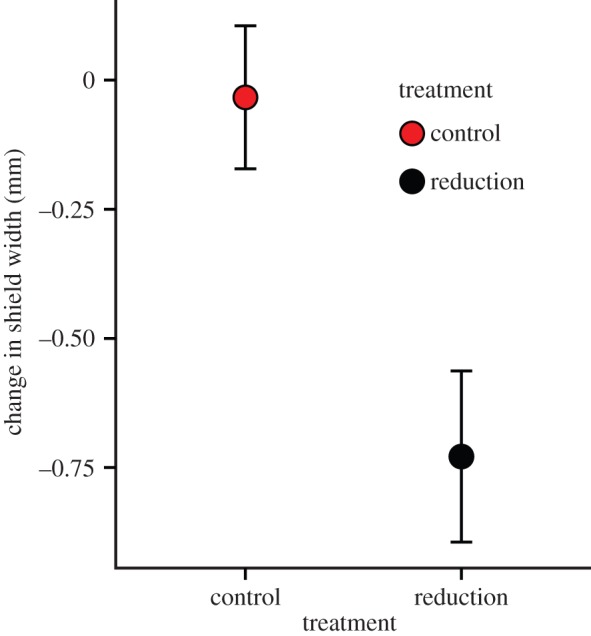
The change in true shield width as a result of apparent shield manipulation. Data shown are the mean change (±s.e.) over a one-week period after manipulation for individuals that received the control (n = 9) and shield reduction treatments (n = 7). See text for details of statistical analysis. (Online version in colour.)
4. Discussion
In this study, we found that frontal shield size is strongly correlated with dominance status in pukeko. Furthermore, the relationship between shield size and dominance remained even after controlling for important confounds that could explain the relationship between ornament size and status. These results, in combination with the fact that frontal shields are prominently displayed during aggressive interactions [23], are highly suggestive that the pukeko's frontal shield acts as a status signal. We also found strong evidence for a dynamic relationship between signal expression and social interactions. Pukeko that had the apparent size of their frontal shield decreased received more aggression and also decreased their true shield relative to individuals who did not have their apparent shield size altered. As our manipulation did not directly affect the receiver-independent costs of signalling, these changes in true shield size must have been mediated by changes in social interactions (see also [19]).
Our results are not surprising, given that previous studies have shown that changes in the social environment can lead to changes in signal expression [10–15], and also that changes in apparent signal expression can cause feedback effects on individual physiology [19]. Proximate control of many avian status signals relies on androgen hormones [7], and androgens are sensitive to social factors [17,19]. It is possible that the increase in aggression received by manipulated individuals leads to a decrease in circulating androgen levels, which could have decreased shield size. While this suggestion is speculative, endocrine physiology can influence both behavioural and morphological traits, and thus is likely to be important to understanding the complex relationships between signals and social environments [20].
There is growing evidence that the social environment has an important influence on status signal expression, and that receiver-dependent costs are important in the enforcement of signal honesty in many species [7,9]. A possible mechanism by which receiver-dependent costs could influence signal expression is through the recognition and punishment of incongruent signals [35–38]. This hypothesis predicts that receivers are sensitive to other cues of quality in addition to badges (e.g. behaviour, body size, individual recognition) and when the various cues to fighting ability are inconsistent, they should challenge signallers to ascertain their true quality. Recognition and punishment of incongruent signalling has been convincingly demonstrated in paper wasps (Polistes dominulus). In this species, individuals who had either their facial markings or behaviour experimentally enhanced (i.e. made more dominant) had increased fight costs in paired interactions with unfamiliar individuals [36,38], whereas individuals who had both their facial markings and behaviour enhanced (and thus displayed congruent signals) did not incur these increased costs [38]. The data presented in the present study are also consistent with recognition and punishment of incongruent signals. Pukeko whose frontal shields were made more subordinate (and thus incongruent with the other signals it was producing) received more aggressive challenges. However, it is also possible that manipulated individuals were simply perceived as subordinates, and were therefore challenged and attacked when they did not yield to other group members. This study cannot discriminate between these two mechanisms, and further studies aimed at understanding how receivers integrate signal phenotypes with other dominance cues will be useful in understanding how receiver-dependent costs contribute to signal honesty.
While status signalling has been demonstrated in a wide variety of taxa (e.g. reptiles [39], insects [36], birds [7], fish [13] and mammals [3]), the correlation between signal traits and the qualities that they signal is likely to vary among species and with the signal modality involved. Signals that are relatively inflexible could become less informative in environments with high temporal heterogeneity because individual condition may change between the time when the signal was produced and when the signal is used. For example, avian plumage badges are a classic example of a conventional signal of dominance. However, the relationship between plumage badge size or colour and social dominance is often weak, especially after controlling for other confounding variables, such as body size, sex and age [7,40–42]. Feather growth typically occurs during discrete life-history stages and is constrained by energetic and physiological limitations. Thus, plumage traits will typically be representative of the condition of the individual at some past time-point when the feathers were grown (although see [43,44] for examples of the dynamic properties of plumage ornaments). However, birds can also signal quality with non-plumage traits (e.g. shields, legs, eye rings, bills and wattles), which are typically vascularized and are therefore able to respond rapidly to changes in individual condition [11,45,46]. While non-plumage traits have a well-established signalling role in mate choice [45–47], their role in a competitive context has been underappreciated (but see [11,48–51]) despite their potential to be more informative to rivals than plumage badges. We suggest that future studies on dynamic status signals (e.g. avian bare-parts) will help in understanding the complex relationships between individual quality, social interactions and signal expression because of the increased opportunity to investigate the direct and indirect factors that mediate signal expression.
Social dominance is important in the lives of many animals, and signals of dominance and fighting ability will often be under strong selection because of their role in determining access to mates and other resources. Social factors are ultimately crucial to understanding honest signalling, not only because the behaviour of signal receivers will determine the benefits of producing a certain signal, but also because there may be receiver-dependent costs that make dishonest signalling unprofitable in a range of species. In this study, we identified a hitherto undescribed status signal (the pukeko's frontal shield) that strongly predicts dominance rank, even after controlling for body size and sex. This strong correlation between signal size and dominance status may be due in part to the ability of bare-part ornaments to dynamically respond to short-term changes in individual condition or the social environment. Furthermore, we show that changes in signal phenotype can influence an individual's social interactions, and also provide feedback on future signal expression. As a result, this study adds to the growing evidence for a dynamic and bidirectional relationship between social interactions and signal phenotype. This study also demonstrates a unique approach to measuring the receiver-dependent costs of signals that could be widely applicable to future studies of dynamic signals.
Acknowledgements
The authors thank Diane Fields, Andrew Green, Adam Snowball, Chris Mariella and Charlene Williams for assistance with data collection. Furthermore, we thank the Tawharanui Open Sanctuary Society and Auckland Council staff, especially Alison Stanes, Colin Wards, Maurice Puckett and Matt Maitland. This manuscript was improved by comments from Constance O'Connor, Associate Editor Rebecca Safran and two anonymous reviewers.
Data accessibility
Data from this study are available from the Dryad Digital Repository: doi:10.5061/dryad.r6797.
Funding statement
This research was financially supported by a National Science and Engineering Research Council (NSERC) Operating grant to J.S.Q. and an NSERC Canadian Graduate Scholarship to C.J.D. Travel was supported by the Society for Integrative and Comparative Biology, the Animal Behavior Society and the McMaster Graduate Student Association.
References
- 1.Searcy W, Nowicki S. 2005. The evolution of animal communication. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Fitch WT. 1997. Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. J. Acoust. Soc. Am. 102, 1213–1222 (doi:10.1121/1.419022) [DOI] [PubMed] [Google Scholar]
- 3.Vannoni E, McElligott AG. 2008. Low frequency groans indicate larger and more dominant fallow deer (Dama dama) males. PLoS ONE 3, e3113 (doi:10.1371/journal.pone.0003113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zahavi A. 1975. Mate selection: a selection for a handicap. J. Theor. Biol. 53, 205–214 (doi:10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 5.Grafen A. 1990. Biological signals as handicaps. J. Theor. Biol. 144, 517–546 (doi:10.1016/S0022-5193(05)80088-8) [DOI] [PubMed] [Google Scholar]
- 6.Johnstone RA, Norris K. 1993. Badges of status and the cost of aggression. Behav. Ecol. Sociobiol. 32, 127–134 (doi:10.1007/BF00164045) [Google Scholar]
- 7.Senar JC. 2006. Color displays as intrasexual signals of aggression and dominance. In Bird coloration, vol. 2: function and evolution (eds Hill GE, McGraw KJ.), pp. 87–136 Cambridge, MA: Harvard University Press [Google Scholar]
- 8.Grose J. 2011. Modelling and the fall and rise of the handicap principle. Biol. Phil. 26, 677–696 (doi:10.1007/s10539-011-9275-1) [Google Scholar]
- 9.Számadó Sz. 2011. The cost of honesty and the fallacy of the handicap principle. Anim. Behav. 81, 3–10 (doi:10.1016/j.anbehav.2010.08.022) [Google Scholar]
- 10.West-Eberhard MJ. 1983. Sexual selection, social competition, and speciation. Q. Rev. Biol. 58, 155–183 (doi:10.1086/413215) [Google Scholar]
- 11.Zuk M, Johnsen TS. 2000. Social environment and immunity in male red jungle fowl. Behav. Ecol. 11, 146–153 (doi:10.1093/beheco/11.2.146) [Google Scholar]
- 12.Karubian J, Lindsay WR, Schwabl H, Webster MS. 2011. Bill coloration, a flexible signal in a tropical passerine bird, is regulated by social environment and androgens. Anim. Behav. 81, 795–800 (doi:10.1016/j.anbehav.2011.01.012) [Google Scholar]
- 13.Rhodes SB, Schlupp I. 2012. Rapid and socially induced change of a badge of status. J. Fish Biol. 80, 722–727 (doi:10.1111/j.1095-8649.2011.03212.x) [DOI] [PubMed] [Google Scholar]
- 14.Setchell JM, Dixson AF. 2001. Changes in the secondary sexual adornments of male mandrills (Mandrillus sphinx) are associated with the gain and loss of alpha status. Horm. Behav. 39, 177–184 (doi:10.1006/hbeh.2000.1628) [DOI] [PubMed] [Google Scholar]
- 15.Bywater CL, Wilson RS. 2012. Is honesty the best policy? Testing signal reliability in fiddler crabs when receiver-dependent costs are high. Funct. Ecol. 26, 804–811 (doi:10.1111/j.1365-2435.2012.02002.x) [Google Scholar]
- 16.Barnard CJ, Behnke JM, Sewell J. 1993. Social behaviour, stress and susceptibility to infection in house mice (Mus musculus): effects of duration and aggressive behaviour prior to infection on susceptibility to Babesia microti. Parasitology 107, 183–192 (doi:10.1017/S0031182000067299) [DOI] [PubMed] [Google Scholar]
- 17.Oliveira RF, Silva A, Canário AV. 2009. Why do winners keep winning? Androgen mediation of winner but not loser effects in cichlid fish. Proc. R. Soc. B 276, 2249–2256 (doi:10.1098/rspb.2009.0132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moller AP. 1989. Viability costs of male tail ornaments in a swallow. Nature 339, 132–135 (doi:10.1038/339132a0) [Google Scholar]
- 19.Safran RJ, Adelman JS, McGraw KJ, Hau M. 2008. Sexual signal exaggeration affects physiological state in male barn swallows. Curr. Biol. 18, R461–R462 (doi:10.1016/j.cub.2008.03.031) [DOI] [PubMed] [Google Scholar]
- 20.Rubenstein DR, Hauber ME. 2008. Dynamic feedback between phenotype and physiology in sexually selected traits. Trends Ecol. Evol. 23, 655–658 (doi:10.1016/j.tree.2008.07.010) [DOI] [PubMed] [Google Scholar]
- 21.Craig JL. 1980. Pair and group breeding behaviour of a communal gallinule, the pukeko, Porphyrio p. melanotus. Anim. Behav. 28, 593–603 (doi:10.1016/S0003-3472(80)80068-6) [Google Scholar]
- 22.Jamieson IG. 1997. Testing reproductive skew models in a communally breeding birds, the pukeko, Porphyrio porphyrio. Proc. R. Soc. Lond. B. 264, 335–340 (doi:10.1098/rspb.1997.0048) [Google Scholar]
- 23.Craig JL. 1977. The behaviour of the pukeko, Porphyrio porphyrio melanotus. New Zeal. J. Zool. 4, 413–433 (doi:10.1080/03014223.1977.9517966) [Google Scholar]
- 24.Jamieson IG, Craig JL. 1987. Dominance and mating in a communal polygynandrous bird: cooperation or indifference towards mating competitors? Ethology 75, 317–327 (doi:10.1111/j.1439-0310.1987.tb00663.x) [Google Scholar]
- 25.Gullion GW. 1951. The frontal shield of the American Coot. Wilson Bull. 63, 157–166 [Google Scholar]
- 26.Eens M, Duyse EV, Berghman L, Pinxten R. 2000. Shield characteristics are testosterone-dependent in both male and female moorhens. Horm. Behav. 37, 126–134 (doi:10.1006/hbeh.1999.1569) [DOI] [PubMed] [Google Scholar]
- 27.Dey CJ, Jamieson I, Quinn JS. 2012. Reproductive skew and female trait elaboration in a cooperatively breeding rail. Ibis 154, 452–460 (doi:10.1111/j.1474-919X.2012.01223.x) [Google Scholar]
- 28.Craig JL, McArdle BH, Wettin PD. 1980. Sex determination of the pukeko or purple swamphen. Notornis 27, 287–291 [Google Scholar]
- 29.R Development Core Team 2013. R: a language and environment for statistical computing. Vienna, Austria R Foundation for Statistical Computing: See http://www.R-project.org/ [Google Scholar]
- 30.David HA. 1987. Ranking from unbalanced paired-comparison data. Biometrika 74, 432–436 (doi:10.1093/biomet/74.2.432) [Google Scholar]
- 31.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 32.Amundsen T. 2000. Why are female birds ornamented? Trends Ecol. Evol. 15, 149–155 (doi:10.1016/S0169-5347(99)01800-5) [DOI] [PubMed] [Google Scholar]
- 33.Dey CJ, Dale J, Quinn JS. 2013. Data from: manipulating the appearance of a badge of status causes changes in true badge expression. Dryad Digital Repository. (doi:10.5061/dryad.r6797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickham H. 2009. ggplot2: elegant graphics for data analysis. New York, NY: Springer; See http://had.co.nz/ggplot2/book [Google Scholar]
- 35.Rohwer SA, Rohwer FC. 1978. Status signalling in Harris’ sparrows: experimental deceptions achieved. Anim. Behav. 26, 1012–1022 (doi:10.1016/0003-3472(78)90090-8) [Google Scholar]
- 36.Tibbetts EA, Dale J. 2004. A socially enforced signal of quality in a paper wasp. Nature 432, 218–222 (doi:10.1038/nature02949) [DOI] [PubMed] [Google Scholar]
- 37.Caryl PG. 1982. Telling the truth about intentions. J. Theor. Biol. 97, 679–689 (doi:10.1016/0022-5193(82)90366-6) [Google Scholar]
- 38.Tibbetts EA, Izzo A. 2010. Social punishment of dishonest signalers caused by mismatch between signal and behavior. Curr. Biol. 20, 1637–1640 (doi:10.1016/j.cub.2010.07.042) [DOI] [PubMed] [Google Scholar]
- 39.Whiting MJ, Nagy KA, Bateman PW. 2003. Evolution and maintenance of social status signalling badges: experimental manipulation in lizards. In Lizard social behavior (eds Fox SF, McCoy JK, Baird TA.), pp. 47–82 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 40.Balph MH, Balph DF, Romesburg HC. 1979. Social status signalling in winter flocking birds: an examination of a current hypothesis. Auk 96, 78–93 [Google Scholar]
- 41.Watt DJ. 1986. A comparative study of status signalling in sparrows (genus Zonotrichia). Anim. Behav. 34, 1–15 (doi:10.1016/0003-3472(86)90001-1) [Google Scholar]
- 42.Whitfield DP. 1987. Plumage variability, status signalling and individual recognition in avian flocks. Trends Ecol. Evol. 2, 13–18 (doi:10.1016/0169-5347(87)90194-7) [DOI] [PubMed] [Google Scholar]
- 43.Møller AP, Erritzøe J. 1992. Acquisition of breeding coloration depends on badge size in male house sparrows Passer domesticus. Behav. Ecol. Sociobiol. 31, 271–277 (doi:10.1007/BF00171682) [Google Scholar]
- 44.Adamik P, Vaňáková M. 2011. Feather ornaments are dynamic traits in the Great Tit Parus major. Ibis 153, 357–362 (doi:10.1111/j.1474-919X.2010.01097.x) [Google Scholar]
- 45.Rosen RF, Tarvin KA. 2006. Sexual signals of the American goldfinch. Ethology 112, 1008–1019 (doi:10.1111/j.1439-0310.2006.01257.x) [Google Scholar]
- 46.Velando A, Beamonte-Barrientos R, Torres R. 2006. Pigment-based skin color in the blue-footed booby: an honest signal of current condition used by females to adjust reproductive investment. Oecologia 149, 535–542 (doi:10.1007/s00442-006-0457-5) [DOI] [PubMed] [Google Scholar]
- 47.Hill GE. 2006. Female mate choice for ornamental coloration. In Bird coloration, vol. 2: function and evolution (eds Hill GE, McGraw KJ.), pp. 137–200 Cambridge, MA: Harvard University Press [Google Scholar]
- 48.Ligon JD, Thornhill R, Zuk M, Johnson K. 1990. Male-male competition, ornamentation and the role of testosterone in sexual selection in red jungle fowl. Anim. Behav. 40, 367–373 (doi:10.1016/S0003-3472(05)80932-7) [Google Scholar]
- 49.Papeschi A, Carroll JP, Dessi-Fulgheri F. 2003. Wattle size is correlated with male territorial rank in juvenile ring-necked pheasants. Condor 105, 362–366 (doi:10.1650/0010-5422(2003)105[0362:WSICWM]2.0.CO;2) [Google Scholar]
- 50.Crowley CE, Magrath RD. 2004. Shields of offence: signalling competitive ability in the dusky moorhen, Gallinula tenebrosa. Aust. J. Zool. 52, 463–474 (doi:10.1071/ZO04013) [Google Scholar]
- 51.Murphy TG, Rosenthal MF, Montgomerie R, Tarvin KA. 2009. Female American goldfinches use carotenoid-based bill coloration to signal status. Behav. Ecol. 20, 1348–1355 (doi:10.1093/beheco/arp140) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from this study are available from the Dryad Digital Repository: doi:10.5061/dryad.r6797.